Abstract
Acyl-coenzymeA:cholesterol acyltransferase (ACAT) catalyzes the intracellular synthesis of cholesteryl esters (CE). Both ACAT isoforms, ACAT1 and ACAT2, play key roles in the pathophysiology of atherosclerosis and ACAT inhibition retards atherosclerosis in animal models. Rimonabant, a type 1 cannabinoid receptor (CB1) antagonist, produces anti-atherosclerotic effects in humans and animals by mechanisms which are not completely understood. Rimonabant is structurally similar to two other cannabinoid receptor antagonists, AM251 and SR144528, recently identified as potent inhibitors of ACAT. Therefore, we examined the effects of Rimonabant on ACAT using both in vivo cell-based assays and in vitro cell-free assays. Rimonabant dose-dependently reduced ACAT activity in Raw 264.7 macrophages (IC50= 2.9 ± 0.38 µM) and isolated peritoneal macrophages. Rimonabant inhibited ACAT activity in intact CHO-ACAT1 and CHO-ACAT2 cells and in cell-free assays with approximately equal efficiency (IC50=1.5 ± 1.2 µM and 2.2 ± 1.1 µM for CHO-ACAT1 and CHO-ACAT2, respectively). Consistent with ACAT inhibition, Rimonabant treatment blocked ACAT-dependent processes in macrophages, oxysterol-induced apoptosis and acetylated-LDL induced foam cell formation. From these results we conclude that Rimonabant is an ACAT1/2 dual inhibitor and suggest that some of the atherosclerotic beneficial effects of Rimonabant are, at least partly, due to inhibition of ACAT.
Keywords: Acyl-coenzymeA:cholesterol acyltransferase (ACAT), atherosclerosis, type 1 cannabinoid receptor (CB1), Rimonabant, foam cell formation, oxysterol-induced apoptosis
Atherosclerosis is a chronic inflammatory disease associated with a buildup of cholesteryl esters (CE) in arterial walls. Intracellular synthesis of CE is carried out by two acyl-coenzyme A:cholesterol acyltransferase (ACAT) isoforms, ACAT1 and ACAT2, using cholesterol and long-chain fatty acyl-coenzyme A as substrates [1]. Both ACAT isoforms have been implicated in the pathogenesis of atherosclerosis. ACAT1 expressed by macrophages is responsible for esterification of cholesterol obtained from the uptake of modified low density lipoproteins (LDL), such as oxidized LDL (oxLDL), in the vascular intima [2]. Macrophages that accumulate large amounts of CE in cytosolic lipid droplets can transform into foam cells, a hallmark of early atherosclerotic lesions [3]. ACAT1 also plays a role in two macrophage apoptosis pathways relevant to atherosclerosis. Oxysterol constituents of oxLDL induce macrophage apoptosis by a mechanism that is, at least partly, dependent upon ACAT1 [4], while loss of ACAT1 activity in macrophages ingesting modified LDL leads to a buildup of free cholesterol which induces apoptosis via the unfolded protein response mechanism [5]. ACAT2 expression is restricted to intestinal enterocytes where it plays a key role in absorption of dietary cholesterol and hepatocytes where it plays a role in CE enrichment of LDL [2]. In animal studies, administration of a nonselective ACAT inhibitor reduces foam cell formation, lowers plasma cholesterol levels and reduces formation of atherosclerotic lesions [6; 7; 8].
Cannabinoids and endocannabinoids produce the majority of their effects by binding to two G-protein coupled receptors, CB1 and CB2. CB1 receptors expressed in the central nervous system are responsible for the psychoactive effects of cannabinoids [9], while CB2 receptors expressed by immune cells, including macrophages, are responsible for the anti-inflammatory and immunosuppressive effects of cannabinoids [10]. CB2 receptors are present in atherosclerotic lesions and exogenous cannabinoid compounds reduce the progression of atherosclerosis in ApoE-null mice by a mechanism that is sensitive to co-administration of a CB2 receptor-selective antagonist [11; 12]. In addition, CB2-deficient macrophages display partial resistance to oxLDL/oxysterol-induced apoptosis [13].
SR141716A (Rimonabant) is an inverse agonist of CB1 initially developed as an anti-obesity drug [14]. In clinical trials, Rimonabant produced cardiovascular beneficial effects beyond that expect from weight loss alone [15; 16] and, in one recent study employing intravascular ultrasonography, a reduction in the total volume of coronary atheromas [17]. Rimonabant significantly reduces the development of atherosclerotic lesions in hyperlipidemic mice by exerting a number of anti-atherosclerotic effects including; reducing serum cholesterol levels, reducing proinflammatory cytokines, inhibiting monocyte/macrophage proliferation and migration, and inducing reverse cholesterol transport in macrophages [18; 19]. However, the precise mechanisms by which Rimonabant exerts these anti-atherosclerotic effects remain to be determined.
Recently, we found that two compounds with structural and pharmacological similarities to Rimonabant, AM251 and SR144528, effectively inhibit ACAT activity in macrophages and prevent foam cell formation [20]. These compounds share structural homology to the diphenylethane backbone of Sandoz compound 58-035, a pharmacophore for ACAT inhibition [21]. The lone structural difference between AM251 and Rimonabant is the substitution of a p-iodo group on the phenyl substituent of C-5 of the pyrazole ring with a p-chloro group. From these observations, we hypothesized that some of the anti-atherosclerotic effects of Rimonabant result from inhibition of ACAT, independent of its effects on cannabinoid receptor signaling. In the present study we test this hypothesis by evaluating the ability of Rimonabant to inhibit cholesteryl ester synthesis in vivo and in vitro.
Materials and methods
Cells and Reagents
Raw 264.7 cells were cultured in RPMI-1640 containing 10% Fetal Bovine Serum (FBS), 2 mM Glutamine, 100 U/mL Penicillin, and 100 µg/mL streptomycin at 37°C in 5% CO2/95% air humidified atmosphere. AC29 cells, mutant CHO cells lacking endogenous ACAT [22], stably transfected with human ACAT1 (CHO-ACAT1) or ACAT2 (CHO-ACAT2), a kind gift from TY Chang (Dartmouth Medical School, Hanover, New Hampshire), were cultured in 1:1 DMEM: Ham’s F12 media containing 5% heat-inactivated FBS, Fungizone, 100 U/mL Penicillin, 100 µg/mL streptomycin, and 200 µg/mL G418. SR141716 (Rimonabant) was obtained from Sanofi-Adventis R&D, Montpellier, France. 9,1022123H Oleic acid and [oleoyl-1-14C]-CoA were from American Radiolabeled Chemicals, Inc. (St. Louis, MO) and acetylated LDL (AcLDL) from Biomedical Technologies, Inc (Stoughton, MA).
Mice
Resident peritoneal macrophages were isolated by peritoneal lavage from WT mice as previously described [13]. All animal procedures were approved and conducted in accordance with the guidelines administered by the Animal Research Facility and the Institutional Animal Care and Usage Committee of East Tennessee State University.
PCR verification of CHO-ACAT1 and CHO-ACAT2
Genomic DNA was isolated from CHO-ACAT1 and CHO-ACAT2 using QIAamp DNA blood mini kit (Qiagen) and subjected to polymerase chain reaction (PCR) analysis using the following primers. ACAT1 upstream primer 5’-TCCTACCGTTGTTTGGACCTGGTGG-3’; ACAT1 downstream primer 5’-TAGGACCAGAACACGAGCGCTGAAG-3’; ACAT2 upstream primer 5’-GCCTGGGCTGTGCGCTTTTA-3’; ACAT2 downstream primer 5’-TGGCAACGTGGCATGCAGGA-3’.
Neutral lipid synthesis
Cells (2×106 / well) seeded in12-well plates were allowed to attach overnight, rinsed with PBS twice and refed media containing 0.5% FBS supplemented with varying amounts of Rimonabant. All wells were adjusted to receive an equivalent volume of vehicle (DMSO). After 1 h each well was adjusted to contain 1.0 µCi/ml [3H]-oleate and the incubation continued for 6 h before the amount [3H]-oleate incorporated into [3H]-cholesteryl oleate was determined as previously described [20]. All treatments were performed in triplicate and the data presented as the percentage of the mean ± SD of untreated controls.
Cell free ACAT Activity Assay
Microsomes were prepared from CHO-ACAT1 and CHO-ACAT2 cells by homogenizing in 3.0 ml of cold buffer A (100mM sucrose, 50mM KCl, 40mM KH2PO4, and 30mM EDTA, pH 7.4) followed by centrifugation at 100,000 × g for 1h at 8°C. The microsomal pellets were resuspended in buffer A to a final concentration of 20 mg/ml protein and stored as aliquots at −80°C until used. Reactions monitoring the incorporation of [14C] oleoyl Coenzyme A into [14C] cholesteryl oleate were performed essentially as we described previously [20] with the following modifications; reactions contained 0.5 mg/ml microsomal proteins and were carried out for 10 minutes.
Caspase-3 Activity Assay
Raw 264.7 cells (2×106/well) in 12-well plates were rinsed with PBS and refed culture media supplemented with varying amounts of Rimonabant 1h prior to supplementation with 7-ketocholesterol (7KC). All wells were adjusted to receive equal amounts of vehicle. Following a 16 h incubation, caspase-3 and caspase 3-like activity was determined, as previously described [20].
Immunoblotting
Raw 264.7 cells (2×106/well) in 12-well plates were treated with or without 7KC (20 µg/ml) in the absence or presence of Rimonabant (1µM or 2µM) for 16h. Cell lysates containing 20 µg of protein were resolved on 4–12% SDS-PAGE gels and transferred to a Polyvinylidene fluoride membrane. After blocking in TBS-T containing 5% non fat dry milk, the membranes were probed with antibodies to cleaved PARP (Cell Signaling, Danvers, MA) and cleaved caspase-3 (Cell signaling, Danvers, MA) (1:1000 dilution) overnight at 4ºC, rinsed and incubated with a goat anti-rabbit secondary antibody conjugated to horse radish peroxidase (1:50,000 dilution) and developed using Super signal-West Pico Chemiluminescent Substrate (Pierce). The membranes were then striped and reprobed with an antibody to β-actin (Neomarkers, Fremont, CA) as a loading control.
Foam Cell Formation
The accumulation of cytosolic lipid droplets determined as previously described [20]. Briefly, Raw 264.7 macrophage were seeded at 1×105 per well in a 4-chamber slide (LabTek), allowed to adhere, rinsed with PBS twice and refed RPMI-1640 + 2% FBS supplemented with and without Rimonabant. After 1h, AcLDL (100 µg/ml) was added to each well and the incubation continued for 16 h at 37 ºC before staining with Oil Red O.
Statistical analysis
Statistical differences were determined by Student’s t-test with a p-value ≤ 0.05 considered significant.
Results
Rimonabant inhibits the synthesis of cholesteryl esters in unstimulated and stimulated macrophages
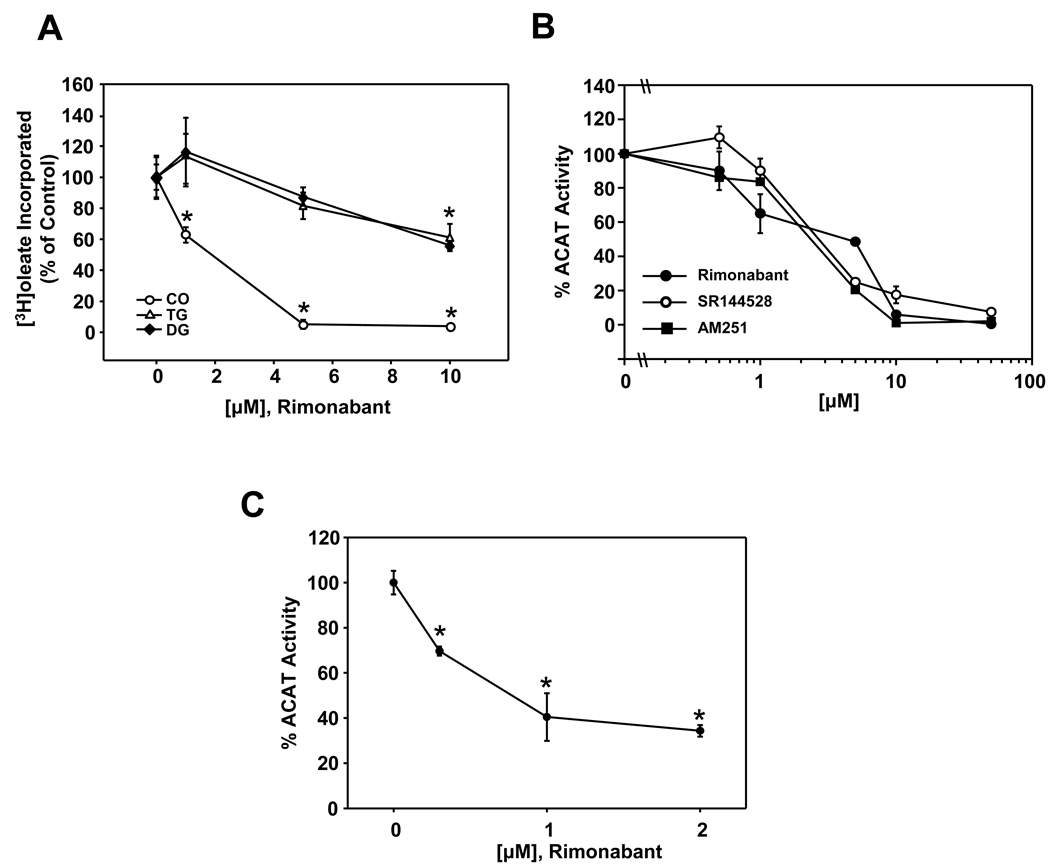
To determine if Rimonabant displays properties consistent with that of an ACAT inhibitor, we monitored the metabolic incorporation of [3H] oleate into cholesteryl [3H] oleate (CO), [3H] triacylglycerol (TG), and [3H] diacylglycerol (DG)] in Raw 264.7 macrophages cultured in the presence of increasing amounts of Rimonabant. Rimonabant dose-dependently inhibited CO synthesis in Raw 264.7 macrophages, with 1 µM producing a significant (~40%) decrease compared to untreated controls and concentrations ≥ 5 µM producing near complete inhibition (Fig. 1A). A small, but significant, reduction of TG and DG synthesis was also observed with Rimonabant at concentrations ≥ 10 µM. Inhibition of CO synthesis in Raw 264.7 macrophages by Rimonabant (IC50 value 2.9 ± 0.38 µM) was very similar to that of AM251 and SR144528 (IC50 value 2.6 ± 0.26 µM and 2.5 ± 0.32 µM, respectively) (Fig. 1B), two related compounds previously demonstrated to be potent ACAT inhibitors. Mouse peritoneal macrophages also displayed significantly reduced CO synthesis in response to Rimonabant treatment (Fig. 1C).
Fig. 1.
Rimonabant selectively inhibits cholesteryl ester synthesis in macrophages. (A) The effect of Rimonabant on incorporation of [3H] oleate into neutral lipids in Raw 264.7 macrophages. Cells were incubated in medium supplemented with increasing concentrations of Rimonabant as indicated for 1 hr prior to addition of [3H] oleate (1 µCi/ml). Following a 6 h incubation, total lipids were extracted and the amount of cholesteryl [3H] oleate (CO), [3H] triacylglycerol (TG), and [3H] diacylglycerol (DG) formed was determined. (B) Comparison of the effect of Rimonabant, AM251 and SR144528 on the incorporation of [3H] oleate into [3H] CO in Raw 264.7 macrophages. C) Effect of Rimonabant on [3H] CO synthesis in isolated peritoneal macrophages. Values are expressed as the percentage of the mean dpm/mg protein ± SD determined for untreated control cells for triplicate samples. Graphs are representative of triplicate experiments each performed independently. Error bars for some data points are smaller than the symbol and are not visible. * = P <0.05.
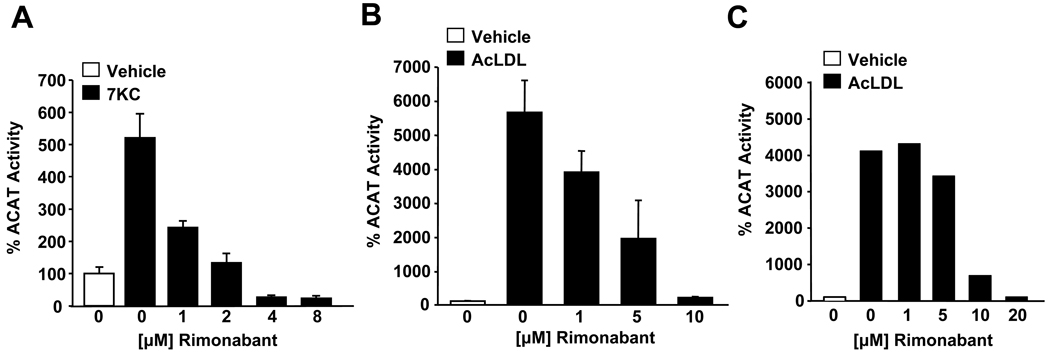
We also examined the ability of Rimonabant to inhibit CO synthesis in macrophages in which ACAT activity was stimulated by the addition of an oxysterol, 7-ketocholesterol (7KC), or acetylated low density lipoproteins (AcLDL) (Fig. 2). 7KC-stimulated CO synthesis in Raw 264.7 macrophages was significantly reduced by Rimonabant at concentrations ≥ 1 µM (Fig 2A). AcLDL-stimulated CO synthesis in Raw 264.7 macrophages (Fig. 2B) and mouse peritoneal macrophages (Fig. 2C) was also significantly inhibited by Rimonabant.
Fig. 2.
Rimonabant inhibits oxysterol-stimulated and AcLDL-stimulated ACAT activity in macrophages. (A) Raw 264.7 macrophages were cultured in the presence of increasing concentrations of Rimonabant as indicated for 1h prior to stimulation of ACAT activity with 7-ketocholesterol (7KC, 20 µg/ml). The formation of [3H] CO from [3H] oleate was then determined as described in fig 1. Raw 264.7 macrophages (B) and isolated peritoneal macrophages (C) were incubated in the presence of increasing amounts of Rimonabant as indicated for 1h prior to stimulation of ACAT activity with AcLDL (100 µg/ml) and [3H] CO synthesis was determined. ACAT activity is expressed as the percentage of the mean dpm/mg protein ± SD determined for untreated controls for triplicate samples. Graphs are representative of duplicate experiments performed independently. * = P <0.05.
Rimonabant is a nonselective dual inhibitor of ACAT isoforms
We next monitored the effect of Rimonabant on sterol esterification in AC29 cells, a mutant Chinese hamster ovary (CHO) cell line lacking endogenous ACAT, stably expressing human ACAT1 or ACAT2 (Fig. 3). Rimonabant at concentrations ≥ 1µM significantly inhibited CO synthesis in CHO-ACAT1 and CHO-ACAT2 cells in a concentration-dependent manner with similar efficiency (IC50 values of 1.5 ± 1.2 µM and 2.2 ± 1.1 µM, respectively, Fig. 3B). Rimonabant also dose-dependently inhibited ACAT activity in vitro, as determined by monitoring the incorporation of [14C] oleoyl Coenzyme A into cholesteryl [14C] oleate, in cell-free assays utilizing mouse liver microsomes (data not shown) and microsomes isolated from CHO-ACAT1 and CHO-ACAT2 cells (Fig. 3C).
Fig. 3.
Rimonabant inhibits ACAT1 and ACAT2 in vivo and in vitro with equivalent efficiency. (A) Genomic DNA isolated from CHO-ACAT1 and CHO-ACAT2 cells (AC29 cells stably expressing human ACAT1 or ACAT2) was subjected to PCR using human ACAT1-specific primers (P1) and human ACAT2-specific primers (P2) as indicated. PCR products were analyzed on agarose gels along with a DNA ladder (Std) for verification of the correct size of the PCR amplification products (550 bp and 446 bp for ACAT1 and ACAT2, respectively). (B) CHO-ACAT1 and CHO-ACAT2 cells were cultured in the presence of increasing amounts of Rimonabant as indicated for 6 h and the formation of [3H] CO from [3H] oleate was determined as described above. (C) Cell-free assay mixtures containing [14C] oleoyl CoA (0.25 µCi/ml) and microsomes isolated from CHO-ACAT1 or CHO-ACAT2 cells were incubated for 10 minutes at 37°C in the presence of increasing concentrations of Rimonabant as indicated. The reactions were terminated and the amount of cholesteryl [14C] oleate formed was determined. Data is presented as the percentage of cholesteryl [14C] oleate formed in the absence of Rimonabant for triplicate samples. Error bars for some data points are smaller than the symbol and are not visible. Graphs are representative of duplicate experiments performed independently. * = P <0.05.
Rimonabant inhibits 7KC-induced apoptosis and AcLDL-induced cytosolic lipid droplet accumulation in macrophages
We next investigated the effects of Rimonabant on ACAT-dependent processes in macrophages relevant to atherosclerosis, including macrophage-derived foam cell formation and oxysterol-induced apoptosis of macrophages [4; 6]. Previous work demonstrated that 7-ketocholesterol (7KC)-induced apoptosis in macrophages is a caspase-3 dependent process that is reliably detected by assaying for caspase-3 activity and immunoblotting for cleavage of procaspase-3 and poly (ADP-ribose) polymerase (PARP) [4]. Rimonabant significantly reduced caspase-3 activity in Raw 264.7 macrophages induced by 7KC treatment (Fig. 4A). Consistent with inhibition of 7KC-induced apoptosis, Raw 264.7 macrophages treated with Rimonabant also contained reduced levels of cleaved (active) caspase-3 and cleaved poly (ADP-ribose) polymerase (PARP) compared to macrophages treated with 7KC in the absence of Rimonabant (Fig 4B). Rimonabant had no effect on capase-3 activity in macrophages cultured in the absence of 7KC (Fig. 4A) or in macrophages undergoing apoptosis induced by staurosporine (Fig 4C). At these concentrations of Rimonabant, no effect on cell viability as evaluated by modified MTT assays was observed (Fig. 4D).
Fig. 4.
Rimonabant inhibits oxysterol-induced macrophage apoptosis and AcLDL-induced foam cell formation. Raw 264.7 macrophages were treated with varying amounts of Rimonabant 1h prior to the addition of 7KC (20 µg/ml) as indicated in the figure. After 16h, the induction of apoptosis was evaluated by (A) caspase-3 activity assays and (B) immunoblotting for cleavage of caspase 3 and PARP. (C) Effect of Rimonabant on induction of apoptosis by staurosporine (1 µM) in Raw 264.7 macrophages as determined by caspase-3 activity. (D) Rimonabant does not affect cell viability as determined by modified MTT assay. (E) Representative photomicrographs of Raw 264.7 macrophages cultured in the absence or presence of Rimonabant (8 µM) for 1hr prior to addition of AcLDL (100 µg/ml) for 16h prior to staining with oil red-O to visualize cytosolic accumulation of neutral lipid droplets. Original magnification = 40×. Bar, 10 µm. * = P <0.05.
Macrophage foam cell formation depends upon ACAT-mediated esterification of LDL-derived cholesterol resulting in the accumulation of CE in cytosolic lipid droplets. Macrophages cultured in the presence of modified LDL develop characteristics of foam cells, including the accumulation of cytosolic lipid droplets. Intracellular cytosolic lipid droplets were readily detected in Raw 264.7 macrophages cultured for 16 h in the presence of AcLDL (100 µg/ml), but were noticeably absent from macrophages cultured in the absence of AcLDL (Fig. 4E). Rimonabant supplementation dramatically decreased the accumulation of cytosolic lipid droplets in response to AcLDL (Fig. 4E). No effects on macrophage morphology or viability were observed under these conditions.
Discussion
In the current study, we have shown that the selective CB1 receptor antagonist, Rimonabant, in low µM concentrations, potently inhibits cholesteryl ester synthesis in intact cells and in cell-free assays. Our results further demonstrate that Rimonabant is a nonselective dual inhibitor of both known ACAT isoforms, ACAT1 and ACAT2, and that treatment of macrophages with Rimonabant reduces foam cell formation and oxysterol-induced macrophage apoptosis, two ACAT-dependent processes associated with the pathophysiology of atherosclerosis. Given that ACAT inhibition is well known to exert beneficial effects on atherogenesis, these results suggest that some of the observed effects of Rimonabant on atherosclerosis may be due; at least in part, to inhibition of ACAT activity.
Low-density lipoprotein receptor knockout mice fed an atherogenic diet supplemented with a high dose (50 mg/kg/day) of Rimonabant displayed significantly decreased atherosclerosis associated with decreased serum levels of total cholesterol, LDL-cholesterol, and triglycerides (TG) and increased HDL cholesterol [18]. Such an effect has been found with oral administration of nonselective ACAT inhibitors which are known to affect hepatic ACAT and lower plasma LDL-cholesterol (LDL-C) and TG levels by suppressing VLDL secretion [8; 23; 24; 25], and affect intestinal ACAT preventing the absorption of dietary cholesterol. Considering that in the current study Rimonabant displayed effective inhibition of ACAT2, the primary ACAT isoform in hepatocytes and intestinal enterocytes, we hypothesize that high doses of Rimonabant may reduce total cholesterol and LDL-C levels by preventing VLDL secretion from the liver and/or preventing absorption of cholesterol in the gut.
Recently, it was reported that AM251 decreases expression of CD36 and increases ATP-binding cassette protein A1 (ABCA1) expression in Raw 264.7 macrophages, and that Rimonabant enhances ATP-binding cassette protein G1 (ABCG1) and scavenger receptor B1 (SR-B1) in THP-1 macrophages [19; 26]. These reports suggest that CB1 receptor blockade enhances reverse cholesterol transport (RCT) in macrophages [19; 26]. ACAT inhibition is known to enhance RTC from macrophages [27; 28; 29; 30], by increasing the pool of free cholesterol available for conversion into oxysterol ligands of the nuclear receptors, LXRα and LXRβ, leading to activation of genes involved in RCT [31]. The observation that Rimonabant efficiently inhibited CO synthesis in cell free assays with nearly the same efficiency as in intact cells demonstrates that Rimonabant directly inhibits ACAT. This suggests that Rimonabant-induced and AM251-induced effects on RCT genes in macrophages are due to inhibition of ACAT, independent of or in addition to, effects resulting from antagonism of CB1 receptors.
Rimonabant inhibits 7KC-induced apoptosis in macrophages at concentrations which significantly reduce ACAT activity. As oxysterol esterification is a required step in the pathway of 7KC-induced apoptosis in macrophages [4], it is likely that the inhibitory effect of Rimonabant on 7KC-induced apoptosis is a consequence of ACAT inhibition. Macrophage apoptosis is advantageous in early lesion formation where it retards lesion development, but detrimental in advanced lesions where it contributes to necrotic core formation and plaque instability [5]. In addition to oxLDL/oxysterol-induced apoptosis, apoptosis induced by accumulation of free cholesterol (FC) loading in macrophages is also affected by ACAT activity. Whereas oxysterol-induced macrophage apoptosis requires ACAT activity, FC loading-induced apoptosis increases as a consequence of reduced ACAT activity. Thus, in the setting of atherosclerosis, Rimonabant would be expected to reduce oxysterol-induced apoptosis and enhance FC-loading induced apoptosis which may produce potentially detrimental effects on plaque stability.
In conclusion, due to its selectivity for the CB1 receptor, the majority of pharmacological effects produced by Rimonabant are likely attributable to its antagonistic properties. However, the observation that Rimonabant is a direct and nonselective ACAT inhibitor suggests a mechanism by which Rimonabant may also produce significant non-cannabinoid receptor mediated affects on pathophysiological conditions involving aberrant cholesterol homeostasis, such as atherosclerosis. Future studies should be aimed at determining the biochemical mechanisms and in vivo relevance of Rimonabant-induced ACAT inhibition. In addition, studies examining the molecular basis of any Rimonabant-induced effects should account for the possible consequences of non-CB receptor mediated inhibition of ACAT.
Research Highlights
Rimonabant reduces cholesteryl ester synthesis in macrophages.
Rimonabant inhibits acyl CoA:cholesterol acyltransferase 1 and 2 in intact cells.
Rimonabant inhibits acyl CoA:cholesterol acyltransferase 1 and 2 in cell free assays.
Rimonabant reduces oxysterol-induced apoptosis in macrophages.
Rimonabant retards macrophage foam cell formation.
Acknowledgments
Supported by NIH National Heart Lung and Blood Institute grant HL085137 (DPT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang TY, Li BL, Chang CC, Urano Y. Acyl-coenzyme A:cholesterol acyltransferases. Am J Physiol Endocrinol Metab. 2009;297:E1–E9. doi: 10.1152/ajpendo.90926.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudel LL, Lee RG, Cockman TL. Acyl coenzyme A: cholesterol acyltransferase types 1 and 2: structure and function in atherosclerosis. Curr Opin Lipidol. 2001;12:121–127. doi: 10.1097/00041433-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb. 1994;14:840–856. doi: 10.1161/01.atv.14.5.840. [DOI] [PubMed] [Google Scholar]
- 4.Freeman NE, Rusinol AE, Linton M, Hachey DL, Fazio S, Sinensky MS, Thewke D. Acyl-coenzyme A:cholesterol acyltransferase promotes oxidized LDL/oxysterol-induced apoptosis in macrophages. J Lipid Res. 2005;46:1933–1943. doi: 10.1194/jlr.M500101-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. 2009;(50 Suppl):S382–S387. doi: 10.1194/jlr.R800032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyazaki A, Sakai M, Sakamoto Y, Horiuchi S. Acyl-coenzyme A:cholesterol acyltransferase inhibitors for controlling hypercholesterolemia and atherosclerosis. Curr Opin Investig Drugs. 2003;4:1095–1099. [PubMed] [Google Scholar]
- 7.Fujiwara Y, Kiyota N, Hori M, Matsushita S, Iijima Y, Aoki K, Shibata D, Takeya M, Ikeda T, Nohara T, Nagai R. Esculeogenin A, a new tomato sapogenol, ameliorates hyperlipidemia and atherosclerosis in ApoE-deficient mice by inhibiting ACAT. Arterioscler Thromb Vasc Biol. 2007;27:2400–2406. doi: 10.1161/ATVBAHA.107.147405. [DOI] [PubMed] [Google Scholar]
- 8.Terasaka N, Miyazaki A, Kasanuki N, Ito K, Ubukata N, Koieyama T, Kitayama K, Tanimoto T, Maeda N, Inaba T. ACAT inhibitor pactimibe sulfate (CS-505) reduces and stabilizes atherosclerotic lesions by cholesterol-lowering and direct effects in apolipoprotein E-deficient mice. Atherosclerosis. 2007;190:239–247. doi: 10.1016/j.atherosclerosis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 10.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 11.Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Karsak M, Zimmer A, Frossard JL, Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434:782–786. doi: 10.1038/nature03389. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Yuan Z, Liu Y, Xue J, Tian Y, Liu W, Zhang W, Shen Y, Xu W, Liang X, Chen T. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J Cardiovasc Pharmacol. 55:292–298. doi: 10.1097/FJC.0b013e3181d2644d. [DOI] [PubMed] [Google Scholar]
- 13.Freeman-Anderson NE, Pickle TG, Netherland CD, Bales A, Buckley NE, Thewke DP. Cannabinoid (CB2) receptor deficiency reduces the susceptibility of macrophages to oxidized LDL/oxysterol-induced apoptosis. J Lipid Res. 2008;49:2338–2346. doi: 10.1194/jlr.M800105-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 15.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. Jama. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 16.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 17.Nissen SE, Nicholls SJ, Wolski K, Rodes-Cabau J, Cannon CP, Deanfield JE, Despres JP, Kastelein JJ, Steinhubl SR, Kapadia S, Yasin M, Ruzyllo W, Gaudin C, Job B, Hu B, Bhatt DL, Lincoff AM, Tuzcu EM. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. Jama. 2008;299:1547–1560. doi: 10.1001/jama.299.13.1547. [DOI] [PubMed] [Google Scholar]
- 18.Dol-Gleizes F, Paumelle R, Visentin V, Mares AM, Desitter P, Hennuyer N, Gilde A, Staels B, Schaeffer P, Bono F. Rimonabant, a selective cannabinoid CB1 receptor antagonist, inhibits atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:12–18. doi: 10.1161/ATVBAHA.108.168757. [DOI] [PubMed] [Google Scholar]
- 19.Sugamura K, Sugiyama S, Fujiwara Y, Matsubara J, Akiyama E, Maeda H, Ohba K, Matsuzawa Y, Konishi M, Nozaki T, Horibata Y, Kaikita K, Sumida H, Takeya M, Ogawa H. Cannabinoid 1 receptor blockade reduces atherosclerosis with enhances reverse cholesterol transport. J Atheroscler Thromb. 17:141–147. doi: 10.5551/jat.2865. [DOI] [PubMed] [Google Scholar]
- 20.Thewke D, Freeman-Anderson N, Pickle T, Netherland C, Chilton C. AM-251 and SR144528 are acyl CoA:cholesterol acyltransferase inhibitors. Biochem Biophys Res Commun. 2009;381:181–186. doi: 10.1016/j.bbrc.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Medina P, Payre BL, Bernad J, Bosser I, Pipy B, Silvente-Poirot S, Favre G, Faye JC, Poirot M. Tamoxifen is a potent inhibitor of cholesterol esterification and prevents the formation of foam cells. J Pharmacol Exp Ther. 2004;308:1165–1173. doi: 10.1124/jpet.103.060426. [DOI] [PubMed] [Google Scholar]
- 22.Chang TY, Hasan MT, Chin J, Chang CC, Spillane DM, Chen J. Chinese hamster ovary cell mutants affecting cholesterol metabolism. Curr Opin Lipidol. 1997;8:65–71. doi: 10.1097/00041433-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Burnett JR, Wilcox LJ, Telford DE, Kleinstiver SJ, Barrett PH, Newton RS, Huff MW. Inhibition of ACAT by avasimibe decreases both VLDL and LDL apolipoprotein B production in miniature pigs. J Lipid Res. 1999;40:1317–1327. [PubMed] [Google Scholar]
- 24.Kitayama K, Tanimoto T, Koga T, Terasaka N, Fujioka T, Inaba T. Importance of acyl-coenzyme A:cholesterol acyltransferase 1/2 dual inhibition for anti-atherosclerotic potency of pactimibe. Eur J Pharmacol. 2006;540:121–130. doi: 10.1016/j.ejphar.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Wrenn SM, Jr, Parks JS, Immermann FW, Rudel LL. ACAT inhibitors CL 283,546 and CL 283,796 reduce LDL cholesterol without affecting cholesterol absorption in African green monkeys. J Lipid Res. 1995;36:1199–1210. [PubMed] [Google Scholar]
- 26.Jiang LS, Pu J, Han ZH, Hu LH, He B. Role of activated endocannabinoid system in regulation of cellular cholesterol metabolism in macrophages. Cardiovasc Res. 2009;81:805–813. doi: 10.1093/cvr/cvn344. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez A, Bachorik PS, Wee SB. Novel effects of the acyl-coenzyme A:Cholesterol acyltransferase inhibitor 58-035 on foam cell development in primary human monocyte-derived macrophages. Arterioscler Thromb Vasc Biol. 1999;19:2199–2206. doi: 10.1161/01.atv.19.9.2199. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez A, Usher DC. Anti-atherogenic effects of the acyl-CoA:cholesterol acyltransferase inhibitor, avasimibe (CI-1011), in cultured primary human macrophages. Atherosclerosis. 2002;161:45–54. doi: 10.1016/s0021-9150(01)00620-7. [DOI] [PubMed] [Google Scholar]
- 29.Cignarella A, Engel T, von Eckardstein A, Kratz M, Lorkowski S, Lueken A, Assmann G, Cullen P. Pharmacological regulation of cholesterol efflux in human monocyte-derived macrophages in the absence of exogenous cholesterol acceptors. Atherosclerosis. 2005;179:229–236. doi: 10.1016/j.atherosclerosis.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 30.An S, Jang YS, Park JS, Kwon BM, Paik YK, Jeong TS. Inhibition of acyl-coenzyme A:cholesterol acyltransferase stimulates cholesterol efflux from macrophages and stimulates farnesoid X receptor in hepatocytes. Exp Mol Med. 2008;40:407–417. doi: 10.3858/emm.2008.40.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]