Abstract
Several studies suggest that prenatal stress is a possible risk factor in the development of autism spectrum disorders. However, many children exposed to stress prenatally are born healthy and develop typically, suggesting that other factors must contribute to autism. Genes that contribute to stress reactivity may, therefore, exacerbate prenatal stress-mediated behavioral changes in the adult offspring. One candidate gene linked to increased stress reactivity encodes the serotonin transporter. Specifically, an insertion/deletion (long/short allele) polymorphism upstream of the serotonin transporter gene correlates with differential expression and function of the serotonin transporter and a heightened response to stressors. Heterozygous serotonin transporter knockout mice show reductions in serotonin transporter expression similar to the human short polymorphism. In this study, the role of prenatal stress and maternal serotonin transporter genotype were assessed in mice to determine whether their combined effect produces reductions in social behavior in the adult offspring. Pregnant serotonin transporter heterozygous knockout and wild-type dams were placed in either a control condition or subjected to chronic variable stress. The adult offspring were subsequently assessed for social interaction and anxiety using a 3-chamber social approach task, ultrasonic vocalization detection, elevated-plus maze and an open field task. Results indicated that prenatal stress and reduced serotonin transporter expression of the dam may have the combined effect of producing changes in social interaction and social interest in the offspring consistent with those observed in autism spectrum disorder. This data indicates a possible combined effect of maternal serotonin transporter genotype and prenatal stress contributing to the production of autistic-like behaviors in offspring.
Keywords: animal model, autism, knockout mouse, stress, prenatal stress, serotonin transporter
1. Introduction
Autism spectrum disorders (ASD) are a set of neurological disorder characterized by deficits in social interaction, social communication, and the presence of restrictive and repetitive behaviors (APA, 2000; DiCicco-Bloom et al., 2006; Prater and Zylstra, 2002; Singhania, 2005; Tuchman, 2003). Recent surveys indicate the prevalence of ASD as high as one child in every 150 (Center for Disease Control, 2007), or possibly higher (Kogan et al., 2009). Despite the increasing prevalence rates and media attention, the etiology of ASD is still unknown (Fombonne, 2005).
The heritability rate for ASD has been estimated as high as 90% (Freitag, 2007), suggesting that ASD is a predominantly genetic condition (Losh et al., 2008; Folstein and Rosen-Sheidley, 2001; Freitag, 2007). Twin studies have discovered that monozygotic twins show approximately a 70% concordance rate, whereas for dizygotic twin the concordance rate is approximately 5% (Steffenburg et al., 1989; Folstein and Rutter, 1977; Bailey et al., 1995). While genetics has a strong influence in ASD, other factors must also contribute.
One potential environmental risk factor in ASD is prenatal stress. Prenatal stress has been shown to be implicated in a variety of behavioral problems that are displayed in ASD, including attention, language, and learning (Mulder et al., 2002; Weinstock, 1997). It has been found that mothers of children of autism have reported high family discord and psychiatric problems during their pregnancies compared to mothers of nonautistic children, suggesting prenatal stress as a potential risk factor of autism (Ward, 1990). In a 2005 study, Beversdorf et al. surveyed mothers of ASD, Down syndrome, and control children on their level of stress during pregnancy. They reported a significantly greater stress during weeks 21 – 32 of gestation in the mothers of children with ASD in comparison to control or Down syndrome mothers, with a peak at 25 – 28 weeks. Similarly, Kinney et al. studied ASD birth rates in the wake of hurricanes in Louisiana (2008). They found an increase in ASD birthrates among mothers subjected to hurricanes during months 5 and 6 of gestation, which covaried with storm intensity. More intense storms during critical development periods resulted in a greater incidence of ASD births. In these studies, however, many other pregnancies associated with stress resulted in children without autism. Therefore, prenatal stress alone does not account for the development of autism.
One candidate gene that might interact with prenatal stress is the serotonin transporter (5-HTT) gene, SLC6A4, which has an associated insertion/deletion polymorphism in the promoter region that modulates expression and function (Lesch et al., 1996). In humans, the short allele (S) has a 44-base pair deletion within the 5-HTT gene-linked polymorphic region (5-HTTLPR), whereas the long allele (L) is full-length. While earlier evidence which suggested a role of the S allele in the relationship between stress and depression (Caspi et al., 2003) has not been corroborated (Risch et al., 2009), other evidence has suggested a role of the S allele in suicidality (Bondy et al., 2006), alcoholism (Feinn et al., 2005; Sander et al., 1997), and susceptibility to anxiety (Lesch et al., 1996). Thus, regardless of the actual functional nature of the S allele, it likely marks a haplotype that has a significant role in stress reactivity. Human imaging studies have found that carriers of the S allele display greater amygdala activation in response to fearful stimuli (Harari et al., 2002) that is dependent upon 5-HTT availability (Rhodes et al., 2007), also supporting a greater reactivity to stress. In addition, 5-HTT functioning is altered, including reduced transcriptional efficiency and decreased expression and serotonin (5-HT) uptake (Lesch et al., 1996). Similar to human short allele carriers, 5-HTT knockout (KO) mice display increased levels of extracellular 5-HT, decreased intracellular concentrations of 5-HT, and reduced transporter binding availability and uptake activity compared to that observed in wild-type and heterozygous 5-HTT mice (Bengel et al., 1998; Kim et al., 2005; Montañez et al., 2003). While the heterozygous 5-HTT KO mice have similar 5-HT levels as wild-type mice, their uptake activity and transporter binding availability is reduced roughly 50% in comparison to wild-type mice (Bengel et al., 1998; Montañez et al., 2003). This reduction is similar to that seen in human studies investigating the short allele of the 5-HTT gene (Lesch et al., 1996). Furthermore, the short allele polymorphism of the 5-HTT gene has been linked to ASD in some but not all studies (Cook et al., 1997; Brune et al., 2006; Losh et al., 2008; Freitag, 2007; Zhong et al., 1999). Additionally, a second polymorphism, a variable number of tandem repeats in intron 2 of the 5-HTT gene has specifically been linked to platelet hyperserotonemia findings in autism (Coutinho et al., 2004). A single nucleotide polymorphism, Gly56Ala, which causes 5-HTT function to become equivalent to that seen in the S allele variant, has also been linked to autism (Prasad et al., 2005).
While numerous studies have investigated the independent effects of prenatal stress on the etiology of autism and others have independently examined genes known to impact stress response in autism, few have investigated the combined effect of these factors (Cheslack-Postava et al., 2007). We hypothesized that a combined effect of prenatal stress and dysfunctional maternal 5-HTT would produce autistic-like behaviors in the offspring. In this study our focus is on the genotype of the dam and not that of the offspring, as Côté et al. (2007) discovered that maternal peripheral 5-HT is crucial in the neuronal development of mice. Additionally, we expected the 5-HTT +/− dams to be more reactive to stressor exposure during gestation, having a greater adverse effect on the offspring. Thus, stress-related changes in 5-HT levels in the dams may have the ability to significantly alter the development of their offspring, manifesting itself in behavioral abnormalities.
2. Experimental Procedures
2.1 Animals
Four male homozygous 5-HTT KO mice with a C57BL/6 background (Bengel et al., 1998) were obtained from Taconic Farms, Inc. (Hudson, NY), and mated with four homozygous C57BL/6 wild-type (WT) females to obtain the 11 heterozygous (+/−) dams used for this study. Animals were kept in rooms with a temperature of 25°C and a light:dark cycle of 14:10 with lights on at 10:00 PM. Males and females were housed separately in groups of 2–5 in 30 × 16 × 13 cm3 hanging Plexiglas cages with corn cob bedding, which served as their home cages. Food and water was available ad libitum.
2.2 Breeding
Single males were moved into the standard cages with single females for a 5 day period for breeding, during which estrous samples were taken every morning. All of the male mice were WT C57BL/6 mice; 11 dams were the previously described (+/−) females and 7 dams were WT mice. A sample was obtained from a 10 microliter vaginal saline wash, and examined microscopically for the presence of sperm in order to determine the day of conception.
2.3 Chronic Variable Stress Paradigm
Five heterozygous and four WT dams that were randomly assigned to the stress paradigm were subjected to chronic variable stress from gestational day 6 to parturition. Stressors included: constant light exposure (38 hour duration), exposure to fox scent (1 hour) during the dark phase, overnight exposure to novel objects (marbles) in their home cage, restraint (10 minutes) during the light phase, overnight exposure to novel noises (radio static), multiple cage changes during light cycle, and overnight exposure to water-saturated bedding. Each of the seven stressors was presented in succession over 7 days, and this pattern was repeated 3 times. Stressors were chosen on the basis that they do not cause pain and have minimal influence on food intake and weight gain (Mueller et al., 2006). Six heterozygous and three WT dams proceeded from gestational day 6 to parturition without stressors.
2.4 Postnatal procedures
Between postnatal days (PD) 0–2, full-litter cross-fostering was implemented to counterbalance any lasting behaviors resulting from genotype- or stress-related changes on early maternal care. Breeding was strategically timed as to allow cross fostering to occur across all experimental groups. While every effort was made to adequately counterbalance the cross-fostering, the small number of total litters resulted in conditions not being fully counterbalanced. However, in some cases births did not occur within a time window that allowed for cross-fostering (i.e. two separate dams giving birth within two days of one another) (Table 1). On PD 7, all offspring were tattooed for later identification with an Aramis Animal Microtattoo System (Brockville, Ontario, Canada). A hypodermic needle penetrated a single toe of the mouse and deposited a small amount of tattoo paste, leaving an easily identified mark. Pups were weaned on PD 28. Pups were housed with sex-matched littermates and were housed with up to five mice per cage.
Table 1.
Cross-fostering conditions with the number of litters in each condition shown. G = across genotype, S = across stress, G&S = across genotype and stress, W/I = within group, NC = litter not crossed.
| Cross-Fostering Condition | |||||||
|---|---|---|---|---|---|---|---|
| G&S | G | S | W/I | NC | Total | ||
| Dam Condition | 5-HTT +/− Control | 1 | 1 | 1 | 2 | 1 | 6 |
| 5-HTT +/− Prenatal Stress | 0 | 1 | 1 | 2 | 2 | 6 | |
| WT Control | 0 | 1 | 0 | 0 | 2 | 3 | |
| WT Prenatal Stress | 1 | 1 | 0 | 0 | 1 | 3 | |
| Total | 2 | 4 | 2 | 4 | 6 | ||
2.5 Ultrasonic Vocalizations
Behavioral testing began with an investigation of spontaneous ultrasonic vocalization (USV), which can indicate a distress call during maternal isolation or separation in order to attempt to assess communication known to be decreased in autism. On PD 7, immediately prior to toe tattoo procedures, USVs emitted during maternal separation were recorded from pups using a Med Associates USV Detector (St. Albans, VT). Each pup was individually placed in a 1000 mL beaker with approximately 1–2 cm of corn cob bedding and an USV detector mounted to the top. The beaker was then placed in a sound- and light-attenuated behavior chamber. Following a 10 s attenuation period, USVs were constantly recorded over a 60 s interval. The total number of USVs, emitted across two frequency ranges (20–40 kHz and 40–100 kHz), were considered for analysis. Typically, maternal separation and social isolation are associated with calls above 40 kHz, while lower frequencies are associated with postpartum sounds, which are typically observed during maternal grooming of the pup (Hahn and Lavooy, 2005). However, these pups were not with the dams at the time of testing in our study. Bedding inside the beaker was replaced after each animal.
2.6 Neurological Exam Battery
On PD 35, all offspring were subject to a neurological exam battery in order to detect any deficits in sensory functioning or gross developmental abnormalities (Crawley, 1999). This was done to confirm that any autism-related behaviors were not due to more general impairments in function. All mice were weighed individually at the beginning of the exam to ensure normal developmental growth (Crawley, 1999). Mice weighing less than 1.5 standard deviations from their sex’s average weight were considered outliers and were excluded from the study. A small piece of food was randomly placed under bedding in a 72 × 40 × 28 cm3 chamber into one of nine visually (but not physically) divided quadrants. The latency to find food was recorded, with a maximum of 5 minutes allowed, in order to test olfactory functioning (Crawley, 1999). To test neuromuscular strength, mice were individually placed on a wire lid above their home cage. The lid was then gently turned upside down 6 inches above their home cage. Latency of the mouse to fall was recorded, with a maximum of 60 seconds allowed (Crawley, 1999). Mice were then individually tested in a clean mouse cage to test acoustic startle. The experimenter clapped their hands approximately 2 feet away from the cage and observed whether the mouse startled to the noise, with mice failing to respond excluded from further study (Crawley, 1999). Finally, mice were individually lowered slowly towards a wire cage lid by holding on to their tail. Experimenters recorded whether a mouse reached for the lid in order to test the mouse’s vision, with mice failing to respond excluded from further study (Crawley, 1999). Mice that failed any of the above criteria were excluded from further study.
2.7 Elevated-Plus Maze
Subsequent behavioral testing of the offspring began on PD 60 with the elevated-plus maze, which measures anxiety-like behavior in order to determine whether any social effects detected are due to general anxiety. The maze is constructed with black Plexiglas and is raised off the floor 75 cm, with a stand fitted to the maze. The maze consisted of two open arms (35 × 6.25 × 0.25 cm3) and two closed arms (35 × 6.25 × 21 cm3) that extend from a common central platform (5 × 5 cm2). Opaque Plexiglas sections were fitted into the floor of the maze and were easily removable for cleaning. Mice were individually recorded by Logitech video recorders and later scored. Investigators placed each mouse in the center of the four arms and exited the testing area. Mice were given a total of 5 minutes to explore the maze under low light conditions (260 lux). The maze was wiped down with 30% ethanol alcohol between each testing subject. The experimenter later recorded the amount of time a mouse spent in the open arms as well as the closed arms (Lister, 1987). Open arm ratio (total time spent on open arm / (time spent on open arm + time spent in closed arm)) was calculated for each mouse.
2.8 Open Field
The open field is also a validated anxiety assessment in rodents, and measures the willingness of the mouse to explore a novel environment (Treit and Fundytus, 1988). Animals were tested individually on PD 61 in the open field task. The apparatus was constructed out of clear Plexiglas and measured 45 × 45 × 22 cm3. The apparatus was overlaid on an 8×8 grid. Mice were tested during the dark cycle under red lighting conditions (15 lux) and were recorded individually for a 10 minute period using Logitech video recorders and later scored by hand. No observers were present in the room during testing. The field was wiped down with 30% ethanol alcohol between each testing subject. Time spent in inner squares (4×4 central grid) and time spent in the outer perimeter were recorded.
2.9 Social Approach
To test social novelty seeking, recognition of social cues and social interaction in mice, animals were individually tested on PD 62 using the 3-chambered social approach task originally described by Nadler et al. (2004). The apparatus was constructed from clear Plexiglas and measured 54.5 × 41.5 × 22 cm 3 with 0.5 cm thick walls. The apparatus was evenly divided into three chambers measuring 17.5 × 41.5 × 22 cm3. The two divider walls contained an opening measuring 10.25 × 2.5 cm2 so that the mice could freely move between the three chambers. Removable partitions were present to cover up the openings to restrict the mouse to the central chamber when necessary. Non-experimental mice (“strangers”) that were never exposed to the experimental mice were habituated to small wire cages (Galaxy Cup, Spectrum Diversified Designs, Inc.) for 5 minutes per day for 5 days before the start of the task. Stranger mice were sex-matched and age-matched C57BL/6 mice. Prior to the task, experimental mice were habituated to the central chamber for 10 minutes. They were then allowed to explore the 3 empty chambers for 10 minutes to detect the presence of a side bias. In the first trial, to assess preference to social approach, one stranger mouse was randomly assigned to a non-central chamber, while an empty, identical small wire cage, which served as a novel object, was placed in the other non-central chamber (Figure 1). The experimental mouse was allowed to freely explore the 3 chambers for 10 minutes. Social interest and interaction in this trial can be interpreted as spending significantly more time with the stranger mouse than with the novel object, as assessed by time spent in their respective chambers. In the second trial, to assess preference to novel social approach, an additional novel stranger mouse was placed under the previously empty wire cage. The other, now familiar, stranger mouse remained in the other wire cage. The experimental mouse was allowed to freely explore the chambers for another 10 minutes. In this trial, social interest and social interaction can be interpreted as social novelty seeking behavior, which is quantified as spending significantly more time in the chamber containing the novel stranger than the chamber containing the familiar stranger mouse. All testing occurred during the dark cycle under red lighting conditions (15 lux). The apparatus was wiped down with 30% ethanol alcohol between each testing subject. The side-bias trial, the stranger versus object trial, and the two stranger trial were all individually video recorded for each mouse using the Logitech video recorders and were manually scored by hand for time spent in each chamber.
Figure 1.
The 3-Chambered social approach task used to quantify social interaction in mice offspring. Mice were first habituated to the central chamber for 10 minutes before they were allowed to explore all 3 empty chambers for an additional 10 minutes. Mice were then relocated to the central chamber for a third trial, in which a novel stranger was placed in one side chamber while the remaining side chamber contained a novel object (shown). The task concluded with a final trial, in which an additional stranger mouse is added to the previously empty novel object.
2.10 Euthanasia
On PD 90, all animals were euthanized via CO2 asphyxiation.
2.11 Statistical analysis
All behavioral outcome measures were analyzed using a 2×2×2 ANOVA (maternal gene x stress x offspring sex). Subsequent one-way and two-way ANOVAs and post-hoc tests, including t-tests, were then conducted to specify the effects of significant interactions revealed by the ANOVA. Furthermore, hypothesis-driven t-tests were performed comparing the offspring of stressed 5-HTT +/− mice to offspring of wild type mice for social approach. All statistical analyses were conducted using SPSS software.
3. Results
3.1 Neurological exam battery
One offspring was excluded from the remaining behavioral assays based on the exam battery performance and low body weight. All other offspring displayed normal sensori-motor function.
3.2 Ultrasonic Vocalization
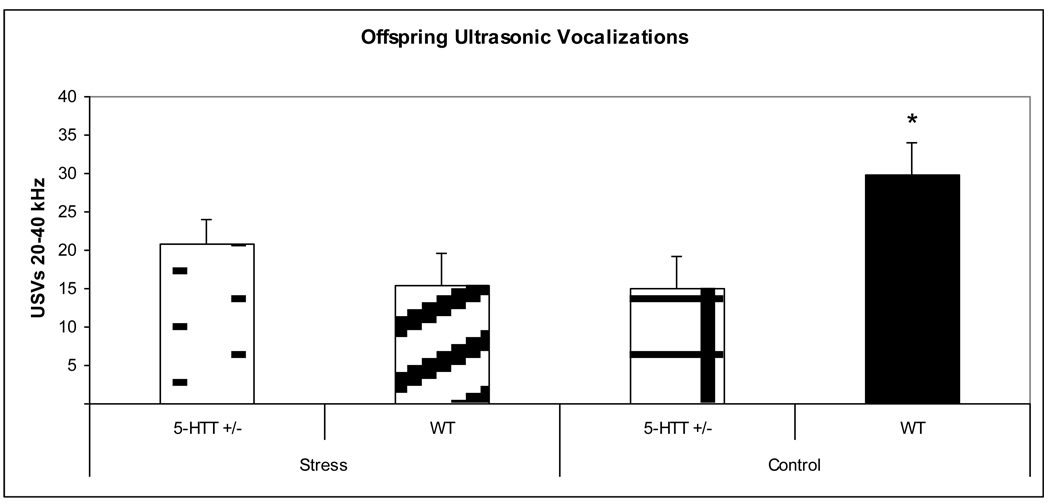
USVs were measured for 31 total offspring (15 prenatally stressed offspring of 5-HTT +/− dams, 9 control offspring of 5-HTT +/− dams, 9 prenatally stressed offspring of WT dams, 9 control offspring of WT dams; approximately equal numbers of male and female mice were distributed across all conditions and a proportional number of cross-fostered and non-cross-fostered mice were distributed across all conditions). We expected that prenatally stressed offspring of 5-HTT +/− dams would have decreased vocalizations as compared to control offspring of WT dams. The ANOVA revealed a maternal genotype x prenatal stress interaction (F3,27 = 14.75, p < 0.001), with control offspring of WT dams vocalizing in the 20–40 kHz range significantly more than all other groups (Figure 2). Additionally, the multivariate ANOVA revealed a main effect of offspring sex (F3,27 = 9.082, p = 0.005), with male pups vocalizing less within the 20–40 kHz range than female pups. An interaction for offspring sex x prenatal stress was also observed, with non-prenatally stressed females vocalizing more across both frequency ranges than other groups (F3, 27 = 5.47, p = 0.025). Findings that did not reach significance were observed towards main effects for both maternal genotype (F3, 27 = 3.407, p = 0.073) and prenatal stress (F3, 27 = 3.407, p = 0.073), with offspring of 5-HTT +/− dams vocalizing less within the 20–40 kHz range than those of WT dams, and prenatally stressed offspring vocalizing less than those not exposed to stress. No other effects were observed for the 40–100 kHz vocalizations.
Figure 2.
A significant maternal genotype x prenatal stress interaction was observed in the 20 – 40 khz range for ultrasonic vocalizations. WT control offspring emitted more vocalizations than all other groups. *p < 0.05
3.3 Elevated-Plus Maze
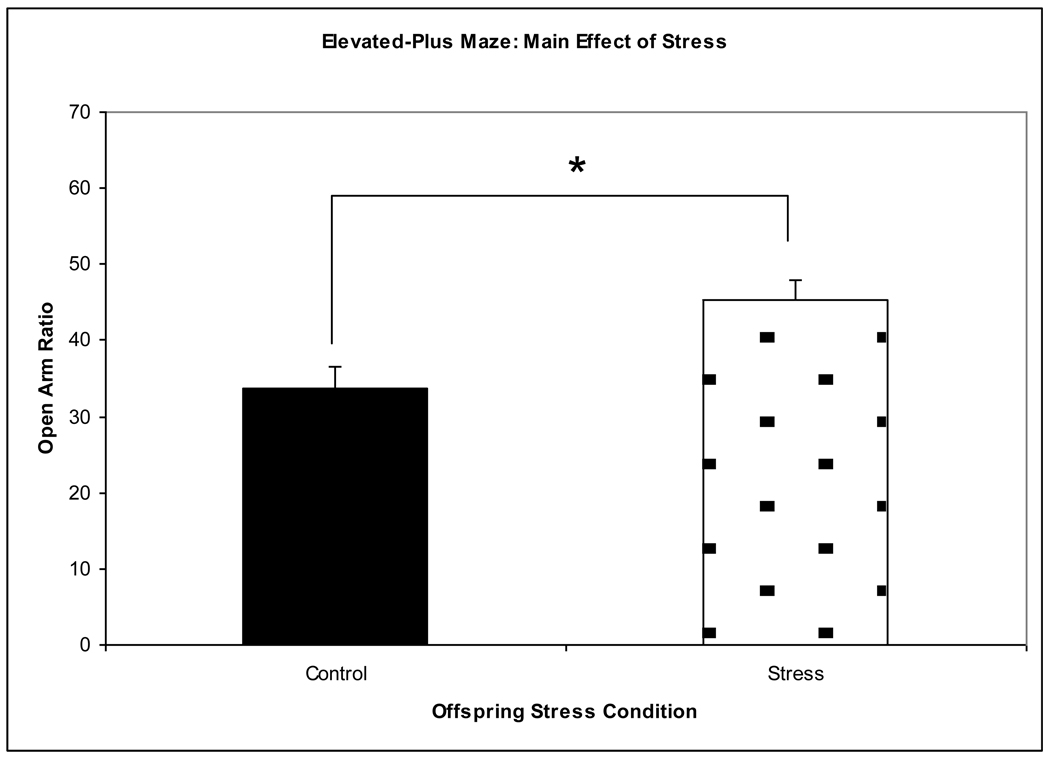
126 behavioral videos were scored and analyzed (43 prenatally stressed offspring of 5-HTT +/− dams, 49 control offspring of 5-HTT +/− dams, 15 prenatally stressed offspring of WT dams, 19 control offspring of WT dams; approximately equal numbers of male and female mice were distributed across all conditions and a proportional number of cross-fostered and non-cross-fostered mice were distributed across all conditions). The ANOVA showed a significant main effect of prenatal stress (F3,122 = 9.316, p = 0.003), with prenatally stressed mice spending significantly more time in the open arms (a higher open arm ratio) than control mice (Figure 3). There was no significant main effect of maternal genotype (F3,122 = 0.915, p = 0.341) or of sex of offspring (F3,122 = 2.336, p = 0.129). There were no significant interactions for maternal genotype x prenatal stress (F3,122 = 0.1, p = 0.752), maternal genotype x offspring sex (F3,122 = 0.006, p = 0.939), prenatal stress x offspring sex (F3,122 = 2.777, p = 0.098), or for maternal genotype x prenatal stress x offspring sex (F3,122 = 1.959, p = 0.164).
Figure 3.
A main effect of prenatal stress was observed in the elevated-plus maze. Overall, prenatally stressed offspring had a higher open arm ratio than control offspring, indicating decreased levels of anxiety-like behavior. *p = 0.001.
3.4 Open Field
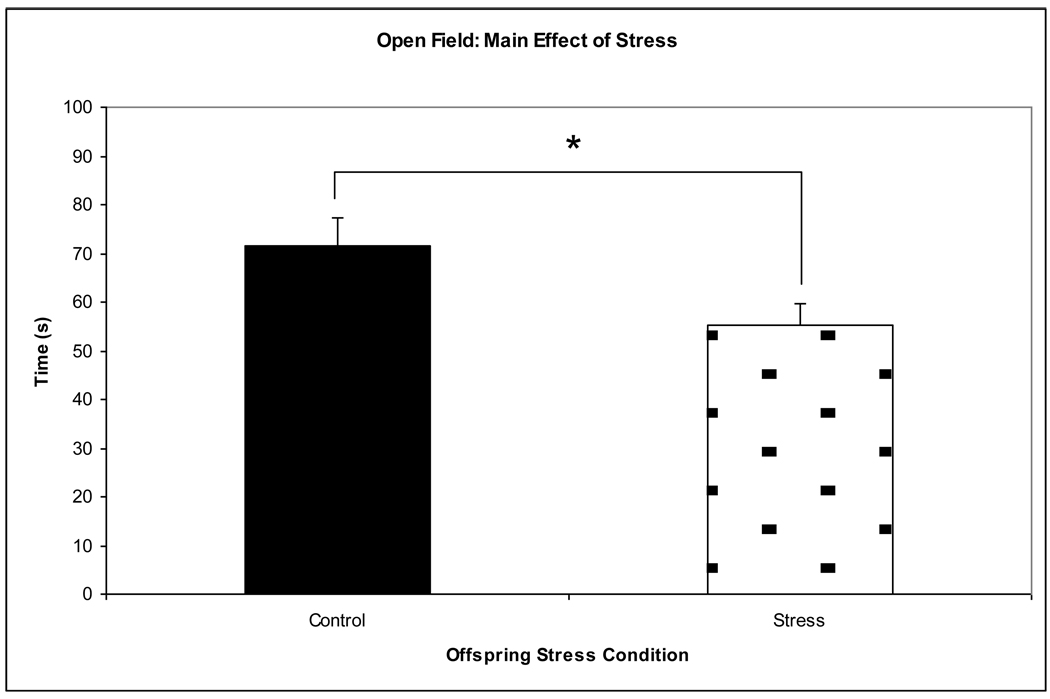
116 behavioral videos were scored and analyzed for anxiety-like behavior (43 prenatally stressed offspring of 5-HTT +/− dams, 48 control offspring of 5-HTT +/− dams, 15 prenatally stressed offspring of WT dams, 10 control offspring of WT dams; approximately equal numbers of male and female mice were distributed across all conditions and a proportional number of cross-fostered and non-cross-fostered mice were distributed across all conditions). A multivariate ANOVA showed a significant main effect of prenatal stress (F3,112 = 4.729, p = 0.032), with the prenatally stressed mice spending less time in the inner region of the field (Figure 4). The multivariate ANOVA also showed a significant maternal genotype x prenatal stress interaction (F3,112 = 5.415, p = 0.022). Subsequent post-hoc t-tests revealed that prenatally stressed mice of WT dams spent significantly less time in the inner region than all other conditions, indicating more anxiety (p < 0.05). The ANOVA revealed no other significant main effects or interactions.
Figure 4.
A main effect of prenatal stress was observed in the open field behavioral apparatus. Overall, prenatally stressed offspring spent less time in the inner region of the field, indicating increased levels of anxiety-like behavior. *p = 0.032.
3.5 Social Approach
3.5a Side Bias
126 behavioral videos were scored and analyzed (same distribution across groups as in the elevated-plus maze). A multivariate ANOVA showed no significant main effects or interactions when comparing time spent across all three chambers (p > 0.50).
3.5b Novel Object vs. Novel Stranger
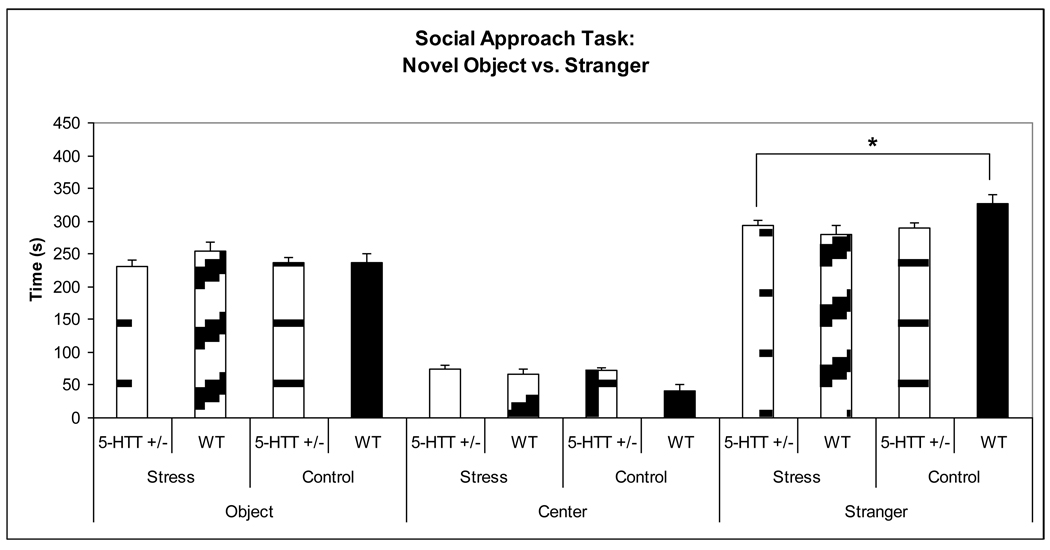
126 behavioral videos were scored and analyzed to assess preference to social approach (same distribution across groups as in the elevated-plus maze). A multivariate ANOVA (with dependent variables being time spent in each of the three chambers) showed a significant main effect for maternal genotype (F3,122 = 4.314, p = 0.006), a significant main effect of stress (F3,122 = 3.131, p = 0.028), and a significant maternal genotype x stress interaction (F3,122 = 3.123, p = 0.029). To address our specific hypothesis that prenatally stressed offspring of 5-HTT +/− dams would have decreased social interaction than control offspring of WT dams, t-testing was conducted. This t-testing showed that these findings were driven by the fact that the WT control group spent significantly more time with the novel stranger (vs. prenatally stressed offspring of 5-HTT +/− dams p = 0.014, vs. control offspring of 5-HTT +/− dams p = 0.003, vs. prenatally stressed offspring of WT dams p = 0.002) and significantly less time in the central chamber (vs. prenatally stressed offspring of 5-HTT +/− dams p < 0.001, vs. control offspring of 5-HTT +/− dams p < 0.001, vs. prenatally stressed offspring of WT dams p = 0.006) than any of the 3 other conditions. This data suggests that control offspring of WT dams display more social-like behavior than all other conditions. Specifically, to address our hypothesis, t-tests revealed that the prenatally stressed offspring of 5-HTT +/− dams spent significantly less time with the novel mouse as compared to the control offspring of WT dams (p = 0.014), suggesting decreased social interest in the stressed 5-HT +/− offspring (Figure 5). This withstood correction for multiple comparisons, performed since we had multiple primary outcome measures for social behavior. A maternal genotype x stress x offspring sex interaction did not reach significance (F3,122 = 2.335, p = 0.077).
Figure 5.
A priori t-testing revealed that WT control offspring spent significantly more time with the novel stranger than prenatally stressed offspring of 5-HTT +/− dams (* p = 0.014).
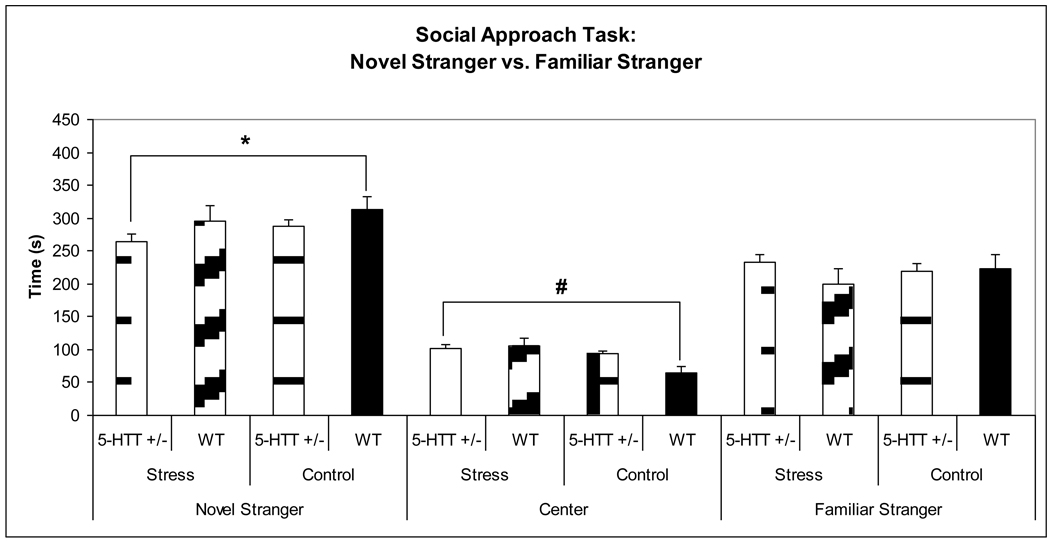
3.5c Novel Stranger vs. Familiar Stranger
125 behavioral videos were scored and analyzed for the social behavior (43 prenatally stressed offspring of 5-HTT +/− dams, 49 control offspring of 5-HTT +/− dams, 15 prenatally stressed offspring of WT dams, 18 control offspring of WT dams; approximately equal numbers of male and female mice were distributed across all conditions and a proportional number of cross-fostered and non-cross-fostered mice were distributed across all conditions) to assess preference to novel social approach. To address our specific hypothesis that prenatally stressed offspring of 5-HTT +/− dams would have decreased social interaction than control offspring of WT dams, t-tests were performed, revealing that prenatally stressed offspring of 5-HTT +/− dams spent significantly less time with the novel stranger as compared to the control offspring of WT dams (p = 0.049), and also spent more time in the center chamber (more time in the chamber containing no mice) as compared to the control offspring of WT dams (p < 0.001), again suggesting decreased social interest in the stressed 5-HT +/− offspring (Figure 6). Among these primary outcome measures, the finding for the central chamber withstood correction for multiple comparisons.
Figure 6.
A priori t-testing revealed that prenatally stressed offspring of 5-HTT +/− dams spent significantly less time with the novel stranger (*p = 0.049) and significantly more time in the central chamber (#p < 0.0005) than WT control offspring.
A significant main effect of stress (F3,121 = 3.284, p = 0.023) was observed, with prenatally stressed mice showing decreased social interaction. Also, a significant main effect of offspring sex was observed (F3,121 = 4.427, p = 0.006), with female offspring showing decreased social interaction. A significant prenatal stress x offspring sex interaction was observed (F3,121 = 3.433, p = 0.019), with prenatally stressed female mice displaying the lowest degree of social interaction. A maternal genotype x offspring sex interaction did not reach significance (F3,121 = 2.324, p = 0.079). The multivariate ANOVA showed a maternal genotype x prenatal stress x offspring sex interaction that did not reach significance (F3,121 = 2.319, p = 0.079).
4. Discussion
The aim of this study was to investigate whether the effects of maternal serotonin transporter expression and prenatal stress combine in such a manner to produce autistic-like behaviors in offspring. Our specific hypothesis was that prenatally stressed offspring of 5-HTT +/− dams would display decreased social interaction in comparison to control offspring of WT dams. Our results seem to support our hypothesis. Prenatally stressed offspring of 5-HTT +/− dams appeared to display social deficits despite having no observed increase in anxious behavior. The neurological exam battery indicated no major developmental abnormalities resulting from our experimental manipulations in the studied mice.
The behavioral battery revealed significant differences between prenatally stressed offspring of 5-HTT +/− dams and control offspring of WT dams. Further study will be needed to fully understand how prenatal stress and maternal genotype interact. During the 3-chamber social approach task comparing interaction with a novel object and stranger mouse, the offspring of prenatally stressed 5-HTT +/− dams spent significantly less time with the stranger mouse than the offspring of WT control dams, suggesting decreased preference for social interaction. Offspring of WT control dams spent significantly more time with both the stranger mouse and the object than prenatally stressed offspring of 5-HTT +/− dams, further indicating that the prenatally stressed offspring of 5-HTT +/− dams have decreased social interaction and social interest. The prenatally stressed offspring of 5-HTT +/− dams appeared to display decreased levels of social novelty seeking compared to control offspring of WT dams, as assessed in the trial comparing interaction with a novel stranger and a familiar stranger, although this comparison did not withstand Bonferroni correction. The prenatally stressed offspring of 5-HTT +/− dams also preferred to stay in the center chamber, and not interact with either mouse, as compared to the other groups, further suggesting a decreased preference for social novelty seeking. Decreased total time spent with the novel stranger as compared to the familiar stranger can be interpreted as decreased social novelty seeking as well as decreased recognition of social cues (Crawley, 2004), and less time spent in the chamber with a novel mouse in comparison to a novel object is indicative of decreased sociability (Moy et al., 2004). In our study, presence of either prenatal stress or maternal 5-HTT +/−genotype alone was sufficient to result in an increase in time spent in the central chamber in the novel stranger versus familiar stranger experiment, since the non-stressed offspring of WT dams spent less time in the central chamber than all other groups. In the novel stranger versus novel object experiment, increased time in the central chamber suggests a decreased preference for novelty in general, since it would result from the combined effects of less approach for both the object and the mouse. Decreased novelty seeking is also part of the increased insistence on sameness that is observed in autism clinically (APA, 2000). The initial trial in which mice explored all of the empty chambers equally suggests that these findings are not due to the mice having a general decrease in exploratory behavior.
It is important to note that in any task assessing levels of social interest, interaction or exploration in the mouse, difficulties arise in attempting to interpret the from data in mice as applicable in humans. In individuals with ASD, not only the amount of social interaction is decreased, which is assessed in this task, the quality of interaction is also decreased. While this task assesses the amount of social interaction between the experimental mouse and the stranger mouse, it does not assess the quality or content of the interactions. Further, interactions between two sex-matched mice can be very different than those of two sex-matched humans. For example, one would expect mice to demonstrate more aggressive, territorial behavior toward each other than would be expected in humans. Therefore, while this task is useful in that it helps shed light on deficits in social interaction, we acknowledge that further social testing is necessary to truly understand the nature of the interactions of these proposed autistic-like mice.
Results from the ultrasonic vocalization measure show a decrease in communication of the prenatally stressed offspring of 5-HTT +/− dams in early development in comparison to control offspring of WT dams, as seen in an interaction of maternal genotype and prenatal stress. Decreased communication is a cardinal sign of ASD. As main effects for prenatal stress or maternal genotype did not reach significance alone, the significant interaction between maternal genotype x prenatal stress further supports our hypothesis that a combined effect between maternal genotype and prenatal stress produces behavioral differences in the prenatally offspring of 5-HTT +/− dams. Overall, the USV results indicate a basic disruption of communication, but further investigation is necessary to better characterize these findings. It is important to note that the decrease in communication was in the range of 20 – 40 kHz, and not calls in the range of 40 – 100 kHz. These findings demonstrate a change in the communicative phenotype, but the social role of these vocalizations is not yet fully understood (Hahn and Lavooy, 2005). Furthermore, while it is generally well agreed upon that social calls are in a frequency range above 40 kHz, the role of vocalizations at any frequency is still in need of further understanding. Therefore, more research is needed to understand the social and communicative relevance of the USV effects detected in our study. As a heating pad was not used as part of the USV procedure, it is possible that the vocalizations observed could be due to a reaction to the cold environment. However, length of time isolated from the nest and dam was minimized to lessen the effects of temperature, and mice in all experimental groups were subjected to the same conditions.
By measuring anxiety-like behaviors, we demonstrated that decreases in social interactions witnessed here are not likely to be an indirect result of greater anxiety. With the elevated-plus maze, stressed mice had significantly higher open arm ratio than control mice, indicating lower levels of anxiety. Thus, according to this task, the decrease in social interaction described above is not likely due to an increase in anxiety. However, stressed offspring spent less time in the inner region during the open field task, suggesting they were more anxious than control offspring. For this task a significant interaction between genotype and stress was observed, driven by reduced open field exploration by the prenatally stressed WT-dam group. It is possible that this is reflected in the first part of the social interaction task, in which male mice of WT dams subjected to prenatal stress show no preference for social interaction versus an inanimate object. However, in both tasks which measure anxiety, offspring of 5-HTT +/− dams that were prenatally stressed displayed low to normal levels of anxiety-like behaviors. While these initial findings require further study to definitively determine the role of maternal 5-HTT expression and prenatal stress on anxiety-like behaviors in adult offspring, they support the notion that any detected social interaction effects observed in the offspring of stressed 5-HTT +/− dams are not likely due to increased overall anxiety.
As no significant differences were observed in maternal care behaviors or in offspring behavior across groups that were cross-fostered, the counterbalancing issues between groups are unlikely to confound our results. Additionally, the lack of any noticeable effect on behavior for cross-fostered groups would also suggest against the possibility of introducing confounding factors as a result of the cross-fostering procedure.
This study generally supports our hypothesis that a combined effect of maternal genotype and prenatal stress produces autistic-like deficits in social interaction in prenatally stressed offspring of 5-HTT +/− dams compared to control offspring of WT dams. These findings may relate to some of the findings in the current literature on 5-HTT polymorphisms in patients with ASD (Cook et al., 1997; Brune et al., 2006; Losh et al., 2008; Freitag, 2007). While some studies have found effects of 5-HTT polymorphisms (Cook et al., 1997), others have not (Maestrini et al., 1999). This may be due to the need for environmental factors to be present in order to augment the effects of the gene. Many of the studies that did not show a relationship between the S allele of the 5-HTT gene and autism predominantly examined families with multiple affected family members (Maestrini et al., 1999), where an environmental interaction would less likely be important. Studies that have revealed a relationship between the S allele of the 5-HTT gene were not typically exclusive to families with multiple affected family members (Cook et al., 1997). This implies that while they may serve as risk factors individually, both 5-HTT polymorphisms and prenatal stress could interact to increase ASD risk.
One limitation of this study is the small number of behavioral tasks used. Additional tasks that measure social interaction need to be considered, such as social transmission of food preference, to further support this hypothesis that global social functioning is present in these animals (Wrenn et al., 2003). Additional communication tasks, anxiety, repetitive and restrictive behaviors amongst others should also be included in future studies to expand upon these initial findings (Moy et al., 2006). In the future, the time that experimental mice spend near the stranger mouse as well as the novel object will be assessed in order to obtain a more accurate reading of social interaction. Larger numbers of animals assigned to each condition can also help with matching the number of animals per group as well as with further defining how the combined effects of genetics and prenatal stress interact. We did not collect offspring genotype information in the present study. This is an important point which will need to be further examined.
The neurobiological underpinnings of the combined effects of maternal stress and 5-HTT genetics in offspring remains to be fully elucidated. Post-mortem analysis of receptor binding and gross anatomical differences will help support the hypothesis that 5-HTT maternal genotype and prenatal stress combine in effect to produce a phenotype in the offspring related to ASD, while helping to delineate the anatomical basis of the observed behaviors, by comparing brain structure and function to the known human ASD abnormalities. For example, as previous structural observations of autism patients have revealed a significant decrease in the number of Purkinje cells, these cells should be studied in a mouse model of autism as well (Bauman and Kemper, 2005). Furthermore, abnormal serotonin (5-HT) function is observed in autism (Pardo and Eberhart, 2007). As receptors for serotonin (5-HT) are present on the Purkinje cells of neonatal rats (Maeshima et al., 2004), further investigation of serotonergic ligand binding in Purkinje cells is needed to assess whether the changes in this animal model resemble those in humans. Finally, deceased glutamic acid decarboxylase (GAD) mRNA is observed in Purkinje cells in autism (Yip et al., 2007), and decreased GABA receptor binding is also seen in the hippocampus in autism (Blatt et al., 2001). Further investigation of the effects of GABA in the cerebellum by performing in situ hybridization for GAD mRNA in the Purkinje cells is needed to further assess whether the changes in this animal model resemble those in humans.
Acknowledgements
The authors thank Michael Tilley, Howard Gu and Brad Martin for their help with the genetic analysis of this research.
Abbreviations
- ASD
Autism spectrum disorder
- +/−
Heterozygous
- KO
Knockout
- PD
Postnatal day
- 5-HT
Serotonin
- 5-HTT
Serotonin transporter
- 5-HTTLPR
Serotonin transporter gene-linked polymorphic region
- S-allele
Short allele
- USV
Ultrasonic vocalization
- WT
Wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition. text revision. Washington, DC: Authors; 2000. [Google Scholar]
- Bailey A, LeCouteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25(1):63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper T. Neuroanatomic observations of the brain in autism. In: Bauman M, Kemper T, editors. The Neurobiology of Autism. Baltimore: Johns Hopkins University Press; 1994. pp. 119–145. [Google Scholar]
- Bauman M, Kemper T. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23(2–3):183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bengel D, Murphy D, Andrews A, Wichems C, Feltner D, Heils A, Mössner R, Westphal H, Lesch K. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53(4):649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Beversdorf D, Manning S, Hillier A, Anderson S, Nordgren R, Walters S, Nagaraja H, Cooley W, Gaelic S, Bauman M. Timing of prenatal stressors and autism. J Autism Dev Disord. 2005;35(4):471–478. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- Blatt G, Fitzgerald C, Guptill J, Booker A, Kemper T, Bauman M. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J Autism Devel Disord. 2001;31:537–543. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- Bondy B, Buettner A, Zill P. Genetics of suicide. Mol Psychiatry. 2006;11(4):336–351. doi: 10.1038/sj.mp.4001803. [DOI] [PubMed] [Google Scholar]
- Brune C, Kim S, Salt J, Leventhal B, Lord C, Cook E. 5-HTTLPR genotype-specific phenotype in children and adolescents with autism. Am J Psychiatry. 2006;163(12):2148–2156. doi: 10.1176/ajp.2006.163.12.2148. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt T, Taylor A, Craig I, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-htt gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control. Prevalence of autism spectrum disorders – Autism and Developmental Disabilites Monitoring Network, 14 sites, United States, 2002. MMWR SS. 2007;56(SS-1):12–28. [PubMed] [Google Scholar]
- Cheslack-Postava K, Fallin M, Avramopoulos D, Connors S, Zimmerman A, Eberhart C, Newschaffer C. Beta2-adrenergic receptor gene variants and risk for autism in the AGRE cohort. Mol Psychiatry. 2007;12(3):283–291. doi: 10.1038/sj.mp.4001940. [DOI] [PubMed] [Google Scholar]
- Cook E, Courchesne R, Lord C, Cox N, Yan S, Lincoln A, Haas R, Courchesne E, Leventhal B. Evidence of linkage between the serotonin transporter and autistic disorder. Mol Psychiatry. 1997;2(3):247–250. doi: 10.1038/sj.mp.4000266. [DOI] [PubMed] [Google Scholar]
- Côté F, Fligny C, Bayard E, Launay J, Gershon M, Mallet J, Vodjdani G. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci U S A. 2007;104(1):329–334. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A, Oliveira G, Morgadinho T, Fesel C, Macedo T, Bento C, Marques C, Ataíde A, Miguel T, Borges L, Vicente A. Variants of the serotonin transporter gene (SLC6A4) significantly contribute to hyperserotonemia in autism. Mol Psychiatry. 2004;9(3):264–271. doi: 10.1038/sj.mp.4001409. [DOI] [PubMed] [Google Scholar]
- Crawley J. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835(1):18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Crawley J. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10(4):248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, Schultz RT, Crawley J, Young LJ. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6898–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinn R, Nellissery M, Kranzler H. Meta-analysis of the association of a functinoal serotonin transporter promoter polymorphism with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;133B(1):79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry. 1977;18(4):297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Folstein S, Rosen-Sheidley B. Genetics of Autism: Complex Aetiology for a Heterogeneous Disorder. Nat Rev Genet. 2001;2(12):943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry. 2005;66:3–8. [PubMed] [Google Scholar]
- Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry. 2007;12(1):2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- Hahn M, Lavooy M. A review of the methods of studies on infant ultrasound production and maternal retrieval in small rodents. Behav Genet. 2005;35(1):31–52. doi: 10.1007/s10519-004-0854-7. [DOI] [PubMed] [Google Scholar]
- Harari A, Mattay V, Tessitore A, Kolachana B, Fera F, Goldman D, Egan M, Weinberger D. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Kim D, Tolliver T, Huang S, Martin B, Andrews A, Wichems C, Holmes A, Lesch K, Murphy D. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology. 2005;49(6):798–810. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Kinney D, Miller A, Crowley D, Huang E, Gerber E. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord. 2008;38(3):481–488. doi: 10.1007/s10803-007-0414-0. [DOI] [PubMed] [Google Scholar]
- Kogan M, Blumberg S, Schieve L, Boyle C, Perrin J, Ghandour R, Singh G, Strickland B, Trevathan E, van Dyck P. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US. Pediatrics. 2009;124:1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- Lesch K, Bengel D, Heils A, Sabol S, Greenberg B, Petri S, Benjamin J, Müller C, Hamer D, Murphy D. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lister R. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92(2):180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Losh M, Sullivan P, Trembath D, Piven J. Current developments in the Genetics of Autism: From Phenome to Genome. J Neuropathol Exp Neurol. 2008;67(9):829–837. doi: 10.1097/NEN.0b013e318184482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima T, Shiga T, Ito R, Okado N. Expression of serotonin 2A receptors in Purkinje cells of the developing rat cerebellum. Neurosci Res. 2004;50(4):411–417. doi: 10.1016/j.neures.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Maestrini E, Lai C, Marlow A, Matthews N, Wallace S, Bailey A, Cook E, Weeks D, Monaco A. Serotonin transporter (5-HTT) and gamma-aminobutyric acid receptor subunit beta3 (GABRB3) gene polymorphisms are not associated with autism in the IMGSA families. The International Molecular Genetic Study of Autism Consortium. Am J Med Genet. 1999;88(5):492–496. doi: 10.1002/(sici)1096-8628(19991015)88:5<492::aid-ajmg11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Montañez S, Owens W, Gould G, Murphy D, Daws L. Exaggerated effect of fluvoxamine in heterozygote serotonin transporter knockout mice. J Neurochem. 2003;86(1):210–219. doi: 10.1046/j.1471-4159.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- Moy S, Nadler J, Perez A, Barbaro R, Johns J, Magnuson T, Piven J, Crawley J. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3(5):287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy S, Nadler J, Magnuson T, Crawley J. Mouse models of autism spectrum disorders: the challenge for behavioral genetics. Am J Med Genet C Semin Med Genet. 2006;142C:40–51. doi: 10.1002/ajmg.c.30081. [DOI] [PubMed] [Google Scholar]
- Mueller B, Bale T. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol Behav. 2006;88(4–5):605–614. doi: 10.1016/j.physbeh.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Mulder E, Robles de, Medina P, Huizink A, Van den, Bergh B, Buitelaar J, Visser G. Prenatal maternal stress: effects on pregnancy and the (unborn) child. Early Hum Dev. 2002;70:3–14. doi: 10.1016/s0378-3782(02)00075-0. [DOI] [PubMed] [Google Scholar]
- Nadler J, Moy S, Dold G, Trang D, Simmons N, Perez A, Young N, Barbaro R, Piven J, Magnuson T, Crawley J. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3(5):303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Pardo C, Eberhart G. The neurobiology of autism. Brain Pathol. 2007;17(4):434–447. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad HC, Zhu CB, McCauley J, Samuvel DJ, Ramamoorthy S, Shelton RC, et al. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 2005;102(32):11545–11550. doi: 10.1073/pnas.0501432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prater C, Zylstra R. Autism: a medical primer. Am Fam Physician. 2002;66(9):1667–1674. [PubMed] [Google Scholar]
- Rhodes R, Murthy N, Dresner M, Selvaraj S, Stavrakakis N, Babar S, Cowen P, Grasby P. Human 5-HT transporter availability predicts amygdale reactivity in vivo. J Neurosci. 2007;27(34):9233–9237. doi: 10.1523/JNEUROSCI.1175-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas K. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander T, Harms H, Lesch K, Dufeu P, Kuhn S, Hoehe M, Rommelspacher H, Schmidt L. Association analysis of a regulatory variation of the serotonin transporter gene with severe alcohol dependence. Alcohol Clin Exp Res. 1997;21(8):1356–1359. [PubMed] [Google Scholar]
- Singhania R. Autism spectrum disorders. Indian Journal of Pediatrics. 2005;72:343–351. doi: 10.1007/BF02724019. [DOI] [PubMed] [Google Scholar]
- Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, Bohman M. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry. 1989;30:405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31(4):959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Tuchman R. Autism. Neurol Clin N Am. 2003;21:915–932. doi: 10.1016/s0733-8619(03)00011-2. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Does prenatal stress impair coping and regulation of hypothalamic-pituitary-adrenal axis? Neurosci Biobehav Rev. 1997;21:1–10. doi: 10.1016/s0149-7634(96)00014-0. [DOI] [PubMed] [Google Scholar]
- Wrenn C, Harris A, Saavedra M, Crawley J. Social transmission of food preference in mice: methodology and application to galanin-overexpressing transgenic mice. Behav Neurosci. 2003;117:21–31. [PubMed] [Google Scholar]
- Yip J, Soghomonian J, Blatt G. Decreased CAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol. 2007;113:559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Zhong N, Ye L, Ju W, Brown W, Tsiouris J, Cohen I. 5-HTTLPR variants not associated with autistic spectrum disorders. Neurogenetics. 1999;2:129–131. doi: 10.1007/s100480050064. [DOI] [PubMed] [Google Scholar]