Abstract
The adsorption and elution of the antimicrobial peptide nisin at silanized silica surfaces coated to present pendant polyethylene oxide chains was detected in situ by zeta potential measurements. Silica microspheres were treated with trichlorovinylsilane to introduce hydrophobic vinyl groups, followed by self assembly of the polyethylene oxide-polypropylene oxide-polyethylene oxide (PEO-PPO-PEO) triblock surfactant Pluronic® F108, or an F108 derivative with nitrilotriacetic acid endgroups. Triblock-coated microspheres were γ-irradiated to covalently stabilize the PPO-surface association. PEO layer stability was evaluated by triblock resistance to elution by SDS, and layer uniformity was evaluated by fibrinogen repulsion. Introduction of nisin to uncoated or triblock-coated microspheres produced a significant positive change in surface charge (zeta potential) as a result of adsorption of the cationic peptide. In sequential adsorption experiments, the introduction of fibrinogen to nisin-loaded triblock layers caused a decrease in zeta potential that was consistent with partial elution of nisin and/or preferential location of fibrinogen at the interface. This change was substantially more pronounced for uncoated than triblock-coated silica, indicating that the PEO layer offers enhanced resistance to nisin elution.
Keywords: nisin adsorption, zeta potential, Pluronic® F108, PEO-PPO-PEO triblock surfactant, EGAP-NTA
Introduction
Nisin is a small (3.4 kDa) amphiphilic peptide with five lanthionine rings. It is cationic at neutral pH, due to an isoelectric point above 8.5. Nisin is an effective inhibitor of Gram-positive bacteria, including the two most frequently encountered biomaterial-associated pathogens Staphylococcus aureus and Staphylococcus epidermidis [1–3], and holds potential for use as an anti-infective agent in medical device coatings [4, 5].
Tai et al. [6] reported results of an ellipsometric analysis of nisin adsorption and elution at surfaces coated with the PEO-PPO-PEO surfactant Pluronic® F108. Those results suggested that nisin adsorption occurred via penetration of and entrapment within the PEO layer, as opposed to adsorption onto the mobile PEO chains. It is generally understood that PEO resists protein interactions, and the protein repellent properties of the F108 layer, if retained after nisin adsorption or integration, would inhibit displacement of the antimicrobial peptide by blood proteins. In this way, nisin loading could impart an active protective function, and increase the effectiveness of such a coating. In this regard Tai et al. [7] evaluated the antimicrobial activity of nisin-loaded, F108-coated polystyrene microspheres and polyurethane catheter segments after incubation with blood proteins for up to one week. F108-coated surfaces were observed to retain more antimicrobial activity than uncoated surfaces, suggesting that the pendant PEO chains inhibited displacement or elution of nisin by contact with blood proteins.
The F108 triblocks used by Tai et al. were bound to the base substrates only by hydrophobic association of the polymer and PPO centerblock. It is thus possible that adsorbing nisin dislocated the adsorbed Pluronic at the surface, rather than being integrated into the brush layer itself. Important conclusions relating to nisin entrapment among PEO chains, as well as the enhanced resistance to elution of nisin bound in this way, have thus remained somewhat tentative. In this paper we describe the individual and sequential adsorption of nisin and fibrinogen at silanized silica surfaces coated with covalently-bound PEO-PPO-PEO triblocks. Zeta potential was recorded after protein adsorption to microsphere suspensions coated with F108, or with F108 that had been end-activated with nitrilotriacetic acid groups (EGAP-NTA).
While an abundant literature describes the protein repelling mechanisms of material surfaces presenting pendant PEO, there are very few reports describing the adsorption of small proteins to PEO layers. It has been argued that once a sufficiently high chain density is achieved, the rejection capacity of the pendant polymer phase is determined by protein size, relative to the average distance between polymer chains [8, 9]. Archambault and Brash [10] suggested that grafting densities consistent with the brush configuration would be required before protein discrimination based on size would become evident. Halperin [11] formulated a model for protein adsorption in a PEO brush based on kinetic and thermodynamic considerations, and predicted two possible modes of protein adsorption: primary adsorption (at the surface itself) and secondary adsorption (at the periphery of the grafted PEO chains). Multilayer formation or integration of protein within the PEO chains is not predicted by this simplified model. However, based on surface force experiments involving compression of PEO brushes by protein-coated surfaces, Sheth and Leckband [12] suggested that polymer chains in a PEO brush may exhibit coexistence between an inner, dense, hydrophobic phase and a dilute hydrophilic phase at the outer edge of the brush. Such coexistence would give rise to an inner region “attractive” for protein adsorption. Nisin adsorption within PEO layers may thus be attributable to its high amphiphilicity, in addition to its small size.
Fang et al. [13] formulated a model for protein interaction with PEO brushes based on a generalized diffusion approach. Their model showed that adsorption and desorption kinetics depend on protein size and brush layer thickness. In particular, when the pendant chain layer thickness is greater than the size of the protein, adsorption and desorption kinetics both decrease with increasing chain length. In fact, their model indicated that the adsorption time is so large that, for any practical purpose, protein adsorption is negligible. A particularly interesting outcome of their approach was that proteins may become “trapped” between the surface and the barrier presented by the pendant chains. Increasing the chain length increases the steric barrier to elution, and the rate of protein desorption is thus decreased. Based on that result, they suggested that such a trapping mechanism could be used in the design of strategies for the controlled release of proteins from surfaces.
Some studies have shown that protein adsorption is insensitive to PEO end group chemistry while others have reported significant effects. Mathematical models of PEO in the brush configuration indicate that it is highly unlikely that end group chemistry would affect interaction with proteins. For example, Halperin [11] showed that chain ends are statistically distributed throughout the brush, with a maximum occurring at a distance about 70% of the chain length from the surface. Unsworth et al. [14] showed experimentally that protein repulsion at PEO brushes was uniquely determined by chain density, independent of chain length and end group chemistry. However, beyond a critical chain density, it was observed that brushes with –OH end groups were observed to remain nonfouling, while brushes with –OCH3 end groups promoted protein adsorption. The authors suggested that the high densities of terminal methoxy groups may have resulted in increased inter-chain association and/or adsorption-induced protein denaturation. The formation of terminal –OCH3 “islands” and defects in the brush layer are also predicted theoretically in a random-sequential-adsorption model advanced by Katira et al. [15].
Materials and Methods
Proteins and surfactants
A commercial purified nisin preparation was obtained from Prime Pharma (Gordons Bay, South Africa), and was dissolved as needed in filtered (0.2 μm), 10 mM monobasic sodium phosphate solution with 150 mM NaCl. To this was added filtered, 10 mM dibasic sodium phosphate with 150 mM NaCl to bring the pH to 7.4. Fibrinogen (MW 340 kDa, Sigma-Aldrich, St. Louis, MO) was dissolved in filtered, 10 mM phosphate-buffered saline (150 mM NaCl, pH 7.4, PBS), incubated at 37 °C for 4 h with gentle mixing, and then passed through a 0.45 μm syringe filter to remove aggregates. All protein solutions were prepared immediately prior to use. BASF Pluronic® triblock surfactant F108 (PEO141–PPO44– PEO141), and an end-group activated form of Pluronic® F108, with the terminal hydroxyl groups of the PEO chains converted to nitrilotriacetic acid groups (EGAP-NTA), were obtained from Allvivo Vascular, Inc. Polyclonal anti-human fibrinogen antibodies modified with horseradish peroxidase (HRP) were purchased from U.S. Biological (Swampscott, MA). All other reagents and solvents were purchased from commercial sources, and were of the highest practical purity. All solutions and buffers were made with HPLC-grade H2O to minimize contamination.
Silica surface modification
Monodisperse, 1 μm silica microspheres (Fiber Optic Center, New Bedford, MA) were used as the base substrate for all zeta potential measurements. The microspheres were washed with H2O:30% NH4OH:30% H2O2 (5:1:1 v/v) at 80 °C for 10 min, followed by H2O:37% HCl:30% H2O2 (5:1:1 v/v) at 80 °C for 10 min to remove organic contaminants [6]. The washed (bare) microspheres were then rinsed with H2O three times, dried at 110 °C, and stored desiccated. The washed microspheres were modified to render the silica surfaces sufficiently hydrophobic for triblock coating with two different, vinyl-containing silanes: trichlorovinylsilane (TCVS, Aldrich, St. Louis, MO), and allyldimethylchlorosilane (ADCS, Alfa Aesar, Ward Hill, MA). In each case, bare silica microspheres were suspended in a freshly-prepared 5% (v/v) solution of either TCVS or ADCS in dry chloroform at room temperature for 3 h. The microspheres were then washed three times each with dry chloroform, dry ethanol and HPLC-grade H2O (the residual ethanol facilitates H2O wetting of the now-hydrophobic surface) [16]. The silanized microspheres were dried overnight at 110 °C, and stored desiccated under inert gas in the dark to prevent oxidation of the vinyl groups.
Silicon wafer disks (1.0 cm2, WaferNet, San Jose, CA) were used as the substrate for enzyme-linked immunosorbent assay (ELISA) experiments. Wafers were washed as described above, and then modified with TCVS by a vapor deposition procedure [17]. Clean, dry wafers were placed in a vapor-phase reactor to which flowing dry nitrogen was introduced for 1 h. The nitrogen stream was then passed through a reservoir containing liquid TCVS at 25 °C to entrain the silane vapor. After about two hours, the TCVS had completely evaporated (leaving a small amount of non-volatile residue), and the nitrogen was allowed to flow for another hour to purge the reactor. The TCVS-modified wafers were stored desiccated in the dark under inert gas.
Surface coating with F108 and EGAP-NTA triblocks
Triblocks were covalently attached to the silanized microsphere surfaces according to methods described by McPherson et al. [16] and Park et al. [18], in which PEO-PPO-PEO triblocks were adsorbed on the hydrophobic surfaces produced by reaction of metal oxides with a vinyl silane, then subjected to γ-irradiation. Absorption of radiation or interaction with water-derived radicals forms surface-bound free radicals, which attack the neighboring adsorbed PPO block, forming covalent bonds between the surface and polymer [16].
TCVS-treated microspheres were coated by overnight incubation with a 0.50% solution of either F108 or EGAP-NTA triblock in PBS (the ADCS-treated samples were coated with F108 only). After incubation, some of the samples were washed three times with PBS prior to γ-irradiation; the remaining samples were kept in the 0.50% triblock coating solution. The surfactant-coated microspheres were irradiated to a dose of 0.3 Mrad by a 60Co source, then washed twice with PBS. Un-irradiated triblock layers (i.e. F108/EGAP-NTA bound to the vinyl-rich microsphere surface by hydrophobic association only) served as controls for layer stability tests. The stability of the triblock/surface association was evaluated by incubation of coated microspheres with 5% SDS in PBS for 1 h to dislocate non-covalently bound surfactant. Zeta potential measurement was used to evaluate the stability of the triblock coating.
Individual and sequential protein adsorption
Silanized, triblock-coated or uncoated microspheres (10% w/v) were incubated for 4 h with PBS or with PBS containing 10 mg/mL nisin or 10 mg/mL fibrinogen, then rinsed with 2 volumes of PBS. The rinsed microsphere suspensions were further incubated with PBS or with fibrinogen in PBS for 4 h. All microsphere samples were then rinsed twice with PBS to remove loosely-bound protein.
Zeta potential analysis
A 10 μL aliquot of a 10% microsphere suspension was diluted into 2 mL of 1 mM KCl (pH 7.55) in a disposable polystyrene cuvette. The diluted sample was then analyzed for 5 cycles of 30 recordings/cycle, using the phase analysis-light scattering (PALS) mode of a ZetaPALS system (Brookhaven Instruments Corp., Holtsville, NY).
Enzyme-linked immunosorbent assay (ELISA)
Flat, TCVS-modified silicon wafers were incubated with F108 (10 mg/mL in PBS) for 4 h, and irradiated in the presence of the F108 coating solution as described above. Wafers with and without F108 coatings were incubated with PBS or a 0.05 mg/mL solution of commercial nisin (Sigma-Aldrich N5764) in PBS for 4 h. F108-coated and uncoated wafers, with and without adsorbed nisin, were then transferred to the bottoms of BSA-blocked wells in a polystyrene micro-test plate. Each sample was covered with fibrinogen (0.01 mg/mL in PBS) for 1 h, and then rinsed three times with PBS. Samples were incubated for 1 h with the HRP-labeled anti-fibrinogen antibody and rinsed again according to manufacturer’s instructions (with the exception that HEPES-buffered saline with BSA was prepared in the absence of Tween 20 to reduce the possibility of elution of loosely-held fibrinogen). Bound HRP was quantified by reaction with o-phenylenediamine and H2O2 for 20 min. The reaction was quenched with sulfuric acid, and the absorbance of each sample (490 nm) used to calculate the adsorbed amount of fibrinogen.
Results and Discussion
Silanization with TCVS vs. ADCS
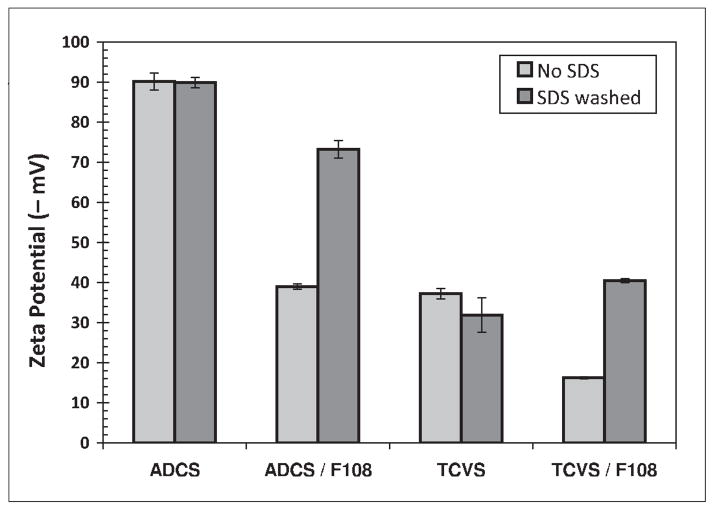
McPherson et al. [16] and Park et al. [18] described the covalent binding of PEO-PPO-PEO triblocks to TCVS-modified glass, metal and pyrolytic carbon surfaces by γ-irradiation. In the present study, triblock immobilization on layers formed by the monofunctional silane ADCS was also evaluated; this reagent cannot polymerize, and thus was expected to produce a smoother, more uniform layer for triblock coating than TCVS [19]. Representative zeta potential measurements of uncoated and F108-coated TCVS/ADCS-treated microsphere suspensions which were not γ-irradiated are shown in Figure 1. Surfaces treated with TCVS consistently showed less negative zeta potentials than their ADCS counterparts. We ascribe this effect to the thicker layers typically produced by polymerization of the trifunctional silane TCVS. Although coating with F108 could be expected to mask variations in surface charge (zeta potential) caused by due differences in silane layer thickness, this effect was not observed (Figure 1). It is possible that the TCVS treatment leaves a relatively rough surface, which better accommodates a dense packing of the triblocks. But whether silanized by TCVS or ADCS, all un-irradiated surfaces coated with F108 exhibited a significantly more negative zeta potential upon SDS challenge, to an extent consistent with near-complete removal of the F108. The zeta potential of uncoated, silanized microspheres remained largely unchanged following treatment with SDS; this is an expected result for reversible binding of SDS on a stable surface layer.
Figure 1.
Effect of SDS washing on measured zeta potential of uncoated and F108-coated microspheres silanized with TCVS or ADCS. These samples were not subjected to γ-irradiation.
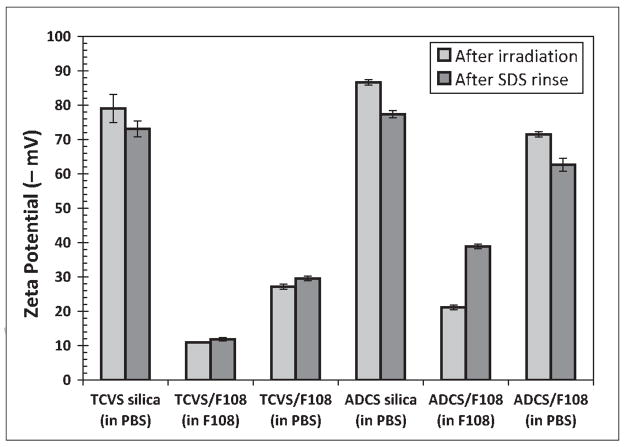
Representative zeta potentials for uncoated and F108-coated microsphere suspensions that were subjected to γ-irradiation are shown in Figure 2. The F108-coated microsphere suspensions were γ-irradiated either in PBS after washing three times with the same buffer (“washed”), or in the presence of the 0.50% F108 solution used for coating (“unwashed”). The washed samples (i.e. those irradiated in PBS) had a consistently more negative zeta potential than their unwashed counterparts, regardless of the silane used for pretreatment. This suggests that irradiation of silanized surfaces in the presence of F108 produced a denser, more uniform triblock coating. Moreover, the TCVS-silica samples irradiated in F108 showed essentially no significant change in zeta potential upon challenge with SDS, while those pretreated with ADCS showed a substantial negative shift upon challenge with SDS. As with the unirradiated samples (Figure 1), the zeta potential of the uncoated samples remained largely unchanged upon treatment with SDS, consistent with reversible SDS binding.
Figure 2.
Effect of SDS washing on measured zeta potential of uncoated and F108-coated microspheres silanized with TCVS or ADCS, and subjected to γ-irradiation. Microspheres were irradiated either in PBS (“washed”) or in the F108 coating solution (“unwashed”).
The zeta potential for uncoated TCVS-treated microspheres (about −37 mV; Figure 1) became significantly more negative (about −79 mV) after γ-irradiation. McPherson et al. attribute this increase in negative surface charge density to radiation-induced loss of the vinyl-rich surface layer itself, exposing the silica substrate. However, the zeta potential of the irradiated uncoated TCVS samples was more negative than that of unmodified silica itself (−70 mV, data not shown), yet stable coatings were also formed in the presence of F108. We speculate that dissolved O2 contributes to the radiation-induced oxidation of the vinyl C=C bonds to form ionizable, hydrophilic species.
The results of Figures 1 and 2 indicate that γ-irradiation of TCVS-treated, F108-coated surfaces in the presence of the F108 coating solution produced denser, more stable F108 layers than washed or ADCS-treated surfaces. Based on these results, all triblock coatings for further individual protein and sequential adsorption experiments were produced by TCVS treatment and γ-irradiation in the triblock coating solution.
Triblock layer stability
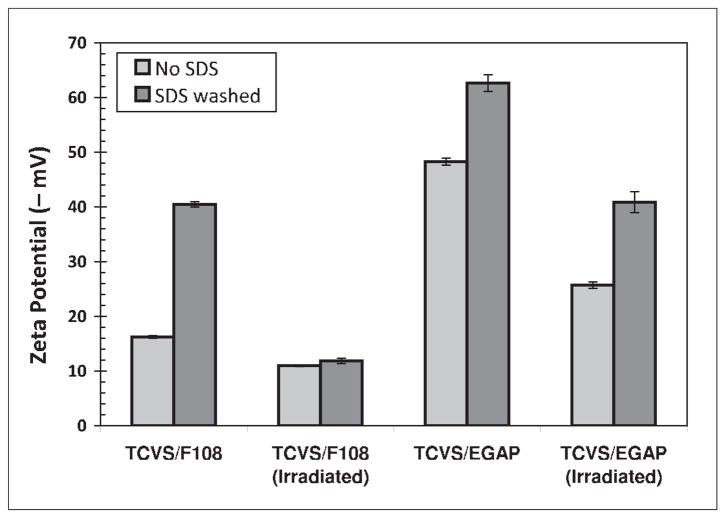
Figure 3 shows the zeta potentials of TCVS-treated microspheres coated with EGAP-NTA, with and without stabilization by γ-irradiation in the presence of 0.50% EGAP-NTA in PBS. For comparison, the analogous data for F108-coated microspheres (Figures 1 and 2) have been redrawn in Figure 3. The more negative zeta potential recorded for microspheres coated with EGAP-NTA relative to F108 is attributed to the highly anionic NTA end group. But while γstabilization of the EGAP-NTA layer was accompanied by a change in zeta potential to a less negative value, these layers appeared to remain somewhat elutable by SDS. Experimentally, the microsphere suspensions coated with EGAP-NTA tended to resist pellet formation upon centrifugation. Following SDS challenge, the efficient washing with PBS was hindered by attempts to minimize bead loss, and so the observed high negative surface charge may be due in part to residual negatively-charged SDS near the interface.
Figure 3.
Effect of γ-irradiation on the resistance of F108 and EGAP-NTA layers to elution by SDS, as determined by zeta potential of TCVS-treated, triblock-coated microspheres. Microspheres were γ-irradiated in the triblock coating solution in each case.
Individual protein and sequential adsorption of nisin and fibrinogen
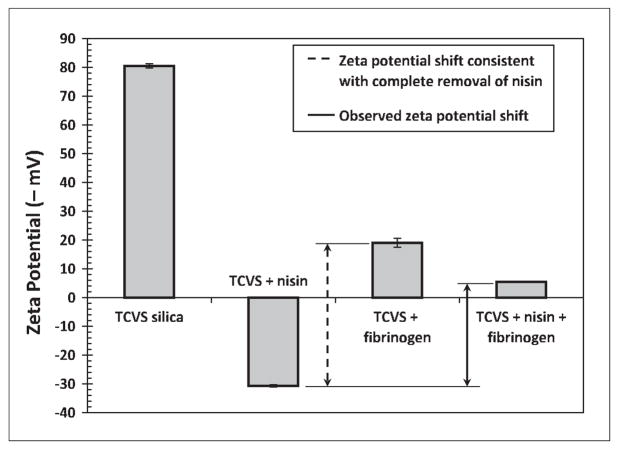
Uncoated, F108-coated and EGAP-NTA-coated microspheres were incubated with nisin or with fibrinogen in independent experiments. Microsphere samples were also incubated with nisin, rinsed and then incubated with fibrinogen (sequential adsorption). Figure 4 shows zeta potential changes due to protein adsorption on uncoated surfaces. The surface charge of uncoated microspheres became positive (+30 mV) after nisin contact, consistent with adsorption of the cationic polypeptide at the surface. The high negative charge density of the uncoated microspheres remained negative, but was masked appreciably after incubation with fibrinogen, consistent with fibrinogen adsorption at the silanized microsphere surface. Fibrinogen has an isolectric point between 5.1 and 6.3, and therefore has a net negative charge at neutral pH.
Figure 4.
Zeta potential detection of protein adsorption to uncoated, TCVS-modified and irradiated microspheres incubated with nisin alone, with fibrinogen alone, and incubated sequentially with nisin followed by fibrinogen. Microspheres were γ-irradiated in PBS (i.e. no triblocks adsorbed) prior to protein contact.
The difference between the second and third bars in Figure 4 (i.e. zeta potential of nisin- and fibrinogen-contacted surfaces) quantifies a shift in potential that would be consistent with the complete replacement of nisin on a TCVS-modified surface by fibrinogen. The difference between the second and fourth bars in Figure 4 (adsorption of nisin vs. sequential adsorption of nisin and fibrinogen) is the actual (observed) shift in zeta potential. It is instructive to compare the observed shift to the maximum possible value. The positive charge density produced by incubation with nisin alone became substantially negative after subsequent incubation with fibrinogen, indicating significant removal of adsorbed nisin. In particular, contact with fibrinogen produced a shift in zeta potential equivalent to 73% of that associated with the complete removal of nisin.
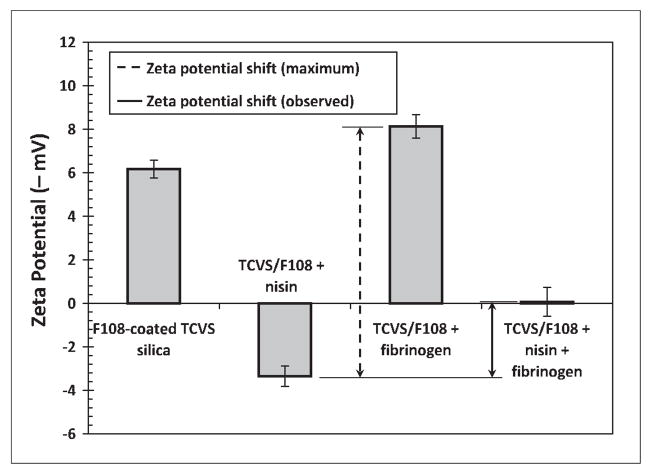
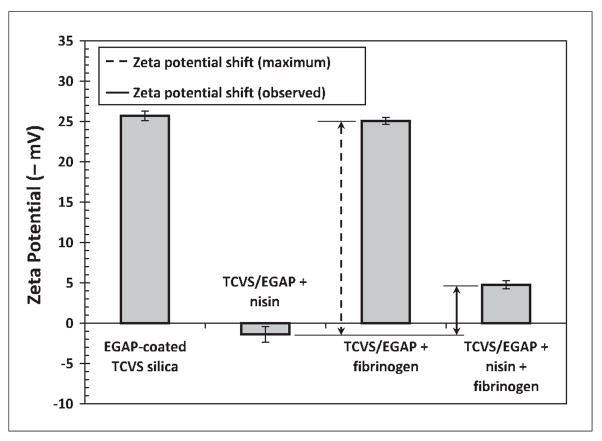
Figures 5 and 6 show zeta potential changes due to protein adsorption on F108- and EGAP-NTA-coated surfaces. Similar to the results just discussed, these results show that the observed surface charge density became positive after nisin contact, indicative of nisin adsorption at these PEO layers. Zeta potentials recorded after incubation with fibrinogen are in each case consistent with good fibrinogen repulsion by each type of PEO layer. The very small shift in zeta potential to a more negative value after fibrinogen contact observed with the F108 layer (Figure 5) can probably be attributed to non-uniformities in the triblock layer [15], giving rise to regions of unprotected hydrophobic silica that allow unhindered adsorption of fibrinogen.
Figure 5.
Zeta potential detection of protein adsorption to F108-coated, TCVS-modified microspheres incubated with nisin alone, with fibrinogen alone, and incubated sequentially with nisin followed by fibrinogen. Microspheres were γ-irradiated in F108 coating solution prior to protein contact.
Figure 6.
Zeta potential detection of protein adsorption to EGAP-NTA-coated, TCVS-modified microspheres incubated with nisin alone, with fibrinogen alone, and incubated sequentially with nisin followed by fibrinogen. Microspheres were γ-irradiated in EGAP-NTA coating solution prior to protein contact.
The presence of nisin entrapped within immobilized PEO was validated in related experiments, using X-ray photoelectron spectroscopy (XPS). In those experiments, silicon wafers were made hydrophobic by treatment with octadecyltrimethoxysilane in ethanol to form a C18 surface coating. Triblocks with a polybutadiene centerblock (PEO-PBD-PEO) were adsorbed on these C18 surfaces and immobilized by γ-irradiation as described above. In this system, the triblocks themselves contain the activated double-bonds that covalently bond with the otherwise inert C18-modified surface [16]. After washing the irradiated surfaces to remove loosely-bound triblocks, the PEO brush layers were challenged with nisin in PBS, as described above, with reference to microsphere samples. After extensive washing with buffer and water to remove excess nisin, the wafers were dried under vacuum and examined by XPS (Thermo-Fisher ESCALAB 250) equipped with a monochromatic Al Kα X-ray source (1486.6 eV). Survey and high-resolution C1s, N1s, O1s, and S2s/2p spectra were recorded at a take-off angle of 0°. The high-resolution peaks were quantified using Shirley background removal and the manufacturer’s sensitivity factors. The C1s peak was deconvoluted using the supplied peak-fitting software. A distinct C1s peak was observed at 286.3 eV on the triblock-coated, irradiated surface; this binding energy corresponds to polyether C-O bonds and is consistent with a stable PEO coating. Following incubation with nisin and several washes, the PEO-coated surfaces exhibited a strong N1s peak and small S2s/2p peaks, indicating the presence of nisin protein at the surface PEO layer. The calculated atom% ratio of N1s:S2p was 5.6 (data not shown), consistent with a N/S ratio of 6.0 calculated from the known composition of nisin. Although taken from a different triblock coating, these XPS results indicate that nisin can be entrapped within immobilized PEO.
Both Figures 5 and 6 show that the positive charge density evident after incubation with nisin alone became negative again following subsequent incubation with fibrinogen, indicating some removal of entrapped nisin in the presence of fibrinogen. But in contrast to uncoated silica, the sequential contact with fibrinogen in these cases produced a smaller shift in zeta potential, only 30% of that consistent with the complete removal of nisin at the F108 layer, and 23% of that consistent with the complete removal of nisin at the EGAP-NTA layer.
These data indicate that nisin integrates into covalently stabilized, fibrinogen-repellent PEO layers. Moreover, we observed nisin to be substantially more resistant to elution by fibrinogen when entrapped in PEO than when simply adsorbed at an uncoated surface. If present at the interface in multilayer quantities, we should expect nisin located nearer the chain ends to be less resistant to elution than nisin located deeper within the PEO [13]. Thus the sequential adsorption results can be taken as consistent with the outermost nisin molecules being eluted while PEO segments extending beyond the level of entrapped nisin retain their steric repulsive character. On the other hand, the results shown in Figures 4–6 do not preclude the possibility of fibrinogen adsorption, presumably at regions of the nisin-loaded PEO layer where electrostatic interactions could be important and the steric repulsive capability of the PEO could be compromised, due to the presence of the peptide.
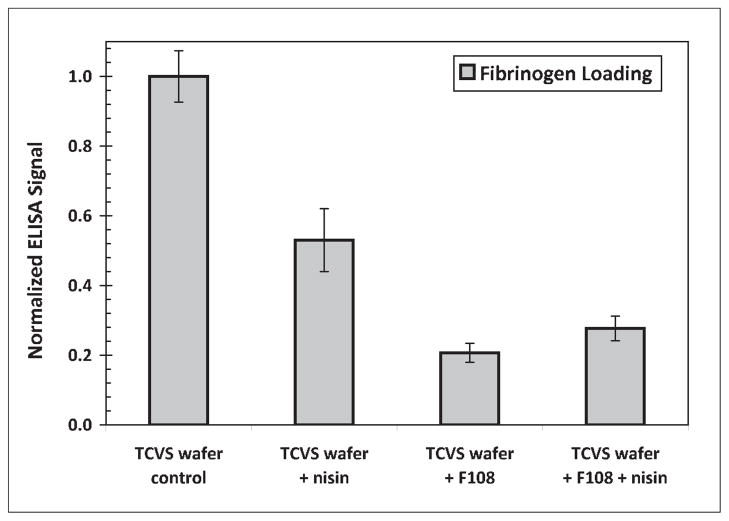
Any preferential location of a procoagulant protein such as fibrinogen at a peptide-loaded PEO layer would significantly reduce the viability of a medical device coating based on this approach. Figure 7 shows results of ELISA experiments performed with uncoated and F108-coated silica samples, in the presence and absence of adsorbed nisin. These results suggest that the presence of nisin in the PEO layer evoked a fibrinogen loading that is not substantially greater than with PEO alone. However, the presence of fibrinogen was apparent on each of the F108-coated surfaces tested. The fibrinogen detected at these surfaces may be explained by the reasonable assumption of PEO layer non-uniformities that compromise fibrinogen repulsion, but may also be an outcome of the ELISA technique itself, including difficulties associated with ensuring the absence of nonspecific adsorption by HRP-labeled anti-fibrinogen. Questions surrounding fibrinogen adsorption in this context warrant further investigation with a more direct, surface analytical approach, and will contribute to the subject of a future report.
Figure 7.
Relative fibrinogen adsorption on uncoated and F108-coated TCVS-modified surfaces in the presence and absence of adsorbed nisin. Uncoated surfaces were γ-irradiated in PBS prior to protein contact; the F108-coated surfaces were γ-irradiated in F108 coating solution. Values shown are normalized to the response of fibrinogen adsorbed to the uncoated, TCVS-treated silica.
Summary
Hydroxyl- and nitrilotriacetic acid-terminated PEO-PPO-PEO triblock coatings adsorbed on silica surfaces modified with TCVS and γ-irradiated in the presence of triblock solution were resistant to elution by SDS and showed good fibrinogen repulsion. Nisin adsorption to these PEO layers was detected by zeta potential measurements. Nisin appeared substantially more resistant to elution in the presence of fibrinogen when entrapped in PEO than when adsorbed at an uncoated surface. Tentatively, the sequential adsorption results reported here are consistent with the partial elution of nisin in the presence of fibrinogen, but retention of the steric repulsive quality of the layer.
RESEARCH HIGHLIGHTS.
Nisin entrapment occurs within fibrinogen repellent, pendant PEO layers
Entrapment within PEO enhances nisin resistance to elution
Sequential adsorption results suggest retention of PEO steric repulsive character
Acknowledgments
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (grant no. 2 R44 DK 072560-02). The authors would like to thank the staff at Allvivo Vascular Inc. for synthesizing the EGAP-NTA used in this work, and Dr. Tom Shellhammer of OSU for use of his ZetaPALS instrument.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dugdale DC, Ramsey PG. Am J Med. 1990;89:137. doi: 10.1016/0002-9343(90)90290-t. [DOI] [PubMed] [Google Scholar]
- 2.Raad I, Narro J, Khan A, Tarrand J, Vartivarian S, Bodey GP. Eur J Clin Microbiol Infect Dis. 1992;11:675. doi: 10.1007/BF01989970. [DOI] [PubMed] [Google Scholar]
- 3.Raad I, Bodey GP. Clin Infect Dis. 1992;15:197. doi: 10.1093/clinids/15.2.197. [DOI] [PubMed] [Google Scholar]
- 4.Bower CK, Bothwell MK, McGuire J. Colloids Surf B: Biointerfaces. 2001;22:259. [Google Scholar]
- 5.Bower CK, Parker JE, Higgins AZ, Oest ME, Wilson JT, Valentine BA, Bothwell MK, McGuire J. Colloids Surf B: Biointerfaces. 2002;25:81. [Google Scholar]
- 6.Tai YC, Joshi P, McGuire J, Neff JA. J Colloid Interface Sci. 2008;322:112. doi: 10.1016/j.jcis.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tai YC, McGuire J, Neff JA. J Colloid Interface Sci. 2008;322:104. doi: 10.1016/j.jcis.2008.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malmsten M, Emoto K, Van Alstine JM. J Colloid Interface Sci. 1998;202:507. doi: 10.1006/jcis.1998.5727. [DOI] [PubMed] [Google Scholar]
- 9.Rovira-Bru M, Giralt F, Cohen Y. J Colloid Interface Sci. 2001;235:70. doi: 10.1006/jcis.2000.7355. [DOI] [PubMed] [Google Scholar]
- 10.Archambault JG, Brash JL. Colloids Surf B Biointerfaces. 2004;33:111. doi: 10.1016/j.colsurfb.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Halperin A. Langmuir. 1999;15:2525. [Google Scholar]
- 12.Sheth SR, Leckband D. Proc Natl Acad Sci USA. 1997;94:8399. doi: 10.1073/pnas.94.16.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang F, Satulovsky J, Szleifer I. Biophys J. 2005;89:1516. doi: 10.1529/biophysj.104.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unsworth LD, Sheardown H, Brash JL. Langmuir. 2008;24:1924. doi: 10.1021/la702310t. [DOI] [PubMed] [Google Scholar]
- 15.Katira P, Agarwal A, Hess H. Adv Mater. 2008;20:1–6. [Google Scholar]
- 16.McPherson TB, Shim HS, Park K. J Biomed Mater Res. 1997;38:289. doi: 10.1002/(sici)1097-4636(199724)38:4<289::aid-jbm1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 17.Popat KC, Johnson RW, Desai TA. Surf Coatings Technol. 2002;154:253. [Google Scholar]
- 18.Park K, Shim HS, Dewanjee MK, Eigler NL. J Biomater Sci Polym Ed. 2000;11:1121. doi: 10.1163/156856200744228. [DOI] [PubMed] [Google Scholar]
- 19.Rajam S, Ho CC. J Membr Sci. 2006;281:211. [Google Scholar]