Abstract
HER2 is highly expressed in a significant proportion of breast cancer, ovarian cancer, and gastric cancer. Since the discovery of its role in tumorigenesis, HER2 has received great attention in cancer research during the past two decades. Successful development of the humanized monoclonal anti-HER2 antibody (Trastuzumab) for the treatment of breast cancer further spurred scientists to develop various HER2 specific antibodies, dimerization inhibitors and kinase inhibitors for cancer therapy. On the other hand, the high expression of HER2 and the accessibility of its extracellular domain make HER2 an ideal target for the targeted delivery of anti-tumor drugs as well as imaging agents. Although there is no natural ligand for HER2, various artificial ligands targeting HER2 have been developed and applied in various targeted drug delivery systems. The emphasis of this review is to elucidate the roles of HER2 in cancer therapy and targeted drug delivery. The structure and signal pathway of HER2 will be briefly described. The role of HER2 in tumorigenesis and its relationship with other tumor markers will be discussed. For the HER2 targeted cancer therapy, numerous strategies including the blockage of receptor dimerization, inhibition of the tyrosine kinase activity, and interruption of the downstream signal pathway will be summarized. For the targeted drug delivery to HER2 positive tumor cells, various targeting ligands and their delivery systems will be described in details.
Keywords: HER2 receptor, tumor marker, targeted therapy, targeted drug delivery, antibody, affibody
I. Introduction
Human Epidermal Growth Factor Receptor 2 (HER2), also known as ErbB2, c-erbB2 or HER2/neu, is a 185 kDa protein (p185) with an intracellular tyrosine kinase domain and an extracelluar ligand binding domain. In humans, HER family includes four structurally related members, HER1 (ErbB1, also known as EGFR), HER2 (ErbB2), HER3 (ErbB3) and HER4 (ErbB4). Although HER2 is the only receptor which has no identified ligand, it is the preferred partner to form heterodimer with other HER members. HER2 involved heterodimerization is the most potent signal transduction pathway among all dimmers formed by the HER family [1]. HER2 plays important roles in cell growth, survival, and differentiation in a complex manner. The major signaling pathways mediated by HER2 involve migtogen-activated protein kinase (MAPK) pathway and phosphatidylinositol 3-kinase (PI3K) pathway. As a key gene for cell survival, HER2 gene amplification and protein overexpression lead to malignant transformation [2]. It directly associates with poor clinical outcomes in breast, ovarian, gastric, prostate and other cancers.
The successful development of trastuzumab (Herceptin™), an anti-HER2 antibody, has had a major impact on the treatment of breast cancer [3]. Since then, a variety of HER2 specific antibodies and small molecular inhibitors have been assessed in clinical trials [4]. Both HER2 receptor and the whole HER2 signaling pathway have been extensively studied as the target for cancer therapy. Both the upstream and downstream of the HER2 signaling pathway are promising targets to block the signal and inhibit the tumor growth. Moreover, as an overexpressed cyto-membrane protein, HER2 is an attractive marker for targeted drug delivery to tumor cells.
The aim of this review is to elucidate the roles of HER2 in cancer therapy and targeted drug delivery. Various therapeutic strategies targeting HER2, as well as targeted drug delivery systems will be discussed.
II. HER2 and cancer
A. HER2 structure and signaling pathways
HER2 structure
Similar to all HER receptors, HER2 is a type 1 transmembrane glycoprotein composed of three distinct regions: an N-terminal extracellular domain (ECD), a single α-helix transmembrane domain (TM), and an intracellular tyrosine kinase domain.
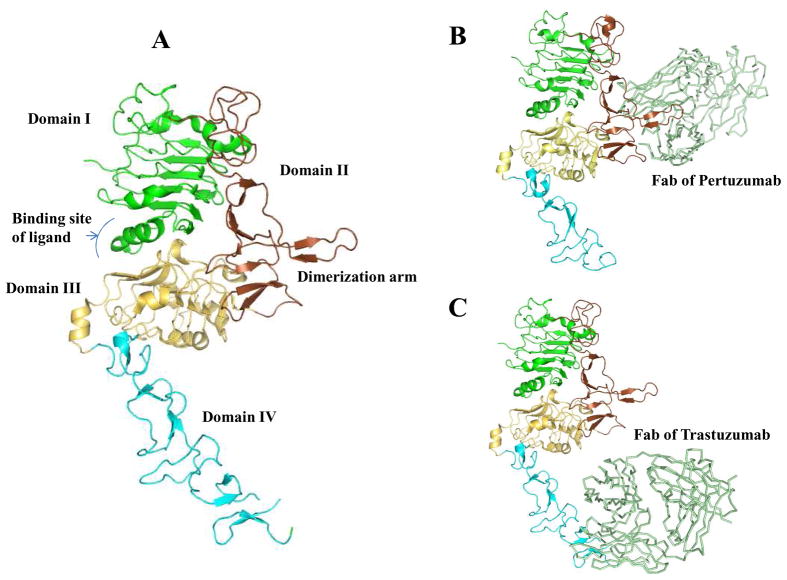
As the largest part of HER2, the N-terminal ECD contains approximately 600 residues (90~110 kD) which could be divided into four subdomains (I–IV) (Fig. 1A). Subdomain I and III can form a binding site for the receptor’s potential ligands [5]. Whereas the cysteine-rich subdomains II and IV are involved in the homodimerization and heterodimerization. Subdomain II, which contains a dimerization arm, is generally believed the main contributor for dimerization [6]. The dimerization arm is a short hairpin loop which protrudes outside of the subdomain II to contact with the dimerization arm of its dimerization partner [7]. Pertuzumab, a HER dimerization inhibitor, binds to the dimerization arm of HER2 and prevents the dimerization of HER2 with other HER family members, leading to the transformation signal blocking (Fig. 1B). Subdomain IV is close to the transmembrane domain, and it has been proven to be the critical binding site for trastuzumab (Fig. 1C) [8].
Fig 1.
The structures of HER2 ECD and its complex with Pertuzumab (PDB Code 1S78) and Trastuzumab fragment (PDB Code 1N8Z). A) The diagram of HER2 ECD. The domain I was highlighted in green, domain II in brown, domain III in lightyellow, and domain IV in cyan. The dimerization arm in domain II is also indicated. B) Fab of Pertuzumab (palegreen) binds to the dimerization arm of HER2 receptor and inhibits its dimerization with other HER receptors. C) The complex of HER2 ECD with the Fab of Trastuzumab (palegreen). The Trastuzumab Fab binds to the domain IV of HER2 ECD.
[Fig 1 was prepared using the program Pymol (DeLano, W.L. The PyMOL Molecular Graphics System (2002) DeLano Scientific, San Carlos, CA, USA)]
There are two conformations of the ECD, the closed configuration (inactive state) and the open configuration (active state) [9]. In the closed configuration, the interaction between domains II and IV occludes its dimerization arm and prevents its association with dimerization arms from other HER receptors. Binding of its native ligand to the receptor induces the association between subdomain I and III, which changes the receptor’s conformation to the open configuration, in which the dimerization arm is protruded outside of the domain II and IV and accessible to other HER receptors’ dimerization arms. Crystallographic data indicates that most of HER2 receptors exist in the open configuration [9], suggesting that the dimerization arm of HER2 is always ready to dimerize with other receptors. This might be the mechanistic explanation for HER2’s role as a prefered dimerization partner for other members in the HER family.
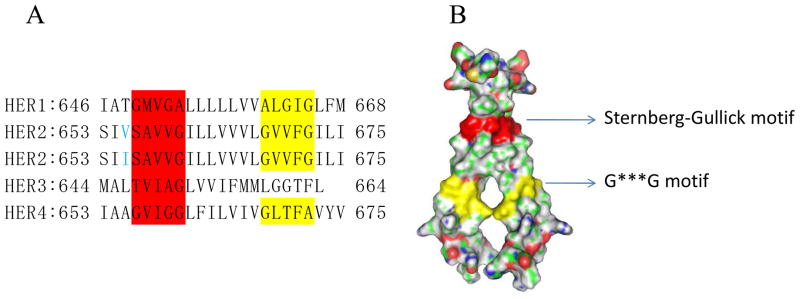
The TM domain of HER2 is a single α-helix comprised of 23 amino acids. Multiple sequence alignment of HER family shows that there are two motifs with a conserved sequence of 5 residues in the TM domain [10]. The Sternberg-Gullick motif is presented in all HER members. A point mutation (Val-664→Glu) in the Sternberg-Gullick motif of rat neu oncogene is known to induce oncogenic transformation [11]. The G***G motif is found in the TM domain of HER1, HER2 and HER4, but not HER3 (Fig. 2A) [12]. These two dimerization motifs in the TM domain might be the major driving force for the receptor dimerization. It is postulated that the strong dimerization forces are generated by van der waal forces and hydrogen bonds between hydrophobic residues in the dimerization motifs [13]. The strong interactions between the motifs have also been proven from the crystal structure of HER2 TM homodimers (Fig. 2B). .
Fig 2.
The sequence and structure of HER receptors’ transmembrane domain. A) The sequences of HER receptors transmembrane domains (~23 residues), the dimerization motifs are highlighted. Sternberg-Gullick motif is colored in red and G***G motif in yellow. The residue in blue presents a Val/Ile single nucleotide polymorphism. B) The surface of a HER2 transmembrane domain homodimer (PDB Code 2JWA). The dimerization is triggered by the strong association forces between Sternberg-Gullick motifs (red) and G***G motifs (yellow).
(Fig. 2A is adapted from Sarel J. F. et al A putative molecular-activation switch in the transmembrane domain of erbB2 PNAS 2002; 99:15937–15940. Copyright permission will be requested.)
[Figure 2B was prepared using the program Pymol (DeLano, W.L. The PyMOL Molecular Graphics System (2002) DeLano Scientific, San Carlos, CA, USA)]
The intracellular domain is approximately 500 residues and comprised of a cytoplasmic juxtamembrane (JM) linker, a tyrosine kinase (TyK) domain and a carboxyl-terminal tail. JM linker is a short flexible linker who connects the TM domain and TyK domain. As the most complicated part of HER2 receptor, TyK domain contains several important loops which form the enzyme active site [14]. The TyK domain has been extensively reviewed and hence will not be covered here. The carboxyl-terminal tail has six tyrosine residues which are available for transphosphorylation, and serve as the docking site for signaling molecules containing Src homology 2 (SH2) or phosphotyrosine binding (PTB) domain [15–16].
HER2 signaling pathway
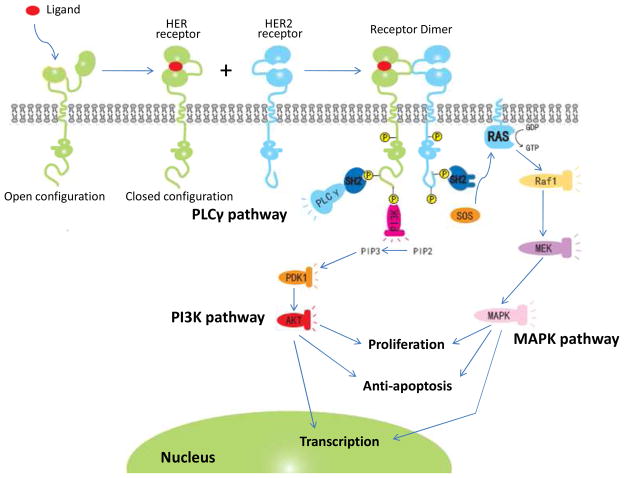
After dimerization, HER2 can signal through at least three different pathways including PI3K, MAPK, and phospholipase C-γ (PLCγ) pathways. The pattern of dimerization significantly influences the downstream signaling pathway. Different dimeric combinations are believed to produce different intracellular signaling cascades [1]. For example, induction of PI3K lipid kinase activity is stimulated by HER2/HER3 heterodimer, which is the most transforming and mitogenic signaling combination. It is possible that tyrosine-phosphorylated HER3 directly couples to PI3K since HER3 has numerous binding domains which can interact with the regulatory subunit p85 of PI3K [17]. Different from the PI3K pathway, all HER2 involved dimerization (HER1/HER2, HER2/HER3, and HER2/HER4) can activate the MAPK pathway. PI3K and MAPK pathways are key signaling pathways to promote cell proliferation and prevent apoptosis. A schematic diagram of the putative HER2 signaling pathways is presented in Fig. 3. More details about these signaling pathways have been reviewed by Yarden et al [18–19].
Fig 3.
The HER2 signaling pathway. Following ligand dependent activation of HER receptor, HER2 dimerizes with the activated HER receptor which results in tyrosine residues phosphorylation and signal transduction. PLCγ, PI3K and MAPK are the most common signaling cascades, whereas PI3K and MAPK are the major pathways involved in tumor growth and antiapoptosis.
Another important aspect of the HER’s signaling pathway is the nuclear localization which has been reported in various cell lines and tissues. These receptors can translocate to the nucleus as intact, spliced or even with receptor ligands. The nuclear-localized receptors probably act as transcription factors for genes such as Cycline D1 [20], COX2 [21] and p53 [22]. It is well documented that the nuclear HER2 activates the COX 2 gene promoter and up-regulates the COX 2 expression in tumor cells [21]. Similar to cytoplasmic HER2, nuclear HER2 may also serve as a prognostic marker of breast carcinoma. Although all four members of HER family have been shown to migrate into the nucleus, HER2 and HER3 are less studied in comparison to HER1 and HER4. However, the intracellular traffic mechanism of HER2 from cell surface to the nucleus is not fully understood, even though it is known that the juxtamembrane region of HER2 receptor harbors a putative nuclear localization sequence (NLS) containing a cluster of basic amino acids (AA 676-689, KRRQQKIRKYTMRR) [23]. The nuclear localization of HER2 is possibly mediated by the transport receptor importin β1 and nuclear pore protein Nup358 [24].
B. The role of HER2 in tumorigenesis
In normal cells, HER2 plays important roles in all stages of cell development. However, the mutation or overexpression of HER2 could directly lead to tumorigenesis as well as metastasis. Although mutation of the neu gene (rodent HER2 gene) is required for tumorigenesis in rodents, human HER2 appears to hold tumorigenic potential through overexpression of the wild-type HER2 gene. Overexpression of HER2 enhances and prolongs signals that trigger cells’ transformation. In human cancers, HER2 is frequently overexpressed in breast cancer, gastric cancer, ovarian cancer and prostate cancer.
HER2 in breast cancer
Overexpression of HER2 usually results in malignant transformation of cells and accounts for ~25% of all breast cancer cases. It is always associated with more aggressive tumor phenotypes, greater likelihood of lymph node involvement, and increased resistance to endocrine therapy [25]. The overall survival rate and relapse time for HER2 positive breast cancer patients are significantly shorter than patients without HER2 overexpression. In vitro studies showed that inhibition of HER2 expression induced significant apoptosis in breast cancer cells [26–27]. Thereafter, HER2 is a logical target for breast cancer therapy. Both prognostic and therapeutic values of HER2 in breast cancer have been established [28]. Most notably, the monoclonal humanized antibody against HER2 (Trastuzumab) was approved in 1998 by FDA for the treatment of HER2 positive breast cancer [29]. In addition, it has been shown in vitro that reduction of HER2 expression by antisense or siRNA resulted in growth inhibition and apoptosis in HER2 positive breast cancer cells [27, 30–31]. All these data indicate the essential role of HER2 in proliferation and anti-apoptosis in HER2 positive breast cancer.
HER2 in grastric cancer
Gastric cancer, also known as stomach cancer, is the second most common cause of cancer death in the world. The first description of HER2 overexpression in gastric cancer was reported in 1986 [32]. Since then, accumulated evidences indicated the association of HER2 overexpression with poor prognosis. However, the rate of HER2 overexpressionin gastric cancer was estimated in a wide range (6%–35%), which was probably due to the small sample set and variation in the scoring system [33–34]. In the ToGA clinical trial which was an international trail conducted at 130 centers world widely, HER2 testing process was established to identify eligible patients following a gastric cancer specific scoring system. The overall HER2 positive rate is about 22% (460 of 2,168 patients) which is similar to that in breast cancer (~24%) [35–36]. Unlike other cancers, HER2 overexpression rate in gastric cancer varies according to the site of the tumor. For example, a higher overexpression rate (36%) was shown in Gastroesophageal Junction(GEJ) tumours in comparison to 21% in gastric tumours [35].
Trastuzumab, the HER2 targeted antibody, has been proven effective in gastric cancer therapy. In combination with chemotherapy, trastuzumab significantly prolonged the overall survival time to 13.8 months compared to 11 months in patients treated with chemotherapy alone [37–38]. Gastric cancer is the second type of cancer in which trastuzumab has proven effective[39]. Based on results of the ToGA clinical trial, European commission approved its use in HER2 positive gastric cancer in 2009.
HER2 in ovarian cancer
Ovarian cancer is the leading cause of gynecological cancer death, and the HER2 overexpressed ovarian cancer varies from 9%~32% of all cases [28, 40–41]. However, the role of HER2 in ovarian cancer is less studied, and not as clear as that in breast cancer [28]. In a study, HER2 overexpression was identified in 27.6% of 148 ovarian tumor specimens using tissue microarray [28]. In contrast, HER2 protein was found overexpressed in all 20 immortal ovarian cancer cell lines derived from stage III and IV of ovarian cancers [42], suggesting that the HER2 overexpression is more frequent in advanced stage of ovarian cancer. Overexpression of HER2 in ovarian cancer cells leads to faster cell growth [43], higher abilities in DNA repairment [44] and colony formation [45]. A cross-talk between HER2 and estrogen receptor (ER) was identified in ovarian cancer cells. Estrogen has been proven to induce the phosphorylation of HER2, and initiate the HER2’s signaling pathway. This explained the observation that pertuzumab, a HER2 dimerizatin blocker, could reverse the estrogen-stimulated changes in ovarian cells [46].
HER2 in prostate cancer
HER2 also plays pivotal roles in prostate cancer and many efforts have been made to examine the HER2 expression in prostate cancer, albeit the result is contradictory and confusing [47–48]. The disparity is partly due to the differences in tumor sample selection, the techniques used to detect HER2, and the definition of “positive” [48]. Signoretti et al. have conducted a comprehensive study to analyze the HER2 level at DNA, RNA and protein levels in tumor samples from different clinical stages. Using an absolute scoring system with a defined “positive” criteria, they found that 25% of untreated primary tumors, 59% of localized tumors after neoadjuvant hormone therapy, and 78% of castrate metastatic tumors overexpressed HER2 [47].
However, the use of antibody targeting HER2 (Trastuzumab) showed little effect on the advanced hormone-refractory prostate cancer in a phase II clinical trial [49]. One possible explanation is that HER2 was not overexpressed in those prostate cancer patients. Nevertheless, several lines of evidence have implicated HER2 as a key mediator in the recurrence of prostate cancer to a hormone-refractory, androgen-independent tumor, which is the hallmark of prostate cancer progress [50]. The driving force for prostate cancer recurrence is the reactivation of androgen receptor (AR), which is a type of nuclear receptor, activated by steroid hormone but ablated in hormonal therapy. Phosphorylation and reactivation of AR stimulate cancer cell growth and trigger tumor progression [51]. It has been observed that overexpression of HER2 kinase enhanced AR function and hormone-independent growth in prostate tumor cells.[52–53] It was postulated that HER2 activated AR through the MAPK pathway [53]. Additionally, the HER2/HER3 dimmer increases AR protein stability and promotes the binding of AR to the promoter region of its target genes, resulting in AR activation in an androgen-depleted environment. As a result, HER2 may be a promising therapeutic target for the treatment of aggressive hormone-refractory prostate cancer [54–55]. For example, blockage of HER2 could reverse tumor cells to androgen dependent phenotype, suggesting the combination of anti-HER2 and anti-androgen therapies might be an effective therapy for prostate cancer [56].
C. The relationship of HER2 with other tumor markers
The inverse relationship between estrogen/progesterone receptor and HER2
Both ER and progesterone receptor (PR), the “female hormone’ receptors, are important predictive and prognostic markers in breast cancer. ER is expressed in 70~95% of invasive lobular carcinomas, and 70~80% of invasive ductal carcinomas, whereas PR is expressed in 60~70% of invasive breast carcinomas[57–58]. Compared to HER2, the expression of ER or PR is always associated with better clinical outcome. However, nearly 50% of recurrent tumors will finally progress to ER/PR negative phenotype which constitutively overexpressses other growth-promoting genes, such as HER2 [59]. In general, expression of HER2 is inversely correlated with ER and PR expression. The down-regulation of ER might be mediated through the hyper-activation of MAPK by HER2 [60]. The elevation of NFκB activity in HER2 amplified tumors also contributes to ER down-regulation [61]. Abrogation of HER2 signal transduction by HER2 tyrosine kinase inhibitor or HER2 specific antibody could reverse the down-regulation of ER/PR and restore their activities [62]. Comparison of the variation among ER, PR and HER2 in breast cancers has been proven to be a useful strategy to predict the pathological feature and clinical outcome. Patients with ER+/PR+/HER2− always have the best survival advantage under chemotherapy[63]. ER−/PR−/HER2+ and ER−/PR−/HER2− subtypes confer a similar survival outcome and pathological feature, although one study showed that ER−/PR−/HER2+ had a better survival than ER−/PR−/HER2− subtype [64]. In a study conducted in 61,309 patients with primary invasive breast cancer, the 5-years survival rate for women with ER−/PR−/HER2+ cancer was 75.9%, whereas 96.4% for ER+/PR+/HER2− patients [63]. Thereafter, immunohistological analysis for these three markers may provide a more reliable prediction to the clinical prognosis.
Induction of VEGF
There are extensive preclinical and clinical evidence to support the association of angiogenesis with tumor growth and spreading in breast cancer [65]. As the most potent pro-angiogenic signal, VEGF has been identified as the key angiogenic growth factor in breast cancer. High level of VEGF is associated with greater risk of recurrence [66], as well as decreased response to hormonal therapy [67] and chemotherapy [68]. Therefore, inhibition of VEGF is an effective anticancer approach for breast cancer treatment [69–72]. On the other hand, HER-2 positive breast cancer is more likely to overexpress VEGF [71, 73–74]. In a clinical study involving 611 breast cancer patients, 87.7% of HER2 positive breast cancers were found associated with overexpressed VEGF [75]. It is believed that VEGF partly contributes to the biologically aggressive phenotype of HER2 overexpressing breast cancer [75].
Given the preclinical and clinical evidence described above, the combined blockade of HER2 and VEGF is expected to have a superior antitumor efficacy [76]. Treatment with a combination of VEGF-Trap (a humanized decoy protein targeting VEGF) and monoclonal antibodies directed against HER-2 (Trastuzumab) resulted in significant inhibition of HER2 positive BT474 tumor growth than individual agent alone [5]. In addition, a phase I/II clinical trial has been conducted using trastuzumab and bevacizumab (an anti-VEGF antibody) [76–77]. The phase II clinical trial showed promising activity in HER-2 positive recurrent or metastatic breast cancer [77].
Induction of CXCR4
CXCR4 (CXC chemokine receptor), also called fusin, is a chemokine receptor which is specific for chemokine stromal-derived-factor-1 (SDF-1). It is well documented that CXCR4 is positively correlated with HER2. MDA-MB-435 cells transfected with HER2 gene showed 2.8 fold higher expression of CXCR4 than that of control cells. Similar results were also observed in NIH 3T3 cells [78]. Surprisingly, the up-regulation of CXCR4 is not mediated by amplifying the mRNA of CXCR4, but by increasing its protein synthesis speed. Moreover, HER2 inhibits the CXCR4 ubiquitination, and prevents it from ligand induced degradation [78].
Both CXCR4 and SDF-1 have been shown to be involved in breast cancer metastasis to the lung [79–80]. HER2-induced lung metastasis might be contributed by the up-regulation of CXCR4, because CXCR4 plays an important role in recruiting cancer cells to metastatic organs in which SDF-1 is enriched [80]. In addition, activation of CXCR4 with its ligand, SDF-1, enhances the phosphorylation of HER2 [81].
The important role of CXCR4 in metastasis of breast cancer makes it a novel target in breast cancer therapy. Although no clinical trial has been performed to demonstrate its essential role in therapy, mounting evidences have confirmed its potential clinical implication. Silencing CXCR4 gene by siRNA successfully blocked the breast cancer metastasis in the animal model[82]. In addition to its role in breast cancer metastasis, Lapteva et al found that CXCR4 blockage inhibited the tumor cell growth in vitro, and abrogated the tumorigenesis of cancer cells in vivo [83]. However, CXCR4 is not always correlated with HER2 overexpression. In ovarian cancer, CXCR4 is independent of HER2 expression, and it had no prognostic influence on the overall survival. It is hypothesized that a different HER2 downstream pathway might exist in ovarian cancer and breast cancers [28]. Nevertheless, more extensive studies are expected to confirm this hypothesis.
Suppression of e-cadherin
E-cadherin is a transmembrance glycoprotein that is specifically involved in epithelial cell-cell adhesion [84]. It has been postulated that e-cadherin functioned as an important invasion suppressor gene [85]. Loss of e-cadherin expression has been reported in 50% of invasive ductal carcinomas, and nearly all invasive lobular carcinomas [86]. It is possible that repression of e-cadherin decreases the strength of cellular adhesion in tissue, leading to disruption of the primary tumor architecture and an increase in migration and invasion [87]. Although the correlation of HER2 and e-cadherin expression in primary tumor is still under controversy, it is confirmed that HER2 disrupts the e-cadherin function through β-catenin phosphorylation. The cell-cell adhesion is mainly maintained by the strong association between e-cadherin/β-catenin complex and the cytoskeleton [88]. Whereas, HER2 can directly bind to β-catenin and phosphorylate it, leading to dissociation of e-cadherin/β-catenin complex [89]. In agreement with these findings, inhibiting the interaction between β-catenin and HER2 suppresses the invasion and metastasis potential of gastric cancer cells [90].
III. HER2 as a target for Cancer therapy
Almost immediately after the discovery of its role in tumorgenesis, HER2 was targeted for cancer therapy. Since all the three domains of HER2 are responsible for a specific aspect of the signaling pathway, each domain m can be targeted separately to block the HER2 signaling. Moreover, HER2 can be directly suppressed oligonucleotides including antisense oligonucleotide and siRNA. There are several reasons for HER2 receptor to become an important therapeutic target for cancer therapy. First, upregulated HER2 level causes tumoregenesis and the level of HER2 gene expression is much higher in cancer cells than that in normal adult cells. Second, HER2 overexpression is found in both primary tumors and metastasized organs [91]. Moreover, HER2 is the preferred dimerization partner for other HER receptors in the activation of HER signaling pathways, and the HER2 containing heterodimers have the highese mitogenic potential among all HER complexes. Inhibition of HER2 dimerization prevents the activation of several intracellular signaling cascades including the PI3K and MAPK pathways which can cause carcinogenesis [92–94]. Although HER2 are found overexpressed in various cancers, most of the cancer therapy targeting HER2 are developed for breast cancer.
A. Antibody targeting the extracellular domain
HER2 ECD exists in an open conformation and therefore it is continuously available for dimerization with other HER receptors. The dimerization causes the activation of downstream signaling pathways by phosphorelating the intracellular tyrosine kinase domain. The activated HER family receptors lead to many cellular effects such as cell growth, decreased apoptosis, proliferation, cellular migration and angiogenesis.
Monoclonal antibodies targeting the extracellular domain of HER2 can be used to suppress its dimerization with other HER family members. Since HER2 is the preferred dimerization partner for all other HER family receptors, preventing dimerization of HER2 could block the activation of all HER2 containing dimmers as well as its associated downstream signaling pathways. The binding of antibody would also prevent phosphorylation of the tyrosine kinase domain, and thereafter slow down the initiation of downstream signaling pathway.
Trastuzumab as such is a humanized monoclonal antibody that targets the ECD of HER2. It acts by binding to the extracellular domain of HER2 and exhibits therapeutic efficacy in HER2 over-expressing metastatic and early stage breast cancer [94–95]. Trastuzumab has been shown to induce apoptosis in breast cancer cells through antibody-dependent cellular cytoxicity (ADCC). However, not all HER2 positive patients respond to trastuzumab therapy. The phase II clinical trial of trastuzumab monotherapy only showed low response ranging from 12–34% for an average duration of 9 months. In addition, HER2 over-expressing tumors develop intrinsic resistance to trastuzumab monotherapy [94]. In contrast, combinational therapy of trastuzumab along with other anti-cancer drugs such as paclitaxel, docetaxel, lapatinib and anthracyclin provides a better and complete therapeutic effect. Trastuzumab also acts through the inhibition of angiogenesis, leading to reduced micro vessel formation in vivo [96], and reduced endothelial cell migration in vitro [97]. The lack of efficiency in trastuzumab treatment is possibly due to its inability to block the dimerization of HER2 with other HER family members such as HER3 and EGFR. An international, multicenter, randomized clinical trial was conducted to evaluate the efficacy of trastuzumab therapy after adjuvant chemotherapy in HER2 positive breast cancer. 5081 patients were involved in this study and transtuzumab was given every three weeks after the primary treatment including surgery, chemotherapy, and/or radiation therapy. One year of trastuzumab therapy significantly improved the disease-free survival among those patients [98]. In another similar clinical trial, women with surgically removed HER-2 positive breast cancer received a 52-week trastuzumab/paclitaxel therapy after the treatment with doxorubicin and cyclophosphamide. Transtuzumab treatment reduced the risk of death by 33 percent [99]. However, cardiac related side effects were observed in both clinical trials and it may raise a safety concern in its future application [98–99].
Pertuzumab is another recombinant humanized monoclonal antibody that is specifically designed as a HER2 dimerization inhibitor [93, 100]. Known as a HER dimerization inhibitor, pertuzumab binds to HER2 ECD and sterically blocks its dimerization with other HER receptors. Pertuzumab has shown growth inhibition in breast, ovarian and prostate cancer cells in preclinical studies. Phase I and II clinical trials also demonstrated good safety and promising activity in HER2 positive breast cancer patients. Since pertuzumab and trastuzumab bind to different subdomains of the HER2 ECD, combinational therapy of these two antibodies has been explored, and demonstrated enhanced antitumor activity [101]. Trastuzumab and pertuzumab together synergistically inhibit the growth of breast cancer cells, BT474, leading to reduced proliferation and enhanced apoptosis of the cells. This growth inhibition effect is mainly due to the deceleration in cell cycle progress which transfers cells into quiescence [102–103]. Although it has been reported that trastuzumab is more efficient than pertuzumab in inhibiting tumor growth, pertuzumab potentiates the effect of trastuzumab [102]. A phase III clinical trial of pertuzumab in combination with[74][73][72] trastuzumab is underway [93, 104]. Although further studies need to be done to assess the safety, side effect and cytotoxicity, pertuzumab is found to be safer than trastuzumab [93] as it does not require the antibody related cellular toxicity for its efficacy [100, 105]. In addition, presence of the HER2 containing heterodimers is found to be more responsive to pertuzumab in breast cancer and non-small-cell lung carcinoma [93, 106]. This suggests that prtuzumab has a wider application than trastuzumab for the treatment of various cancers. Subsequently, inhibition of HER dimerization may provide a novel and effective approach for future cancer therapy.
B. HER2 Tyrosine kinase inhibitor
The intracellular domain (tyrosine kinase) is another target in the HER2 signaling pathway. Small molecules have been developed to prevent the phosphorylation of tyrosine kinase. Among them, lapatinib is a small molecular inhibitor of HER2 and HER1 tyrosine kinase domain. It has been developed as a safe and orally effective drug for the treatment of HER1 or/and HER2 overexpressing breast cancer, metastasized tumor and HER1 overexpressing head and neck squamous cell carcinoma [107]. Lapatinib sensitizes HER2 positive breast cancer cells to radiation and chemotherapy. Lapatinib acts through a different mechanism from other tyrosine kinase inhibitors (TKI), in that the interaction between lapatinib and HER1 or HER2 is reversible with a very slow dissociation, allowing for a prolonged inhibition of the tyrosine phosphorylation in tumor cells [94, 108–109]. Lapatinib interrupts signal transduction from the HER2 and HER1 receptors by competing with ATP for the intracellular ATP-binding domain of these receptor tyrosin kinases. Lapatinib has shown to inhibit tumor growth both in vitro and in vivo models with HER1 and HER2 overexpression [110]. Lapatinib interrupts the activity of HER2 and HER1 to block the downstream signaling. Also it is reported to inhibit downstream signaling molecules such as phosphorelated ERK1/2, Akt and cyclin D [111]. In addition, lapatinib induces apoptosis and inhibits insulin like growth factor receptor type 1 (IGF-1R) signaling. Since trastuzumab and lapatinab act through different routes, lapatinib can be used with trastuzumab to produce enhanced and synergistic effect in trastuzumab resistant cancers. For example, a combination therapy of lapatinib with anti-HER2 antibody enhanced the apoptosis of HER2 overexpressing breast cancer cells [112]. Similarly, Konecny et al. reported that lapatinib in combination with trastuzumab exhibited synergistic effect in four different HER2 positive tumor cells. In addition, lapatinib retained significant activity in tumor cells which are resistant to trastuzumab [113].
HER2 positive patients have increased chances of developing central nervous system (CNS) disease, especially if they are treated with trastuzumab. Combination therapy as well as monotherapy with lapatinib has shown to prevent the CNS progression. As a small molecule, lapatinib can cross the blood brain barrier and hence show its activity against CNS disease. Lapatanib has also been used in combination therapy along with other chemotherapey drugs such as paclitaxel, anthracyclin and docetaxel [95]. Moreover, clinical trials demonstrated that lapatinib is a safe drug with no serious and symptomatic cardiotoxicity [95].
C. Silencing of HER2 by oligonucleotides
Instead of targeting the three domains of HER2 receptor, HER2 gene expression can be directly inhibited by oligonucleotides including antisense oligonucleotide and siRNA. Antisense oligonucleotide acts by binding to the complementary mRNA sequence through Watson-Crick base pairing, leading to its degradation by endogenous nuclease RNase H (Green et al., 2000). The resultant downregulation of HER2 mRNA causes inhibition of proliferation of HER2 overexpressing cancer cells [30, 114]. In addition, antisense oligonucleotide suppresses HER2 expression in a dose dependent, sequence specific and target specific manner [30, 115]. It was demonstrated that the combinational therapy of antisense oligonucleotide and chemotherapy drug doxorubicin produced synergistic effect in inhibiting proliferation and activating apoptosis of HER2 overexpressing breast cancer cells [30, 114].
siRNA acts through the RNA interference mechanism where the antisense strand of siRNA binds the complementary sequence of target mRNA and triggers its degradation.[116] Potent knockdown of specific gene sequence makes siRNA a promising therapeutic approach for cancer therapy.[31, 117] Inhibition of HER2 expression by a retrovirus-mediated shRNA (short hairpin RNA) resulted in cell cycle arrest at G0/G1, increased apoptosis, reduced proliferation, and growth inhibition in breast and ovarian tumor cells overespressing HER2.[27] Similar effects have been observed by other groups in HER2 positive breast cancer cells using synthetic siRNA.[26, 118] In addition, we recently studied the effect of HER2 siRNA on tumor cells invasiveness properties including cell morphology change, in vitro migration, cell spreading, and adhesion to extracellular matrix (ECM). Our data demonstrated for the first time that HER2 siRNA could inhibit cell migration and invasion abilities. The HER2 siRNA also exhibited dramatic suppression on cell spreading and adhesion to ECM.[31]
IV. Targeted delivery of drugs to HER2 positive tumor cells
HER2 is highly expressed in a significant proportion of breast cancer, ovarian cancer and gastric cancer cases. The overexpression of HER2 is also clearly associated with more aggressive tumor phenotypes and poor prognosis, thus making it an attractive target for cancer therapy. Moreover, the overexpression of HER2 receptor on tumor cells and accessibility of the extracellular domain of HER2 make it an ideal marker for the receptor mediated, targeted drug delivery systems [119]. Although there is no natural ligand for HER2, artificial ligands including antibody, Fab, ScFv, affibody and peptide have been developed for HER2 targeted drug delivery. The comparison of different artificial ligands are listed in Table 1.
Table 1.
The properties of different artificial anti-HER2 ligands.
| Properties | Antibody | Fab′ | scFv | Affibody | Peptide |
|---|---|---|---|---|---|
| Molecular Wt | 150K | 55K | 27K | 5~6K | ~1K |
| Immunogenicity | Yes | No | No | No | No |
| Ligand/antigen affinity (Kd) | pM~nM | nM | nM | nM~μM | μM |
| T1/2 blooda | 2~3 d | ~4 h | 30~60 min | 10~60min | ~5 min |
| Tissue penetration ability | poor | moderate | moderate | good | excellent |
| Clearance organ | Liver | Kidney | Kidney | Kidney | Kidney |
In blood of nude mice.
A. Using antibody and its fragments as the targeting ligand Anti-HER2 antibody mediated nano-scaled systems
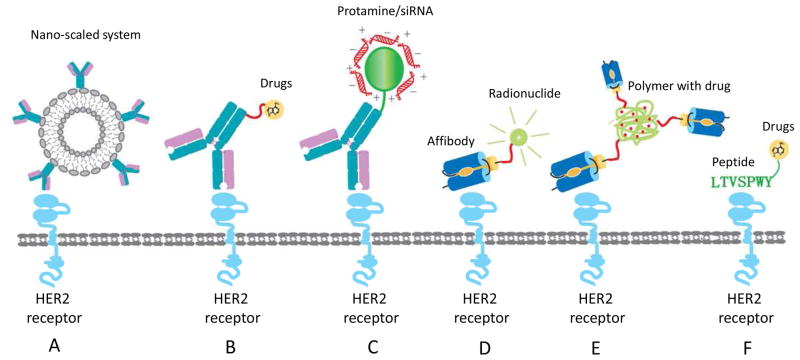
The most intensively studied drug delivery system targeting HER2 receptor is the antibody conjugated nano-scaled systems including nanoparticles and immunoliposomes (Fig. 4A). To recognize cancer cells overexpressing HER2, surface of the nano-scaled system could be modified with anti-HER2 antibodies or its fragments (Fab or ScFv). These nano-scaled systems can be used to deliver small molecule drugs as well as nucleic acid drugs. For example, anti-HER2 antibody/ScFv conjugated, PEG stabilized immunoliposomes displayed a long-term circulation, and selective delivery of the encapsulated drug, doxorubicin, into HER2 positive tumors [120–121]. Paclitaxel has also been selectively delivered to metastatic prostate cancer by an anti-HER2 cationic immunoemulsion [122]. These anti-HER2 delivery systems greatly increased the therapeutic index by enhancing the anti-tumor efficacy and reducing the systemic toxicity.
Fig 4.
Various strategies for targeted drug delivery to HER2 positive cells. A) Anti-HER2 antibody mediated nanoscaled system. B) Anti-HER2 Antibody-Drug Conjugate (ADC). C) Anti-HER2 antibody fusion protein can be used for nucleic acids delivery. D) Radionuclide is conjugated to anti-HER2 affibody for tumor imaging. E) Affibody conjugated drug delivery system. F) Anti-HER2 peptide mediated drug delivery.
Target delivery of nucleic acids to HER2 overexpressing tumor cells was achieved by conjugating anti-HER2 Fab′ fragments to liposome/DNA complexes [123]. In addition to immunoliposome, genosphere is another novel nucleic acids delivery system. It is a type of nanoparticles prepared by assembling the nucleic acids and cationic lipids from an aqueous/organic liquid monophase. Genosphere nanoparticles are homogeneous in size (80~110nm) and affords excellent protection for the entrapped nucleic acids in the presence of plasma [124]. Moreover, genosphere can be stably stored under a variety of conditions without size change and nucleic acids release. HER2 targeted genospheres have been shown to specifically deliver nucleic acids to HER2 positive SK-BR-3 cells [125–126].
Anti-HER2 antibody-drug conjugates (ADC)
Although trastuzumab represents one of the most successful applications of antibody for cancer therapy, its therapeutic efficacy as a single agent has been proven to be limited. Trastuzumab only benefits part of patients who manifest +3 level of HER2 overexpression. Consequently, adjuvant chemical drugs or antibody-drug conjugates (ADC) have been developed to circumvent these drawbacks (Fig. 4B).
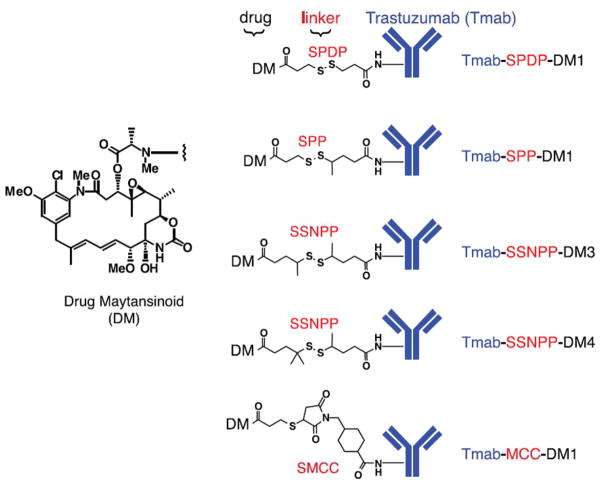
One successful application of ADC is the Trastuzumab-DM1, a covalent coupling of trastuzumab with maytansinoid which is a derivative of the antimitotic drug maytansine (Fig. 5). The disulfide-linked Trastuzumab-DM1 was initially designed to release the cytotic drug after intracellular reduction, and Trastuzumab-SPDP-DM1 with the least hindered disulfide bond was firstly designed. Although the expected anti-proliferation activity was achieved in vitro, pharmacokinetic analysis showed that Trastuzumab-SPDP-DM1 was quickly cleared from the blood and was undetectable at day 3. Addition of a methyl group on one or both sides of the S-S bond generated a more hindered disulfide bond with increased resistance to cleavage. For example, Trastuzumab-SPP-DM1 with one -CH3 on the antibody side of the S-S bond showed that 11% of the ADC was remained in circulation at day 7 post-administration. Addition of a second -CH3 on the S-S (Trastuzumab-SSNPP-DM3) further increased the circulation time, and 55% of the dose remained in the blood after 7 days. Trastuzumab-SSNPP-DM4 with three -CH3 groups showed the highest stability in the circulation with 70% of ADC was detected at day 7.
Fig 5.
Structures of Trastuzumab-maytansinoid (Tmab-DM1). Maytansinoid was conjugated to Trastuzumab via various disulfide bonds and thioether bonds. While Tmab-SSNPP-DM4 and Tmab-MCC-DM1 showed the highest stablility, Tmab-MCC-DM1 exhibited more potent activity in vivo.
(Adapted from Lewis Phillips GD et al Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody–Cytotoxic Drug Conjugate Cancer Res 2008; 68: (22). 9280–9290. Copyright permission will be requested.)
The linker also affects the pharmacokinetics and activity of the ADC. For example, Trastuzumab-MCC-DM4 with a nonreduced linker showed similar stability as Trastuzumab-SSNPP-DM4. However, Trastuzumab-MCC-DM4 displayed higher in vivo activity than other ADCs with disulfide linkers. Moreover, trastuzumab conjugated to DM1 through another nonreducible linker, ADCs with SMCC, was better tolerated in mice than ADCs with disulfide linkeage. Unlike Trastuzumab-DM with disulfide linkers, Trastuzumab-MCC-DM1 undergoes intracellular proteolytic cleavage to release the active maytandinoid. Accordingly, the activity of Trastuzumab-MCC-DM1 was found blocked by protease inhibitors. Trastuzumab-MCC-DM1 has shown potent activity in both Trastuzumab-sensitive and Trastuzumab-insensitive cancer models with overexpressed HER2 [119]. Phase I/II clinical studies of the Tratuzumab-DM1 have been conducted in patients with advanced HER2 positive metastatic breast cancer (MBC), and no cardiac-specific toxicity was observed [127–128].
Several other ADCs using anti-HER2 antibody have also been developed and showed potent antitumor activity.[129–130] For example, a novel vitamin D analog 1α(OH)D5 (D5) has been demonstrated to suppress the development and metastasis of various tumor cells in vitro and in vivo [130–131]. Conjugating of the D5 to HER2 antibody (clone 9G6.10, ab-2) specifically delivered the D5 to HER2 overexpressing breast cancer cells with enhanced in vivo growth-inhibitory effect [132].
Despite the successful reports of the ADC strategy, it is important to note that this strategy has some limitations. The first one is the limited reproducibility of the chemical conjugation due to the fact that there are numerous coupling sites in a single antibody molecule. Second, chemically modified antibody shows greater tendency to aggregate, especially when multiple drugs are conjugated to a single antibody. For example, conjugation technology has been advanced so much that as many as 16 doxorubicin molecules can be linked to the same antibody. However, the conjugates are highly prone to aggregate via noncovalent bonds [133]. Furthermore, it is challenge to remove the unconjugated antibody molecules from the conjugated ADCs.
Anti-HER2 antibody fusion proteins
With the development of recombinant DNA technology, multivalent fusion protein composed of a targeting ligand and a therapeutic portion, becomes a promising therapeutic approach for HER2 positive cancer. Compared to the ADC strategy, the most attractive advantage of the fusion protein is that the targeting ligand and antitumor protein can be produced directly as a single molecule, thus avoids the laborious chemical conjugation steps.
Yang et al. have developed an expression plasmid to express a fusion protein, named as immunoGrB, which contains an anti-HER2 scFV and granzyme B (GrB), a granular serine proteases produced by natural killer cells and cytotoxic T lymphocytes. Injection of the immunoGrB expression plasmid specifically killed HER2 positive tumor cells both in vitro and in vivo in a nude mouse model [134]. Using similar strategy, Jia et al. developed the immunocasp-3, a fusion protein of the anti-HER2 scFv with the active caspase-3. Expression of this immunocasp-3 fusion protein selectively destroyed tumor cells with overexpressed HER2 in a mouse xenograft model [135]. Anti-HER2 antibody and its fragments have also been fused with endostatin [136], apoptosis-inducing factor (AIF) [137], and cytokine IL-12 [138] for cancer therapy.
On the other hand, anti-HER2 antibody and its fragments can be fused with cationic proteins to form a targeted delivery carrier for nucleic acids (Fig. 4C). Human protamine and histone are commonly used cationic proteins to form the complex with negatively charged nucleic acids. Recombinant fusion proteins of anti-HER2 antibody with protamine/histone have been developed for siRNA and plasmid DNA delivery. Song et al. constructed a fusion protein composed of anti-HER2 scFV (ML39 scFv) and protamine. The fused protamine (ML39 scFv-P) formed complex with siRNA and selectively delivered a FITC-siRNA to HER2 positive SK-BR-3 cells, but not the HER2 negative MCF-7 cells. Furthermore, the fusion protamine specifically delivered Ku70 siRNA to SK-BR3 cells and silenced the Ku70 expression, but it was not effective in the MCF-7 cells [139]. Using the same HER2 specific scFv, Li et al. developed a fusion protein comprised of scFv and a truncated form of protamine, called scFv-P-S. This fusion protein delivered a luciferase plasmid DNA into SK-BR-3 cells with a 8~10 fold increase of luciferase expression in comparison to MCF-7 cells [140].
B. Using affibody as the targeting ligand
Affibody molecules are small, stable, 58 amino-acid Z-domain scaffolds, derived from the IgG binding domains of staphylococcal protein A. The binding pocket of an affibody is composed of 13 amino acids, which can be randomized to bind a variety of targets. In contrast to monoclonal antibody, affibody has following advantages as a targeting ligand. First, the small size of affibody (MW:6kDa) guarantees its tissue/cell penetration ability (Table 1). Second, its functional end groups for chemical conjugation are distanced from its binding site. Moreover, affibody has a robust structure, and can be easily synthesized in a large-scale manner [141]. All of these advantages make the affibody a valuable ligand for targeted drug delivery and tumor imaging.
Wikman et al., for the first time, identified an affibody (His6-ZHER2/neu:4) which can specifically bind to the HER2 extracellular domain with a nanomolar affinity (~50 nM). The His6-ZHER2/neu:4 affibody also showed selective binding to native HER2 on breast cancer cells [142]. Since then, anti-HER2 affibody has been widely used as an efficient tumor imaging tool after conjugating with radionuclide (Fig. 4D). The traditional antibody conjugated imaging molecules always have a long biodistribution time, poor tumor penetration ability and slow blood clearance. Although improved imaging agents have been obtained by using ScFv, its size (27~54kDa) is still not small enough to achieve fast distribution, penetration and blood clearance [143]. In contrast, high affinity (up to nM) and small size of the anti-HER2 affibody result in a rapid clearance from plasma, yielding a high tumor-to-background image contrast. Orlova et al. radiolabled 111In to a 1,4,7,10-tetra-azacylododecane-N′N″N‴N″″-tetraacetic acid (DOTA) chelator, which is conjugated with the anti-HER2 affibody ZHER2:342-pep2. The 111In-DOTA-ZHER2:342-pep2 was specific for HER2 positive xenografts and showed a high tumor-to-blood ratio of >7.5 as well as high-contrast gamma camera images 1 h after administration. Pretreatment with trastuzumab did not interfere with the tumor imaging, whereas the degradation of HER2 receptor obscured the tumor imaging [144]. Moreover, two identical affibody molecules can be linked together to enhance the binding affinity [145].
Due to the short plasma circulation and fast blood clearance, affibodys are optimal for tumor imaging, but not for the affibody drug conjugates and radiotherapeutics [146]. Therefore, extension of the affibody survival time might be a prerequisite for affibody mediated targeted therapy. The albumin binding technology has been used to extend the plasma half life of affibody. Fusing affibody to the Albumin Binding Domain (ABD), a small protein domain (5 kDa), has been shown to elongate the half-life of affibody in mice[146–147].
Recently, anti-HER2 affibody was also employed as a targeting ligand for nano-scaled drug delivery systems (Fig. 4E) [141],[148]. Alexis et al. conjugated the anti-HER2 affibody to poly-(D,L-lactic acid)-poly(ethylene glycol)-maleimide (PLA-PEG-Mal) copolymer, which was used to prepare paclitaxel encapsulated nanoparticles. This nanoparticle formulation was specifically internalized to HER2 positive tumor cells, and subsequently demonstrated cellular toxicity [148]. Furthermore, an adenovirus capsid was modified with anti-HER2 affibody to change the natural tropism of the adenovirus vector. The adenovirus fiber was redesigned to include the anti-HER2 affibody without affecting the virion formation. The modified adenoviral vector selectively delivered a dual-function transgene into HER2 positive breast cancer cells [141, 148].
C. Using peptide as the targeting ligand
Compared to antibody, ScFv, and affibody, peptide represents a more appropriate targeting moiety because of its small molecular weight (~1kd), excellent tissue/cell penetration, ease of production, and flexibility in chemical conjugation (Table 1). Remarkable efforts have been made to screen or design anti-HER2 peptides. Using a structure-based procedure, Murali et al. designed a 1.5kDa anti-HER2 peptide mimic (AHNP), which specifically binds to HER2 with a high affinity and exhibits similar potency to the full-length of Trastuzumab [149]. Since then, AHNP has been employed as a targeting moiety to specifically deliver therapeutic agents to HER2 positive cancer cells [150–153]. For example, cell-penetrating peptide (CPP) is very efficient in crossing cell membranes and delivering various agents into cells, but the lack of specificity limits its therapeutic application. By coupling the AHNP to CPP, a novel peptide carrier (P3-AHNP) was developed to target HER2 positive breast cancer cells in vitro and in vivo [153]. A signal transducer and activator of transcription 3 (STAT3) was linked to this peptide carrier to form the P3-AHNP-STAT3. The in vivo studies demonstrated a higher accumulation of P3-AHNP-STAT3 in HER2 positive tumor xenografts in comparison to HER2 negative xenografts. Accordingly, a more significant reduction of proliferation and increased apoptosis were observed in HER2 positive tumor xenografts [153]. Similarly, AHNP was directly conjugated to a mitochondriotoxic, proapoptotic peptide (PAP) to form a bifunctional peptide, which is biologically active and capable of targeting HER2 positive tumor cells. This approach efficiently inhibited tumor growth in a HER2 positive xenografts model [151].
Peptide LTVSPWY is another ligand which was identified using a biopanning procedure, which is a selection technique using a peptide expression phage library [154]. It has been successfully used to identify peptides against various targets via affinity selection[155]. Targeted delivery of an antisense oligonucleotide to HER2 positive tumor cells was achieved by conjugating the LTVSPWY peptide to the oligonucleotide [154]. In addition, a proapoptotic α-tocopheryl succinate (α-TOS) was selectively delivered to HER2 overexpressing cancer cell after conjugating with the LTVSPWY (Fig. 4F). The α-TOS-LTVSPWY rapidly accumulated in HER2 positive cells, induced rapid apoptosis, and efficiently inhibited tumor growth [156].
Recently, in silico methods have been applied to design anti-HER2 peptide directly by analyzing the 3D structure of HER2-antibody complex. A small peptide HRAP was identified using this computer-aided structure-based drug design (SBDD) method. The HRAP inhibited proliferation of HER2 positive breast cancer cells without induction of apoptosis [157]. This peptide may provide another targeting moiety for the targeted drug delivery to HER2 positive tumor cells.
V. Conclusions
Since the discovery of its important roles in tumorigenesis, HER2 has attracted enormous attention as a target for cancer therapy. The discovery of trastuzumab represents one of the most successful advances in breast cancer therapy in the past two decades. Although trastuzumab benefits a lot of patients with HER2 overexpresion, moderate potency, drug resistance and toxic side-effects compromise its therapeutic effect. As a result, many other agents targeting the HER2 signaling pathway, or even the gene silencing of HER2 have been explored, and some of them demonstrated promising activity in preclinical as well as clinical studies.
On the other hand, the overexpressed HER2 receptor is an ideal marker for targeted drug delivery systems. The anti-HER2 ligand can be linked directly to a cytotoxic agent, or attached to a nanoscaled system with encapsulated therapeutic agents. Since HER2 does not have natural ligands, one of the major obstacles in this application is the identification/generation of HER2 specific, artificial ligands with high affinity, and good stability in the blood circulation. HER2 specific antibody and its fragments, affibody, and peptide have been developed to delivery various agents to HER2 positive tumor cells. The molecular technology has advanced to the point where ligand affinity can be dramatically increased by large scale screening and point mutation. Taken together, the importance of HER2 in cancer therapy is not only from its role in tumorigenesis, but also from its role as a maker for targeted delivery of various therapeutic agents.
VII. Search strategy
To summarize the most recent advance in HER2 related topics, MEDLINE and CAS databases were searched using Pubmed (National Library of Medicine) and Scifinder (Chemical Abstracts Service). The search included all the papers and conference abstracts published between January 2000 and May 2009. To supplement to the result, several important original papers before Jan. 2000 were also collected, but the total number was less than 10. Search words included HER2, pathway, trastuzumab, herceptin, breast cancer, gastric cancer, prostate cancer, ovarian cancer, lapatinib, HER2 nanoparticle, HER2 antibody fusion protein, tumor imaging, HER2 affibody, and HER2 binding peptide. The search should include at least one of the above search words in the title, abstract or key words. Articles which had high impact factors were preferred. Low impact factor journals were also considered if the papers are innovative.
Acknowledgments
This work was supported by a Concept Award (W81XWH-08-1-0603) from the Department of Defense Breast Cancer Research Program, a grant (1R21CA143683-01) from the National Cancer Institute at NIH, and a start-up package at the University of Missouri-Kansas City.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol. 2001;12(Suppl 1):S3–8. doi: 10.1093/annonc/12.suppl_1.s3. [DOI] [PubMed] [Google Scholar]
- 2.Neve RM, Lane HA, Hynes NE. The role of overexpressed HER2 in transformation. Ann Oncol. 2001;12(Suppl 1):S9–13. doi: 10.1093/annonc/12.suppl_1.s9. [DOI] [PubMed] [Google Scholar]
- 3.Tan AR, Swain SM. Ongoing adjuvant trials with trastuzumab in breast cancer. Semin Oncol. 2003;30(5 Suppl 16):54–64. doi: 10.1053/j.seminoncol.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Spector N, et al. HER2 therapy. Small molecule HER-2 tyrosine kinase inhibitors. Breast Cancer Res. 2007;9(2):205. [Google Scholar]
- 5.Lax I, et al. Localization of a major receptor-binding domain for epidermal growth factor by affinity labeling. Mol Cell Biol. 1988;8(4):1831–4. doi: 10.1128/mcb.8.4.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pietras RJ, et al. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995;10(12):2435–46. [PubMed] [Google Scholar]
- 7.Di Fiore PP, et al. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987;237(4811):178–82. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- 8.Park JW, et al. Unraveling the biologic and clinical complexities of HER2. Clin Breast Cancer. 2008;8(5):392–401. doi: 10.3816/CBC.2008.n.047. [DOI] [PubMed] [Google Scholar]
- 9.Garrett TP, et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell. 2003;11(2):495–505. doi: 10.1016/s1097-2765(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 10.Fleishman SJ, Schlessinger J, Ben-Tal N. A putative molecular-activation switch in the transmembrane domain of erbB2. Proc Natl Acad Sci U S A. 2002;99(25):15937–40. doi: 10.1073/pnas.252640799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharpe S, Barber KR, Grant CW. Val(659)-->Glu mutation within the transmembrane domain of ErbB-2: effects measured by (2)H NMR in fluid phospholipid bilayers. Biochemistry. 2000;39(21):6572–80. doi: 10.1021/bi000038o. [DOI] [PubMed] [Google Scholar]
- 12.Mendrola JM, et al. The single transmembrane domains of ErbB receptors self-associate in cell membranes. J Biol Chem. 2002;277(7):4704–12. doi: 10.1074/jbc.M108681200. [DOI] [PubMed] [Google Scholar]
- 13.Bazley LA, Gullick WJ. The epidermal growth factor receptor family. Endocr Relat Cancer. 2005;12(Suppl 1):S17–27. doi: 10.1677/erc.1.01032. [DOI] [PubMed] [Google Scholar]
- 14.Telesco SE, Radhakrishnan R. Atomistic insights into regulatory mechanisms of the HER2 tyrosine kinase domain: a molecular dynamics study. Biophys J. 2009;96(6):2321–34. doi: 10.1016/j.bpj.2008.12.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulze WX, Deng L, Mann M. Mol Syst Biol. Vol. 1. 2005. Phosphotyrosine interactome of the ErbB-receptor kinase family; p. 2005 0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones RB, et al. A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature. 2006;439(7073):168–74. doi: 10.1038/nature04177. [DOI] [PubMed] [Google Scholar]
- 17.Hellyer NJ, Cheng K, Koland JG. ErbB3 (HER3) interaction with the p85 regulatory subunit of phosphoinositide 3-kinase. Biochem J. 1998;333(Pt 3):757–63. doi: 10.1042/bj3330757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 19.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–16. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 20.Lin SY, et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3(9):802–8. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 21.Wang SC, et al. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004;6(3):251–61. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Williams CC, et al. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J Cell Biol. 2004;167(3):469–78. doi: 10.1083/jcb.200403155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu SC, Hung MC. Characterization of a novel tripartite nuclear localization sequence in the EGFR family. J Biol Chem. 2007;282(14):10432–40. doi: 10.1074/jbc.M610014200. [DOI] [PubMed] [Google Scholar]
- 24.Giri DK, et al. Endosomal transport of ErbB-2: mechanism for nuclear entry of the cell surface receptor. Mol Cell Biol. 2005;25(24):11005–18. doi: 10.1128/MCB.25.24.11005-11018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engel RH, V, Kaklamani G. HER2-positive breast cancer: current and future treatment strategies. Drugs. 2007;67(9):1329–41. doi: 10.2165/00003495-200767090-00006. [DOI] [PubMed] [Google Scholar]
- 26.Faltus T, et al. Silencing of the HER2/neu gene by siRNA inhibits proliferation and induces apoptosis in HER2/neu-overexpressing breast cancer cells. Neoplasia. 2004;6(6):786–95. doi: 10.1593/neo.04313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang G, et al. Inhibition of breast and ovarian tumor growth through multiple signaling pathways by using retrovirus-mediated small interfering RNA against Her-2/neu gene expression. J Biol Chem. 2004;279(6):4339–45. doi: 10.1074/jbc.M311153200. [DOI] [PubMed] [Google Scholar]
- 28.Pils D, et al. In ovarian cancer the prognostic influence of HER2/neu is not dependent on the CXCR4/SDF-1 signalling pathway. Br J Cancer. 2007;96(3):485–91. doi: 10.1038/sj.bjc.6603581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shawver LK, Slamon D, Ullrich A. Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell. 2002;1(2):117–23. doi: 10.1016/s1535-6108(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 30.Roh H, Pippin J, Drebin JA. Down-regulation of HER2/neu expression induces apoptosis in human cancer cells that overexpress HER2/neu. Cancer Res. 2000;60(3):560–5. [PubMed] [Google Scholar]
- 31.Tai W, Qin B, Cheng K. Inhibition of breast cancer cell growth and invasiveness by dual silencing of HER-2 and VEGF. Mol Pharm. doi: 10.1021/mp9002514. [DOI] [PubMed] [Google Scholar]
- 32.Sakai K, et al. Expression of epidermal growth factor receptors on normal human gastric epithelia and gastric carcinomas. J Natl Cancer Inst. 1986;77(5):1047–52. [PubMed] [Google Scholar]
- 33.Yonemura Y, et al. Evaluation of immunoreactivity for erbB-2 protein as a marker of poor short term prognosis in gastric cancer. Cancer Res. 1991;51(3):1034–8. [PubMed] [Google Scholar]
- 34.Uchino S, et al. Overexpression of c-erbB-2 protein in gastric cancer. Its correlation with long-term survival of patients. Cancer. 1993;72(11):3179–84. doi: 10.1002/1097-0142(19931201)72:11<3179::aid-cncr2820721108>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 35.JLeón-Chong FL, Kang YK, Park SR, Bang YJ, Sawaki A, Van Cutsem E, Stoss O, Jordan BW, Feyereislova A. HER2 positivity in advanced gastric cancer is comparable to breast cancer. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings. 2007 [Google Scholar]
- 36.Kang YYB, Lordick F, Park S, Sawaki A, Chung H, Shen L, Xu JM, Leon-Chong J, Van Cutsem E. Incidence of gastric and gastro-esophageal cancer in the ToGA trial: Correlation with HER2 positivity. 2008 Gastrointestinal Cancers Symposium; 2008. [Google Scholar]
- 37.Van Cutsem E, YK, Chung H, Shen L, Sawaki A, Lordick F, Hill J, Lehle M, Feyereislova A, Bang Y. Efficacy results from the ToGA trial: A phase III study of trastuzumab added to standard chemotherapy (CT) in first-line human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (GC). 2009 ASCO Annual Meeting; 2009. [Google Scholar]
- 38.Petrelli NJ, et al. Clinical Cancer Advances 2009: major research advances in cancer treatment, prevention, and screening--a report from the American Society of Clinical Oncology. J Clin Oncol. 2009;27(35):6052–69. doi: 10.1200/JCO.2009.26.6171. [DOI] [PubMed] [Google Scholar]
- 39.Roukos DH. Targeting gastric cancer with trastuzumab: new clinical practice and innovative developments to overcome resistance. Ann Surg Oncol. 2010;17(1):14–7. doi: 10.1245/s10434-009-0766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bookman MA, et al. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol. 2003;21(2):283–90. doi: 10.1200/JCO.2003.10.104. [DOI] [PubMed] [Google Scholar]
- 41.Steffensen KD, et al. The prognostic importance of cyclooxygenase 2 and HER2 expression in epithelial ovarian cancer. Int J Gynecol Cancer. 2007;17(4):798–807. doi: 10.1111/j.1525-1438.2006.00855.x. [DOI] [PubMed] [Google Scholar]
- 42.Hellstrom I, et al. Overexpression of HER-2 in ovarian carcinomas. Cancer Res. 2001;61(6):2420–3. [PubMed] [Google Scholar]
- 43.Juhl H, et al. HER-2/neu is rate-limiting for ovarian cancer growth. Conditional depletion of HER-2/neu by ribozyme targeting. J Biol Chem. 1997;272(47):29482–6. doi: 10.1074/jbc.272.47.29482. [DOI] [PubMed] [Google Scholar]
- 44.Pietras RJ, et al. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene. 1994;9(7):1829–38. [PubMed] [Google Scholar]
- 45.Bartsch R, et al. HER-2-positive breast cancer: hope beyond trastuzumab. BioDrugs. 2007;21(2):69–77. doi: 10.2165/00063030-200721020-00001. [DOI] [PubMed] [Google Scholar]
- 46.Mullen P, et al. Sensitivity to pertuzumab (2C4) in ovarian cancer models: cross-talk with estrogen receptor signaling. Mol Cancer Ther. 2007;6(1):93–100. doi: 10.1158/1535-7163.MCT-06-0401. [DOI] [PubMed] [Google Scholar]
- 47.Signoretti S, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000;92(23):1918–25. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 48.Scher HI. HER2 in prostate cancer--a viable target or innocent bystander? J Natl Cancer Inst. 2000;92(23):1866–8. doi: 10.1093/jnci/92.23.1866. [DOI] [PubMed] [Google Scholar]
- 49.Ziada A, et al. The use of trastuzumab in the treatment of hormone refractory prostate cancer; phase II trial. Prostate. 2004;60(4):332–7. doi: 10.1002/pros.20065. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, et al. Inhibition of HER-2/neu kinase impairs androgen receptor recruitment to the androgen responsive enhancer. Cancer Res. 2005;65(8):3404–9. doi: 10.1158/0008-5472.CAN-04-4292. [DOI] [PubMed] [Google Scholar]
- 51.Jia L, et al. Androgen receptor activity at the prostate specific antigen locus: steroidal and non-steroidal mechanisms. Mol Cancer Res. 2003;1(5):385–92. [PubMed] [Google Scholar]
- 52.Craft N, et al. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5(3):280–5. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 53.Yeh S, et al. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci U S A. 1999;96(10):5458–63. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mellinghoff IK, et al. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6(5):517–27. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 55.Freeman MR. HER2/HER3 heterodimers in prostate cancer: Whither HER1/EGFR? Cancer Cell. 2004;6(5):427–8. doi: 10.1016/j.ccr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 56.Small EJ, et al. Docetaxel, estramustine, plus trastuzumab in patients with metastatic androgen-independent prostate cancer. Semin Oncol. 2001;28(4 Suppl 15):71–6. doi: 10.1016/s0093-7754(01)90159-9. [DOI] [PubMed] [Google Scholar]
- 57.Sastre-Garau X, et al. Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer. 1996;77(1):113–20. doi: 10.1002/(SICI)1097-0142(19960101)77:1<113::AID-CNCR19>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 58.Zafrani B, et al. High sensitivity and specificity of immunohistochemistry for the detection of hormone receptors in breast carcinoma: comparison with biochemical determination in a prospective study of 793 cases. Histopathology. 2000;37(6):536–45. doi: 10.1046/j.1365-2559.2000.01006.x. [DOI] [PubMed] [Google Scholar]
- 59.Johnston SR, et al. Changes in estrogen receptor, progesterone receptor, and pS2 expression in tamoxifen-resistant human breast cancer. Cancer Res. 1995;55(15):3331–8. [PubMed] [Google Scholar]
- 60.Oh AS, et al. Hyperactivation of MAPK induces loss of ERalpha expression in breast cancer cells. Mol Endocrinol. 2001;15(8):1344–59. doi: 10.1210/mend.15.8.0678. [DOI] [PubMed] [Google Scholar]
- 61.Holloway JN, Murthy S, El-Ashry D. A cytoplasmic substrate of mitogen-activated protein kinase is responsible for estrogen receptor-alpha down-regulation in breast cancer cells: the role of nuclear factor-kappaB. Mol Endocrinol. 2004;18(6):1396–410. doi: 10.1210/me.2004-0048. [DOI] [PubMed] [Google Scholar]
- 62.Munzone E, et al. Reverting estrogen-receptor-negative phenotype in HER-2-overexpressing advanced breast cancer patients exposed to trastuzumab plus chemotherapy. Breast Cancer Res. 2006;8(1):R4. doi: 10.1186/bcr1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parise C, MB, Bauer K, Caggiano V, Caggiano V. Variation among the ER, PR, and HER2 breast cancer subtypes in California. 2007 Breast Cancer Symposium; 2007. [Google Scholar]
- 64.Onitilo AA, et al. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7(1–2):4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–8. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 66.Kinoshita J, et al. Clinical significance of vascular endothelial growth factor-C (VEGF-C) in breast cancer. Breast Cancer Res Treat. 2001;66(2):159–64. doi: 10.1023/a:1010692132669. [DOI] [PubMed] [Google Scholar]
- 67.Manders P, et al. Vascular endothelial growth factor is associated with the efficacy of endocrine therapy in patients with advanced breast carcinoma. Cancer. 2003;98(10):2125–32. doi: 10.1002/cncr.11764. [DOI] [PubMed] [Google Scholar]
- 68.Lissoni P, et al. Changes in circulating VEGF levels in relation to clinical response during chemotherapy for metastatic cancer. Int J Biol Markers. 2003;18(2):152–5. doi: 10.1177/172460080301800209. [DOI] [PubMed] [Google Scholar]
- 69.Davidoff AM, Nathwani AC. Antiangiogenic gene therapy for cancer treatment. Curr Hematol Rep. 2004;3(4):267–73. [PubMed] [Google Scholar]
- 70.Sledge GW, Rugo HS, Burstein HJ. The role of angiogenesis inhibition in the treatment of breast cancer. Clin Adv Hematol Oncol. 2006;4 Suppl 21(10):1–12. [PubMed] [Google Scholar]
- 71.Zelnak AB, O’Regan RM. Targeting angiogenesis in advanced breast cancer. BioDrugs. 2007;21(4):209–14. doi: 10.2165/00063030-200721040-00001. [DOI] [PubMed] [Google Scholar]
- 72.Im SA, et al. Inhibition of breast cancer growth in vivo by antiangiogenesis gene therapy with adenovirus-mediated antisense-VEGF. Br J Cancer. 2001;84(9):1252–7. doi: 10.1054/bjoc.2000.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Linderholm B, et al. Overexpression of c-erbB-2 is related to a higher expression of vascular endothelial growth factor (VEGF) and constitutes an independent prognostic factor in primary node-positive breast cancer after adjuvant systemic treatment. Eur J Cancer. 2004;40(1):33–42. doi: 10.1016/s0959-8049(03)00673-7. [DOI] [PubMed] [Google Scholar]
- 74.Finkenzeller G, et al. Activated Neu/ErbB-2 induces expression of the vascular endothelial growth factor gene by functional activation of the transcription factor Sp 1. Angiogenesis. 2004;7(1):59–68. doi: 10.1023/B:AGEN.0000037332.66411.f0. [DOI] [PubMed] [Google Scholar]
- 75.Konecny GE, et al. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res. 2004;10(5):1706–16. doi: 10.1158/1078-0432.ccr-0951-3. [DOI] [PubMed] [Google Scholar]
- 76.Pegram MD, Reese DM. Combined biological therapy of breast cancer using monoclonal antibodies directed against HER2/neu protein and vascular endothelial growth factor. Semin Oncol. 2002;29(3 Suppl 11):29–37. doi: 10.1053/sonc.2002.34053. [DOI] [PubMed] [Google Scholar]
- 77.Pegram M, et al. Phase II combined biological therapy targeting the HER2 proto-oncogene and the vascular endothelial growth factor using trastuzumab (T) and bevacizumab (B) as first line treatment of HER2-amplified breast cancer. Breast Cancer Res Treat. 2006;100(Supplement 1):S28–29. [Google Scholar]
- 78.Li YM, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6(5):459–69. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 79.Liotta LA. An attractive force in metastasis. Nature. 2001;410(6824):24–5. doi: 10.1038/35065180. [DOI] [PubMed] [Google Scholar]
- 80.Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 81.Chinni SR, et al. CXCL12/CXCR4 transactivates HER2 in lipid rafts of prostate cancer cells and promotes growth of metastatic deposits in bone. Mol Cancer Res. 2008;6(3):446–57. doi: 10.1158/1541-7786.MCR-07-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang Z, et al. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65(3):967–71. [PMC free article] [PubMed] [Google Scholar]
- 83.Lapteva N, et al. CXCR4 knockdown by small interfering RNA abrogates breast tumor growth in vivo. Cancer Gene Ther. 2005;12(1):84–9. doi: 10.1038/sj.cgt.7700770. [DOI] [PubMed] [Google Scholar]
- 84.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65(23):3756–88. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wong AS, Gumbiner BM. Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin. J Cell Biol. 2003;161(6):1191–203. doi: 10.1083/jcb.200212033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5(6):R217–22. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Margulis A, et al. E-cadherin suppression accelerates squamous cell carcinoma progression in three-dimensional, human tissue constructs. Cancer Res. 2005;65(5):1783–91. doi: 10.1158/0008-5472.CAN-04-3399. [DOI] [PubMed] [Google Scholar]
- 88.Lim SC, Lee MS. Significance of E-cadherin/beta-catenin complex and cyclin D1 in breast cancer. Oncol Rep. 2002;9(5):915–28. [PubMed] [Google Scholar]
- 89.Schroeder JA, et al. ErbB-beta-catenin complexes are associated with human infiltrating ductal breast and murine mammary tumor virus (MMTV)-Wnt-1 and MMTV-c-Neu transgenic carcinomas. J Biol Chem. 2002;277(25):22692–8. doi: 10.1074/jbc.M201975200. [DOI] [PubMed] [Google Scholar]
- 90.Shibata T, et al. Dominant negative inhibition of the association between beta-catenin and c-erbB-2 by N-terminally deleted beta-catenin suppresses the invasion and metastasis of cancer cells. Oncogene. 1996;13(5):883–9. [PubMed] [Google Scholar]
- 91.Niehans GA, et al. Stability of HER-2/neu expression over time and at multiple metastatic sites. J Natl Cancer Inst. 1993;85(15):1230–5. doi: 10.1093/jnci/85.15.1230. [DOI] [PubMed] [Google Scholar]
- 92.Franklin MC, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5(4):317–28. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 93.Agus DB, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23(11):2534–43. doi: 10.1200/JCO.2005.03.184. [DOI] [PubMed] [Google Scholar]
- 94.Nahta R, et al. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3(5):269–80. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 95.Cameron DA, Stein S. Drug Insight: intracellular inhibitors of HER2--clinical development of lapatinib in breast cancer. Nat Clin Pract Oncol. 2008;5(9):512–20. doi: 10.1038/ncponc1156. [DOI] [PubMed] [Google Scholar]
- 96.Izumi Y, et al. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416(6878):279–80. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 97.Klos KS, et al. Combined trastuzumab and paclitaxel treatment better inhibits ErbB-2-mediated angiogenesis in breast carcinoma through a more effective inhibition of Akt than either treatment alone. Cancer. 2003;98(7):1377–85. doi: 10.1002/cncr.11656. [DOI] [PubMed] [Google Scholar]
- 98.Piccart-Gebhart MJ, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 99.Romond EH, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 100.Agus DB, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127–37. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 101.Scheuer W, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69(24):9330–6. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 102.Brockhoff G, et al. Differential impact of Cetuximab, Pertuzumab and Trastuzumab on BT474 and SK-BR-3 breast cancer cell proliferation. Cell Prolif. 2007;40(4):488–507. doi: 10.1111/j.1365-2184.2007.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64(7):2343–6. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 104.Kristjansdottir K, Dizon D. HER-dimerization inhibitors: evaluating pertuzumab in women’s cancers. Expert Opin Biol Ther. 2009 doi: 10.1517/14712590903514090. [DOI] [PubMed] [Google Scholar]
- 105.Clynes RA, et al. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6(4):443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 106.Birgit Bossenmaier MH, Koll Hans, Fiebig Heinz Herbert, Akita Robert W, Sliwkowski Mark X, Müller Hans-Joachim. Presence of HER2/HER3 heterodimers predicts antitumor effects of pertuzumab (Omnitarg) in different human xenograft models. Proc Am Assoc Cancer Res. 2004 [Google Scholar]
- 107.Reid A, et al. Dual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu) Eur J Cancer. 2007;43(3):481–9. doi: 10.1016/j.ejca.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 108.Wood ER, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64(18):6652–9. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 109.Nahta R, Esteva FJ. Trastuzumab: triumphs and tribulations. Oncogene. 2007;26(25):3637–43. doi: 10.1038/sj.onc.1210379. [DOI] [PubMed] [Google Scholar]
- 110.Rusnak DW, et al. The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: potential therapy for cancer. Cancer Res. 2001;61(19):7196–203. [PubMed] [Google Scholar]
- 111.Xia W, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21(41):6255–63. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 112.Xia W, et al. Combining lapatinib ( GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene. 2005;24(41):6213–21. doi: 10.1038/sj.onc.1208774. [DOI] [PubMed] [Google Scholar]
- 113.Konecny GE, et al. Activity of the dual kinase inhibitor lapatinib ( GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66(3):1630–9. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 114.Roh H, et al. Synergistic antitumor effects of HER2/neu antisense oligodeoxynucleotides and conventional chemotherapeutic agents. Surgery. 1999;126(2):413–21. [PubMed] [Google Scholar]
- 115.Roh H, et al. Antisense oligonucleotides specific for the HER2/neu oncogene inhibit the growth of human breast carcinoma cells that overexpress HER2/neu. J Surg Res. 1998;77(1):85–90. doi: 10.1006/jsre.1998.5353. [DOI] [PubMed] [Google Scholar]
- 116.Cheng K, Qin B. RNA Interference for Cancer Therapy. In: Lu Y, Mahato RI, editors. Pharmaceutical Perspectives of Cancer Therapeutics. AAPS-Springer publishing program; 2009. [Google Scholar]
- 117.Cheng K, Mahato RI. siRNA delivery and targeting. Mol Pharm. 2009;6(3):649–50. doi: 10.1021/mp900094j. [DOI] [PubMed] [Google Scholar]
- 118.Choudhury A, et al. Small interfering RNA (siRNA) inhibits the expression of the Her2/neu gene, upregulates HLA class I and induces apoptosis of Her2/neu positive tumor cell lines. Int J Cancer. 2004;108(1):71–7. doi: 10.1002/ijc.11497. [DOI] [PubMed] [Google Scholar]
- 119.Lewis Phillips GD, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–90. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 120.Park JW, et al. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8(4):1172–81. [PubMed] [Google Scholar]
- 121.Siwak DR, Tari AM, Lopez-Berestein G. The potential of drug-carrying immunoliposomes as anticancer agents. Commentary re: J. W. Park et al., Anti-HER2 immunoliposomes: enhanced efficacy due to targeted delivery. Clin. Cancer Res., 8: 1172–1181, 2002. Clin Cancer Res. 2002;8(4):955–6. [PubMed] [Google Scholar]
- 122.Goldstein D, et al. Anti-HER2 cationic immunoemulsion as a potential targeted drug delivery system for the treatment of prostate cancer. Cancer Res. 2007;67(1):269–75. doi: 10.1158/0008-5472.CAN-06-2731. [DOI] [PubMed] [Google Scholar]
- 123.Lee CH, et al. Enhanced gene delivery to HER-2-overexpressing breast cancer cells by modified immunolipoplexes conjugated with the anti-HER-2 antibody. J Biomed Sci. 2003;10(3):337–44. doi: 10.1007/BF02256453. [DOI] [PubMed] [Google Scholar]
- 124.Hayes ME, et al. Assembly of nucleic acid-lipid nanoparticles from aqueous-organic monophases. Biochim Biophys Acta. 2006;1758(4):429–42. doi: 10.1016/j.bbamem.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 125.Hayes ME, et al. Genospheres: self-assembling nucleic acid-lipid nanoparticles suitable for targeted gene delivery. Gene Ther. 2006;13(7):646–51. doi: 10.1038/sj.gt.3302699. [DOI] [PubMed] [Google Scholar]
- 126.Hayes ME, et al. Increased target specificity of anti-HER2 genospheres by modification of surface charge and degree of PEGylation. Mol Pharm. 2006;3(6):726–36. doi: 10.1021/mp060040v. [DOI] [PubMed] [Google Scholar]
- 127.Burris HA, SV, Rugo HS, Vogel C, Borson R, Tan-Chiu E, Birkner M, Holden SN, Girish S, Klencke B, O’Shaughnessy J. A Phase II Study of Trastuzumab-DM1 (T-DM1), A HER2 Antibody-Drug Conjugate, in Patients with HER2-Positive Metastatic Breast Cancer. ASCO Breast Cancer Symposium; Washington, DC. 2008. [Google Scholar]
- 128.Beeram M, HAB, Modi S, Birkner M, Girish S, Tibbitts J, Holden SN, Lutzker SG, Krop IE. A phase I study of trastuzumab-DM1 (T-DM1), a first-in-class HER2 antibody-drug conjugate (ADC), in patients (pts) with advanced HER2+ breast cancer (BC). 2007 ASCO Annual Meeting Proceedings; 2007. [Google Scholar]
- 129.Mandler R, et al. Herceptin-geldanamycin immunoconjugates: pharmacokinetics, biodistribution, and enhanced antitumor activity. Cancer Res. 2004;64(4):1460–7. doi: 10.1158/0008-5472.can-03-2485. [DOI] [PubMed] [Google Scholar]
- 130.Mehta RR, et al. Differentiation of human breast carcinoma cells by a novel vitamin D analog: 1alpha-hydroxyvitamin D5. Int J Oncol. 2000;16(1):65–73. doi: 10.3892/ijo.16.1.65. [DOI] [PubMed] [Google Scholar]
- 131.Lazzaro G, et al. Induction of differentiation by 1alpha-hydroxyvitamin D(5) in T47D human breast cancer cells and its interaction with vitamin D receptors. Eur J Cancer. 2000;36(6):780–6. doi: 10.1016/s0959-8049(00)00016-2. [DOI] [PubMed] [Google Scholar]
- 132.Punj V, Graves JM, Mehta RR. Effect of vitamin D analog (1alpha hydroxy D5) immunoconjugated to Her-2 antibody on breast cancer. Int J Cancer. 2004;108(6):922–9. doi: 10.1002/ijc.11590. [DOI] [PubMed] [Google Scholar]
- 133.King HD, et al. Monoclonal antibody conjugates of doxorubicin prepared with branched peptide linkers: inhibition of aggregation by methoxytriethyleneglycol chains. J Med Chem. 2002;45(19):4336–43. doi: 10.1021/jm020149g. [DOI] [PubMed] [Google Scholar]
- 134.Zhao J, et al. Secreted antibody/granzyme B fusion protein stimulates selective killing of HER2-overexpressing tumor cells. J Biol Chem. 2004;279(20):21343–8. doi: 10.1074/jbc.M312648200. [DOI] [PubMed] [Google Scholar]
- 135.Jia LT, et al. Specific tumoricidal activity of a secreted proapoptotic protein consisting of HER2 antibody and constitutively active caspase-3. Cancer Res. 2003;63(12):3257–62. [PubMed] [Google Scholar]
- 136.Cho HM, et al. Enhanced inhibition of murine tumor and human breast tumor xenografts using targeted delivery of an antibody-endostatin fusion protein. Mol Cancer Ther. 2005;4(6):956–67. doi: 10.1158/1535-7163.MCT-04-0321. [DOI] [PubMed] [Google Scholar]
- 137.Xia WY, et al. Expression of PEA3 and lack of correlation between PEA3 and HER-2/neu expression in breast cancer. Breast Cancer Res Treat. 2006;98(3):295–301. doi: 10.1007/s10549-006-9162-7. [DOI] [PubMed] [Google Scholar]
- 138.Helguera G, et al. Long-term immunity elicited by antibody-cytokine fusion proteins protects against sequential challenge with murine mammary and colon malignancies. Cancer Immunol Immunother. 2007;56(9):1507–12. doi: 10.1007/s00262-007-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Song E, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23(6):709–17. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 140.Li X, et al. Single-chain antibody-mediated gene delivery into ErbB2-positive human breast cancer cells. Cancer Gene Ther. 2001;8(8):555–65. doi: 10.1038/sj.cgt.7700337. [DOI] [PubMed] [Google Scholar]
- 141.Alexis F, et al. HER-2-targeted nanoparticle-affibody bioconjugates for cancer therapy. ChemMedChem. 2008;3(12):1839–43. doi: 10.1002/cmdc.200800122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wikman M, et al. Selection and characterization of HER2/neu-binding affibody ligands. Protein Eng Des Sel. 2004;17(5):455–62. doi: 10.1093/protein/gzh053. [DOI] [PubMed] [Google Scholar]
- 143.Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79(3):703–61. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- 144.Orlova A, et al. Synthetic affibody molecules: a novel class of affinity ligands for molecular imaging of HER2-expressing malignant tumors. Cancer Res. 2007;67(5):2178–86. doi: 10.1158/0008-5472.CAN-06-2887. [DOI] [PubMed] [Google Scholar]
- 145.Steffen AC, et al. In vitro characterization of a bivalent anti-HER-2 affibody with potential for radionuclide-based diagnostics. Cancer Biother Radiopharm. 2005;20(3):239–48. doi: 10.1089/cbr.2005.20.239. [DOI] [PubMed] [Google Scholar]
- 146.Tolmachev V, et al. Radionuclide therapy of HER2-positive microxenografts using a 177Lu-labeled HER2-specific Affibody molecule. Cancer Res. 2007;67(6):2773–82. doi: 10.1158/0008-5472.CAN-06-1630. [DOI] [PubMed] [Google Scholar]
- 147.Lee SB, et al. Affibody molecules for in vivo characterization of HER2-positive tumors by near-infrared imaging. Clin Cancer Res. 2008;14(12):3840–9. doi: 10.1158/1078-0432.CCR-07-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Belousova N, et al. Modification of adenovirus capsid with a designed protein ligand yields a gene vector targeted to a major molecular marker of cancer. J Virol. 2008;82(2):630–7. doi: 10.1128/JVI.01896-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park BW, et al. Rationally designed anti-HER2/neu peptide mimetic disables P185HER2/neu tyrosine kinases in vitro and in vivo. Nat Biotechnol. 2000;18(2):194–8. doi: 10.1038/72651. [DOI] [PubMed] [Google Scholar]
- 150.Guillemard V, et al. HER2-mediated internalization of a targeted prodrug cytotoxic conjugate is dependent on the valency of the targeting ligand. DNA Cell Biol. 2005;24(6):350–8. doi: 10.1089/dna.2005.24.351. [DOI] [PubMed] [Google Scholar]
- 151.Fantin VR, et al. A bifunctional targeted peptide that blocks HER-2 tyrosine kinase and disables mitochondrial function in HER-2-positive carcinoma cells. Cancer Res. 2005;65(15):6891–900. doi: 10.1158/0008-5472.CAN-05-0395. [DOI] [PubMed] [Google Scholar]
- 152.Afshar S, Asai T, Morrison SL. Humanized ADEPT comprised of an engineered human purine nucleoside phosphorylase and a tumor targeting peptide for treatment of cancer. Mol Cancer Ther. 2009;8(1):185–93. doi: 10.1158/1535-7163.MCT-08-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tan M, et al. Selective inhibition of ErbB2-overexpressing breast cancer in vivo by a novel TAT-based ErbB2-targeting signal transducers and activators of transcription 3-blocking peptide. Cancer Res. 2006;66(7):3764–72. doi: 10.1158/0008-5472.CAN-05-2747. [DOI] [PubMed] [Google Scholar]
- 154.Shadidi M, Sioud M. Identification of novel carrier peptides for the specific delivery of therapeutics into cancer cells. Faseb J. 2003;17(2):256–8. doi: 10.1096/fj.02-0280fje. [DOI] [PubMed] [Google Scholar]
- 155.Newton J, Deutscher SL. Phage peptide display. Handb Exp Pharmacol. 2008;185(Pt 2):145–63. doi: 10.1007/978-3-540-77496-9_7. [DOI] [PubMed] [Google Scholar]
- 156.Wang XF, et al. A peptide conjugate of vitamin E succinate targets breast cancer cells with high ErbB2 expression. Cancer Res. 2007;67(7):3337–44. doi: 10.1158/0008-5472.CAN-06-2480. [DOI] [PubMed] [Google Scholar]
- 157.Nakajima H, et al. Development of HER2-antagonistic peptides as novel anti-breast cancer drugs by in silico methods. Breast Cancer. 2008;15(1):65–72. doi: 10.1007/s12282-007-0018-8. [DOI] [PubMed] [Google Scholar]