Abstract
In this review, we describe water balance requirements of blood-feeding arthropods, particularly contrasting dehydration tolerance during the unfed, off-host state and the challenges of excess water that accompany receipt of the bloodmeal. Most basic water balance characteristics during the off-host stage are applicable to other terrestrial arthropods, as well. A well-coordinated suite of responses enable arthropods to conserve water resources, enhance their desiccation tolerance, and increase their water supplies by employing a diverse array of molecular, structural and behavioral responses. Water loss rates during the off-host phase are particularly useful for generating a scheme to classify vectors according to their habitat requirements for water, thus providing a convenient tool with potential predictive power for defining suitable current and future vector habitats. Blood feeding elicits an entirely different set of challenges as the vector responds to overhydration by quickly increasing its rate of cuticular water loss and elevating the rate of diuresis to void excess water and condense the bloodmeal. Immature stages that feed on blood normally have a net increase in water content at the end of a blood-feeding cycle, but in adults the water content reverts to the prefeeding level when the cycle is completed. Common themes are evident in diverse arthropods that feed on blood, particularly the physiological mechanisms used to respond to the sudden influx of water as well as the mechanisms used to counter water shortfalls that are encountered during the nonfeeding, off-host state.
Keywords: water balance, dehydration resistance, blood feeding, diuresis, disease vectors
1. Introduction
A blood-feeding lifestyle imposes an interesting set of water balance challenges for arthropods. Though the blood meal is a rich nutrient source, the huge amount of water it contains demands special mechanisms for quick extraction and expulsion of excess water. And, contrasting mechanisms for conserving water are required during the long periods of off-host life. The life of a blood feeder is a continual cycle of overhydration during blood feeding followed by the suppression of dehydration while off the host (Wharton, 1985; Hadley, 1994; Ribeiro, 1996). This implies that throughout its life a blood feeder goes through multiple cycles switching from water conservation to elimination of unwanted water.
Many water balance attributes of the off-host phase of the blood feeder’s life cycle are common to all terrestrial arthropods, and excellent earlier reviews by Edney (1977), Wharton (1985) and Hadley (1994) provide good summaries of the basic principles of water balance that are germane to the off-host phase of the life cycle. Several excellent reviews also discuss various aspects of the rapid processing of water removal during blood feeding: transport mechanisms in the gut and Malpighian tubules (Beyenbach 2003; Lehane, 2005), and the hormonal regulation of diuresis (Coast et al., 2002).
In this review we provide an update of off-host water balance, the changes that occur during blood feeding, and the responses observed when the arthropod again reverts to the non-feeding state. This review focuses only on the hematophagous stages of blood-feeding arthropods (e.g. adult mosquitoes, both nymphal and adult stages of ticks), not the immature stages that do not feed on blood (e.g. mosquito larvae). We suggest that the off-host water balance traits impact the distribution of hematophagous arthropods and offer predictive value for delineating future population expansion.
2. Blood-feeding arthropods
Blood feeding has evolved in diverse groups of arthropods (Table 1). The vast taxonomic distances among blood feeders and the different methods they employ to obtain a bloodmeal suggest that blood feeding evolved numerous times among the arthropods (Waage, 1979; Klowden, 1996). More than 14,000 species representing 400 genera utilize blood from vertebrate hosts (Graça-Souza et al., 2005). Blood feeding possibly evolved by three distinct routes (Klowden, 1996). In the first scenario arthropods living in close association with vertebrates fed on exfoliated skin or other host by-products (Kim, 1985; Lehane, 1991; Klowden, 1996). As the association progressed these arthropods began to utilize nutrient-rich blood due to the development of chewing or piercing mouthparts capable of penetrating the vertebrate epidermis. Those feeding on protein-rich blood were then favored due to increased egg production (Lehane, 1991). This difference is apparent among lice; blood-feeding Anoplura produce significantly more eggs than skin-feeding Mallophaga (Marshall, 1981). An alternative possibility is that arthropods capable of piercing plants switched to blood feeding to obtain a meal richer in protein (Waage, 1979), a scenario exemplified by the vampire moth, Calpe eustrigata, a noctuid that has a modified proboscis capable of penetrating fruit rinds as well as the vertebrate skin. A third possibility is that entomophagous insects, having mouthparts capable of penetrating the cuticle of arthropods, could evolve into forms capable of blood ingestion (Lehane, 1991), as exemplified by Lyctocoris campestris, a hemipteran that resides in bird nests and primarily feeds on other insects but will also utilize blood from nearby birds (Stys and Daniel, 1957).
Table 1.
List of representative blood-feeding arthropods
| Class |
Order |
Family |
Common name* |
|---|---|---|---|
| Arachnida | Acarina | Dermanyssidae Halarachnidae Macronyssidae Rhinonyssidae Ixodidae Argasidae |
Poultry mite Dog nasal mite Rat mite Lung mite Hard tick Soft tick |
| Insecta | Hemiptera | Cimicidae Polyctenidae Reduviidae |
Bed bugs Bat bugs Kissing bugs |
| Phthiraptera† | Philopteridae Haematopididae Hoplopleuridae Linognathidae Pediculidae Polyplacidae Pthiridae |
Bird lice Ungulate lice Armored lice Pale lice Body lice Spiny rat lice Pubic lice |
|
| Lepidoptera | Noctuidae | Vampire moth | |
| Siphonaptera† | Ceratophyllidae Ctenophthalmidae Leptopsyllidae Pulicidae |
Chicken flea Rodent flea Mouse flea Cat and dog flea |
|
| Diptera | Psychodidae Ceratopogonidae Culicidae Simuliidae Rhagonidae Tabanidae Muscidae Glossinidae Nycteribiidae Hippoboscidae Streblidae |
Sand fly Biting midge Mosquito Black fly Snipe fly Horse fly Stable fly Tsetse fly Bat fly Louse fly Bat fly |
denotes the common name for the entire group or the most prevalent groups within the family
indicates that there are more families that feed on blood within the order but only the most common families are listed
Blood feeding is not a trivial task. Many inhibitory factors need to be overcome before an arthropod vector can utilize blood. First, it must locate a host. To accomplish this, the arthropod either actively quests for a host or employs an ambush strategy, waiting in a particular area for a host to appear (Bowen, 1991; Klowden, 1996). In both strategies, host-based chemical cues are utilized. Those that actively search move toward the host, prompted by chemical cues such as host volatiles and carbon dioxide, as well as visual cues in some cases (Gillies, 1980; Lehane, 2005). Those that wait passively usually reside in areas frequented by their hosts, often indicated by the presence of host feces, urine or other chemical cues (Klowden, 1996; Benoit et al., 2008). Host seeking behavior is terminated by the acquisition of a bloodmeal, a behavioral switch triggered by abdominal distension and hormones released by the ovaries during egg maturation (Klowden, 1990; Brown et al., 1994). After penetrating the vertebrate integument, the arthropod must suppress the host immune response, increase vasodilation, prevent the accumulation of toxic compounds, and suppress platelet aggregation and general coagulation in order to successfully blood feed (Ribeiro, 1995; Ribeiro, 1996; Stark and James, 1996; Ribeiro and Francischetti, 2003; Graça-Souza et al., 2005). For insects that feed only on blood, such as bed bugs and tsetse flies, microbial symbionts are needed to synthesize required micronutrients, such as B vitamins and other factors (Romoser, 1996; Ribeiro, 1996; Beard et al., 2002; Akman et al., 2002; Aksoy and Rio, 2005). Additionally, a huge blood meal represents an enormous mass change that not only makes individuals vulnerable to predation but also elicits a significant osmotic stress due to the amount of water and ions present within the bloodmeal (Adams, 1999; Beyenbach, 2003). Thus, utilization of vertebrate blood is a significant feat, from locating the host to dealing with stress induced by the blood meal, but the substantial nutrients available for development and egg production make blood feeding highly rewarding (Beyenbach and Petzel, 1987; Lehane, 2005).
3. Basic concepts of water balance
The maintenance of water balance can be expressed as the difference between water gain and water loss (Wharton, 1985). Routes of water loss include cuticular loss, respiratory loss, defecation, and secretion (Wharton, 1985; Hadley, 1994). Gain is accomplished by ingestion, water vapor absorption and the release of water during metabolism (Hadley, 1994). For an insect to survive and reproduce water balance has to be maintained for extended periods.
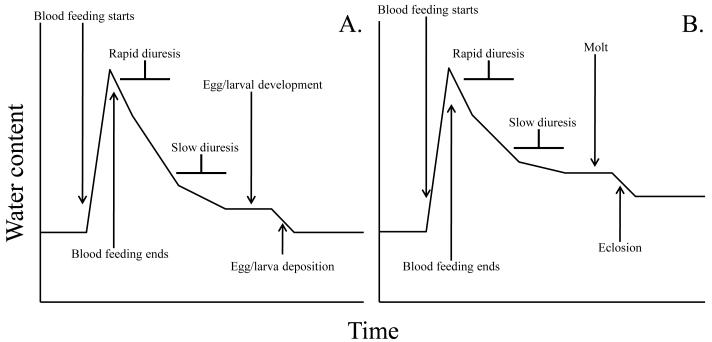
In relation to water balance, there are two distinct types of blood feeding. The first includes adult feeding patterns noted in most dipterans, lice, and bugs. Individuals become engorged with blood and return to their normal hydration state within a distinct period, usually after diuresis, blood digestion and subsequent egg production (Fig. 1a). Thus, water content and insect size are similar before and after blood feeding. Ixodid ticks represent an exception since the adults die after mating and egg laying, thus failing to return to the pre-feeding state (Sonenshine, 1991). The second situation is when blood feeding prompts advancement to the next stage (Fig. 1b). In this case, blood feeding triggers a molt rather than egg production, thus requiring a completely different set of water balance parameters. Additionally, once the next developmental stage is attained, individuals are more resistant to dehydration due to their larger size (Benoit, 2010).
Fig. 1.
The dynamics of water content during a blood feeding cycle in (A) adults and (B) immature stages. Note that the adult feeding cycle typically does not result in a net increase in water content, while a feeding cycle in immature stages (nymphs and larvae) results in a net increase in water content.
4. Off-host water balance maintenance
Between bloodmeals, the challenge is to maintain sufficient body water. In this regard, blood-feeding arthropods can be classified into two distinct groups: those that have the ability to increase their water pool when off-host and those that are unable to do so. Arthropod vectors that do not drink free water include many species of bugs, fleas and some ticks (Hadley, 1994; Thiemann et al., 2003; Benoit et al., 2007a,b). The group that takes up water while off-host can be further subdivided into those that drink free water, e.g. mosquitoes, and those that condense water directly from water vapor present in air, e.g. ticks. Vectors with the ability to increase their water pool when off-host have a tendency to be less resistant to dehydration when compared to those that rely solely on water from their bloodmeal. In this section, we discuss how blood-feeding arthropods, when off-host, reduce water loss, increase their water pool, and prevent dehydration stress.
4.1. Reduction of water loss
To retain water more efficiently, arthropods utilize mechanisms to reduce water loss through the cuticle and during respiration, as well as increase absorption from the alimentary canal. Water lost through the cuticle accounts for a significant portion of the water lost by insects (Hadley, 1994; Chown and Nicolson, 2004). Commonly, individuals that frequent moist habitats have highly permeable cuticles, and those that reside in more xeric regions have cuticles more resistant to water flux (Edney, 1977; Hadley, 1994; Gibbs, 1998; Bradley et al., 1999; Gibbs and Matzkin, 2001). Increases in sclerotization of the cuticle increase the density of the procuticle, thus improving water retention (Benoit et al., 2005), but the cuticular lipids provide the most significant water barrier. Cuticular lipids located on the outer surface of the epicuticle, particularly the wax layer, play a major role in reducing water loss (Blomquist et al., 1987; Hadley, 1981; Lockey, 1988; de Renobales et al., 1991; Hadley, 1994; Gibbs, 1998; 2002). The composition of cuticular lipids varies significantly among arthropods, but the dominant constituents are hydrocarbons (Blomquist et al., 1987; Hadley, 1994; Gibbs, 1998; 2002), and disruption of these lipids increases water loss rates (Noble-Nesbitt 1991, Hadley 1994). Both the quantity and quality of cutcular lipids can impact water loss rate. Increases in the amount of cuticular hydrocarbons result in lower cuticular water loss rates (Hood and Tschinkel, 1990; Yoder and Denlinger, 1991; Hadley, 1994; Benoit and Denlinger, 2007), and shifts in hydrocarbon composition toward more long-chained, saturated lipids with few methyl side chains result in a more effective water barrier (Hadley, 1994; Gibbs, 1998).
Along with water loss through the cuticle, respiration represents an important route of water loss, accounting for 5-20% of the total water loss for most insects’ (Hadley, 1994; Chown, 2002). Insects residing in arid regions usually lose a higher proportion of water through respiration, up to 70%, mainly a consequence of having a highly water-proofed cuticle (Hadley, 1994; Chown, 2002). During respiration, water is lost rapidly when the spiracles are open due to the steep humidity gradient between the tracheal system and the environment (Hadley, 1994). The most prominent mechanism to reduce respiratory water loss is the most simple, closing the spiracles. By closing or even partially blocking the spiracles, water loss, particularly at low relative humidities, is reduced (Bursell, 1957; Hadley, 1994; Chown, 2002). Discontinuous gas exchange (DSC) can also reduce water loss through the spiracles by limiting gaseous diffusion to short periods when carbon dioxide accumulates at a high level (Lighton et al., 1993; Hadley, 1994). Recent research has questioned the ability of DSC to reduce water loss, indicating that more studies are needed to determine exactly how DSC relates to water loss suppression (Sláma, 1999; Chown, 2002), but clearly a lack of spiracle control results in higher water loss rates (Bursell, 1957; Lighton, 1996; Sláma, 1999; Chown, 2002).
The Malphigian tubules and hindgut are the major sites that regulate salt and water levels, as reviewed by Bradley (1985) and Chown and Nicolson (2004). Briefly, water, along with organic molecules, nitrogenous waste and ions, are absorbed from the hemolymph into the upper portion of the Malphigian tubules, and the fluid is then diluted in the lower water-impermeable portion of the Malphigian tubules by the absorption of KCl. The hindgut, particularly the rectum, acts as the primary site for the absorption of water and select solutes from waste materials. Secretion and absorption by the Malphigian tubules and hindgut absorption are regulated by neuropeptide hormones: diuretic hormones elicit secretion of water into the alimentary canal, thus increasing the net water loss, while anti-diuretic hormones act in the opposite direction to retain water. The major neuropeptides involved in water balance are summarized in papers by Coast et al. (2002), Riehle et al. (2002), Gäde (2004) and Coast (2006; 2007), and include calcitonin-like peptides, corticotropin-releasing factor related peptides (CRF-related), insects kinins, and cardioacceleratory peptides, which all function as diuretic hormones, and chloride transport-stimulating hormone (CTSH) and an ion-transport process peptide (ITP) that function as anti-diuretics (Coast, 2006), as discussed in more detail later in the review.
Uric acid is the dominant nitrogenous waste product generated by terrestrial arthropods, but guanine and other closely-related nitrogenous products are used by ticks and spiders (Hadley, 1994; Benoit et al., 2008). Why use uric acid rather than urea or ammonia? Ammonia is toxic and soluble, thus requiring insects to quickly expel this waste product with large quantities of water. Utilization of ammonia as a metabolic end product is thus usually restricted to aquatic insects. Although urea is significantly less toxic than ammonia, it still has to be eliminated in solution. Uric acid is the least toxic of the potential waste products for insects, and due to its low solubility, excretion of a nearly dry waste product is possible. The low toxicity of uric acid also means that it can be accumulated within the body (storage excretion), a situation that completely prevents the loss of water by defecation.
Behaviorally, blood-feeding arthropods can reduce water loss by multiple methods. Aggregation is a frequent behavioral response associated with reducing water loss, particularly during dormant periods (Benoit, 2010). Formation of an aggregation increases the local relative humidity, suppressing water loss for members of the group (Yoder et al., 1993; Benoit et al. 2007a,b). For example, bed bugs, Cimex lectularius, form aggregations in protective harborages near their hosts; as group size increases, metabolic rate drops and water conservation is enhanced (Benoit et al., 2007a,b). The benefits gained are likely the basis for the frequently observed clusters of off-host blood-feeding arthropods. Another factor that can reduce water loss is the restriction of host seeking to times of the day or to seasons when relative humidities are high, as noted for kissing bugs (Barrozo et al., 2003), mosquitoes (Kessler and Guerin, 2008), and ticks (Crooks et al., 2006). When they are not host seeking, vectors commonly reside in cool, moist habitats (Kessler and Guerin, 2008). Thus, blood-feeding arthropods susceptible to dehydration will most likely seek humid microenvironments, possibly form aggregations, and feed when the relative humidity is high, such as at night or after a rain storm, reducing the likelihood of dehydration. Species more xerically-adapted are likely to be found in drier habitats, but even these species may aggregate to reduce dehydration.
4.2. Increasing the water pool
Ingestion of water is the major mechanism used to replenish water supplies (Hadley, 1994). Many insects simply drink free standing water to rehydrate, and the amount of water consumed is regulated by hemolymph volume (Chown and Nicolson, 2004). In the absence of blood feeding, mosquitoes primarily increase their water pool by drinking free water or nectar or by obtaining fluid from plant tissue, while bed bugs, which are indeed capable of drinking, do not normally do so and only obtain water from blood (Benoit et al., 2007b). Metabolism of food resources also generates water, a source that is immediately transferred to the body’s water pool (Edney, 1977; Hadley, 1994). But, the contribution of metabolic water is rather small for most insects and is probably significant only for species with extremely low water loss rates and for those that engage in extended flight. Quite a few arthropods, including acarines, lice and flea larvae, are able to absorb water from subsaturated air (< 99% RH). A relatively complete list of such arthropods was presented by Edney (1977), with later examples added by Hadley (1994). The mouth and anus are sites most commonly used for active water vapor absorption, but water is also sometimes absorbed directly through the cuticle (O’Donnell and Machin, 1988; Hadley, 1994; Bayley and Holmstrup, 1999). Water vapor uptake is most commonly achieved by exploiting hyperosmotic or hygroscopic secretions from the mouth or anus, which in turn capture water vapor from the air that is then internalized (Knülle, 1984; Hadley, 1994). Oral mechanisms of water vapor absorption are particularly well-known for ticks: larvae absorb water from the lowest relative humidities (80-85% RH), followed by nymphs (85-90% RH), and lastly adults (90-95%; Needham and Teel, 1986; 1991). This allows ticks to reside and then quest for a host in areas with little free water, providing the local relative humidity is high.
4.3. Reducing water stress
Heat shock proteins (Hsps) are among the most studied proteins in relation to arthropod dehydration (Tammariello et al., 1999; Bayley et al., 2001; Hayward et al., 2004; Sinclair et al., 2007; Lopez-Martinez et al., 2009). Expression of three Hsps (smHsp, Hsp70, and Hsp90) has thus far been noted during arthropod dehydration (Hayward et al., 2004; Sinclair et al., 2007; Benoit et al., 2010a). In some cases one suite of Hsps is expressed during dehydration while a different suite responds to rehydration (Hayward et al., 2004; Lopez-Martinez et al., 2009). Hsps act to prevent stress damage due to unwanted biochemical interactions either by repairing damaged proteins or by prompting disassembly and breakdown of proteins damaged during dehydration (Parsell and Lindquist, 1993; Feder and Hofmann, 1999). Recently, we have shown that Hsps are essential in mosquitoes for attaining their maximal dehydration tolerance: using RNA interference to suppress expression of hsp70 and hsp90, we demonstrated a reduction of dehydration tolerance in Aedes aegypti (Benoit et al., 2010a).
Late embryogenesis abundant proteins (LEAs, also known as dehydrins) are also likely players in dehydration tolerance; the LEAs appear to act by stabilizing protein structure as the water content declines (Kikiwada et al., 2008). Antioxidant enzymes, such as catalase and superoxide dismutase (SOD), are elevated during dehydration, presumably to reduce damage from oxygen radicals formed from desiccation-induced stress (França et al., 2007; Lopez Martinez et al., 2009). Changes in membrane and cytoskeletal proteins may also be a fairly common response to dehydration (Li et al., 2009). One critical function of proteins associated with the cell membrane is to restructure the membrane to reduce water movement into and out of the cells as hemolymph osmolality changes. Cytoskeletal proteins serve to stabilize the cells during pressure and size changes caused by the osmotic stress of dehydration . Channel proteins, such as aquaporins, are also extremely important in regulating cellular water levels (Campbell et al., 2008; Spring et al., 2009). Three families of invertebrate aquaporins have been identified (DRIP, BIB, and PRIP families; Campbell et al., 2008), and all appear to be critical for maintaining water content within cells, particularly during feeding. Depending on the type of aquaporin, expression may be constitutive or responsive to cellular water stress, and certain aquaporins appear to be tissue specific (Kaufmann et al., 2005; Campbell et al., 2008; Philip et al., 2008).
Insect hemolymph osmolality ranges between 100 to 1400 mOsm kg−1 with a range of 400-500 mOsm kg−1 typical for most insects (Hadley, 1994). It is important to note that increasing osmolality, even 2-3 fold, reduces water loss only slightly, and the net water flux out of the insect persists unless the local environment is at saturation or above the organism’s internal water activity (Willmer, 1980; Wharton, 1985; Hadley, 1994; Chown and Nicolson, 2004). The alimentary canal efficiently regulates ion content and maintains osmolality in the 200-300 mOsm kg−1 range for most insects that reside in mesic and xeric regions. Poor osmoregulators have osmolalities that may vary nearly 1000 mOsm kg−1 (Hadley, 1994; Benoit, 2010); such insects usually reside in moist microhabitats and are capable of tolerating high levels of water loss. One method for regulating osmotic levels within insects is to sequester ions in the fat body during dehydration and subsequently releasing them back into the hemolymph as the hemolymph volume increases (Hyatt and Marshall, 1977; 1985a,b; Folk and Bradley, 2003). Fluctuations in osmolality are influenced by diverse molecules, including salts (NaCl, KCl), polyols (glycerol), sugars (trehalose), free amino acids (proline, etc.), and free fatty acids.
Many molecules that increase in concentration during dehydration have protective qualities (Goyal et al., 2005). Trehalose and glycerol are two of the most common molecules capable of suppressing water loss and reducing stress (Yoder et al., 2006; Watanabe, 2006). Trehalose is especially important during severe dehydration for its roles in preventing unwanted protein interactions, decreasing metabolism by altering fluid dynamics and protecting proteins and cellular membranes (Crowe et al., 1992; Suemoto et al., 2004; Goyal et al., 2005; Yoder et al., 2006b). Proline, as a free amino acid, may have similar effects (Yancey, 2005; Ignatova and Gierasch, 2006). Proline increases during stress in a few insects (Michaud and Denlinger, 2007; Michaud et al., 2008), but additional studies are needed to determine its exact function during dehydration. Dehydration-induced changes have also been documented for glucose and sorbitol (Hadley, 1994).
Volume regulation and/or compartmentalization is another factor that contributes to regulation of osmolality and water content (Zachariassen and Einarson, 1993; Hadley, 1994; Zachariassen and Pedersen, 2002). For example, a significant portion of water may be lost from one water pool (i.e. the hemolymph), but water content in the organs (e.g. salivary glands, midgut) may remain relatively constant. Typically, water in tissues is conserved at the expense of the hemolymph, as exemplified by the tenebrionid beetle, Onymacris plana, a species that loses weight slowly and predominantly at the expense of the hemolymph (Nicolson, 1980). Even though the exact mechanism for retaining water in a tissue at the expense of the hemolymph is not known it is an extremely important mechanism for retaining the integrity of biologically active tissues. Much of the stress induced by dehydration can be reduced by regulating osmolality and the water pool.
Increase in body size is another important factor for enhancing dehydration tolerance of insects. As noted in selection experiments with D. melanogaster, increased water content (from 25 to 30%) and size, along with respiratory changes, are key factors that increase dehydration resistance (Gibbs et al., 1997; Folk et al., 2001). But such differences are not always evident in the field. For example, a mesic species of Drosophila displays the predicted suite of traits, but a desert-adapted cactophilic species does not (Gibbs and Matzkin, 2001). Size changes have also been noted during dormancy in some insects (Hahn and Denlinger, 2007). Size of the northern house mosquito, Culex pipiens, increases substantially in preparation for winter, but the water pool does not increase (Benoit and Denlinger, 2007). Instead, the size of the differences reflect an increase in dry mass, but even so, a decrease in surface area to volume ratio reduces water loss (Benoit and Denlinger, 2007). Increasing body size is a fairly simple mechanism for increasing dehydration resistance.
Few studies have assessed physiological responses to multiple dehydration exposures, yet we suspect that this may be a fairly common occurrence in the natural world. In Culex pipiens, multiple dehydration bouts result in a reduction of total dry mass if the mosquitoes are not provided with sugar during periods of rehydration (Benoit et al., 2010b). Dry mass reduction is likely due to utilization of nutrient reserves (carbohydrate, glycogen and lipid), and the subsequent reduction in nutrient reserves presumably leads to the decrease in survival observed after multiple bouts of dehydration/rehydration. Additionally, these reduced nutrient reserves result in lower egg production. These observations imply that each response of C. pipiens to dehydration and rehydration requires energy expenditure, and after multiple bouts this significantly depletes the mosquito’s nutrient reserves. These results suggest the potential importance of assessing the effects of multiple bouts of dehydration on the physiology of blood-feeding arthropods, particularly since this can lower egg production.
4.4. Population differences
Recently, population differences have been implicated as an important variable of dehydration resistance in blood-feeding arthropods, as exemplified in Anopheles gambiae (Coluzzi et al., 2002; White et al., 2007; Simard et al., 2009). In this species, the M and S forms display major differences in dehydration tolerance: the M form survives significantly longer than the S form under dry conditions (Lee et al., 2009). 2La and/or 2Rbc chromosomal inversions are possibly responsible for this difference because both inversions have been associated with drought tolerance (Touré et al., 1994; Powell et al., 1999). Gray et al. (2009) demonstrated that the 2La inversion improves dehydration tolerance, particularly during early adulthood. One possibility for the increased dehydration resistance associated with the 2La inversion is that this region of the chromosome is involved in maintenance of cuticular proteins containing the RR-2 consensus (White et al., 2007; Gray et al., 2009). Cuticular proteins containing the RR-2 consensus are extremely important for cuticular hardening and may be upregulated in response to dehydration (Rebers et al., 2001; Zhang and Pelletier, 2008). Additionally, glycogen may be increased to improve dehydration resistance in A. gambiae (Gray et al., 2009), as also demonstrated in D. melanogaster (Graves et al., 1992; Djawdan et al., 1998; Archer et al., 2007). This may be due to water stored within the glycogen that can be released when water stores are low. Population differences in A. gambiae may be critical for dry season survival, but we still know little about the capability of A. gambiae for aestivation and the dehydration resistance that would be expected to go along with such a potential form of dormancy (Charlwood et al., 2000). Thus, significant differences may exist within species that allow certain populations to colonize more arid habitats.
4.5. Classification based on water loss rates
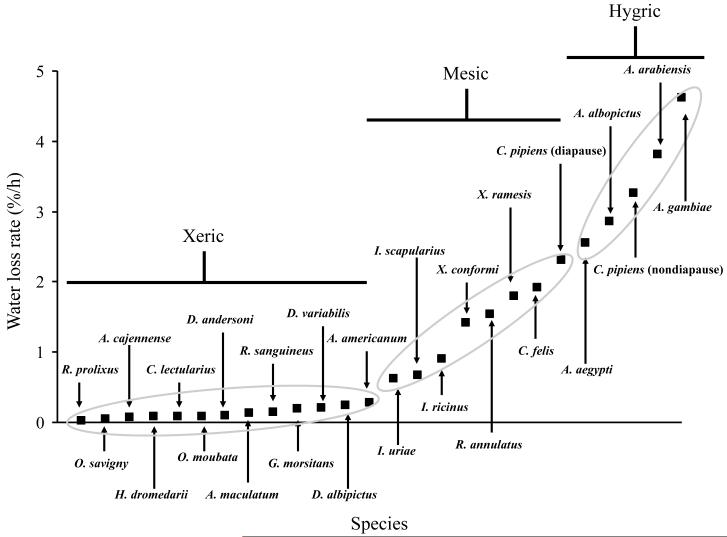
Figure 2 summarizes water loss rates for a number of arthropod vectors during their off-host phase. This classification system is based on that proposed for terrestrial arthropod by Hadley (1994): hygric = high water loss rates, usually over 2.0%/h, mesic = moderate water loss rates, 0.8-2.0%/h, and xeric = low water loss rate, less than 0.8%/h. Certain trends are evident for blood-feeding arthropods: mosquitoes are hygric, with fairly high water loss rates, hemipterans are fairly resistant to dehydration with water loss rates consistently below 0.8%/h, and ticks represent an intermediate having water loss rates that vary from extremely low (0.05%/h) to 1.5%/h. Rhipicephalus (= Boophilus) annulatus is the tick with the highest water loss rate, and this is likely due to the fact that it is a one-host species that spends most of its life attached to its host and thus has nearly continual access to blood for most of its life (Needham and Teel, 1991). Fleas likewise have intermediate loss rates. Interestingly, the tsetse fly, unlike other Diptera, falls into the xeric category, thus this higher dipteran clearly differs from lower Diptera such as mosquitoes in relation to its water balance characteristics. It is important to note that water loss rates are not the only factor involved in maintaining water balance: the ability to uptake water from the air and tolerance of high levels of dehydration are also influential aspects for establishing habitat preferences in relation to water balance. Additionally, even within groups, such as mosquitoes, significant differences have been noted between even closely related species, and as shown for C. pipiens, there are important differences between diapausing and nondiapausing adults (Benoit and Denlinger, 2007). Yet, the results presented in Fig. 2 suggest that general categories of water loss rates can be predicted for species that have not yet been evaluated.
Fig. 2.
Comparison of the water loss rates for off-host adult female hematophagous arthropods. Classification of xeric, mesic, and hygric is based on Hadley (1994). List in order from lowest to highest water loss rates: Rhodnius prolixus (Hemiptera; Hadley, 1994), Ornithodoros savigny (Argasidae; Hafez et al., 1970), Amblyomma cajennense (Ixodidae; Needham and Teel, 1991) Hyalomma dromedarii (Ixodidae; Hafez et al., 1970), Cimex lectularius (Hemiptera; Benoit et al., 2007b), Ornithodoros moubata (Argasidae Lees, 1947), Dermacentor andersoni (Ixodidae; Yoder et al., 2007), Amblyomma maculatum (Ixodidae; Yoder et al., 2009), Rhipicephalus sanguineus (Ixodidae; Yoder et al., 2006a), Glossina morsitans (Diptera; Bursell, 1960), Dermacentor variabilis (Ixodidae; Yoder et al., 2004), Dermacentor albipictus (Ixodidae; Needham and Teel, 1991), Amblyomma americanum (Ixodidae; Yoder et al., 2006c), Ixodes uriae (Ixodidae; Benoit et al., 2007a), Ixodes scapularius (Ixodidae; Yoder and Spielman, 1992), Ixodes ricinus (Ixodidae; Lees, 1946), Xenopsylla confromi (Siphonaptera; Fielden et al., 2002), Rhipicephalus annulatus (Ixodidae; Needham and Teel, 1991), Xenopsylla ramesis (Siphonaptera; Fielden et al., 2002), Ctenocephalides felis (Siphonaptera; Thiemann et al., 2003), Culex pipiens (Diptera; Benoit and Denlinger, 2007), Aedes aegypti (Diptera; Benoit et al., 2009), Aedes albopictus (Diptera; Benoit, J.B. unpublished observation), Anopheles arabiensis (Diptera; Gray and Bradley, 2005), Anopheles gambiae (Gray and Bradley, 2005).
5. Water balance after blood feeding
5.1. Diuresis following blood feeding
Blood feeding causes immediate and drastic changes in arthropod physiology, as noted from experiments conducted primarily on Rhodnius prolixus and mosquitoes. The mass of R. prolixus nymphs and adult female mosquitoes increases 10-12 and 2-3 fold, respectively, following a bloodmeal (Coast, 2009). Although this bloodmeal represents an excellent source of nutrients, a large portion of this resource contains excess Na+ and Cl−, along with a surplus of water. If it does not get rid of the excess liquid quickly, the insect remains corpulent, relatively immobile, and hence vulnerable to predation (Lehane, 2005). Briefly, excess water is directly passed through the alimentary canal or is absorbed into the hemolymph by the midgut, where it is transported into the Malpighian tubules and removed during diuresis as urine. Postprandial diuresis yields a condensed meal leaving mostly blood cells and proteins that are digested quickly in the case of mosquitoes, or slowly in R. prolixus (Lehane, 2005). Diuresis in blood-feeding arthropods is regulated by a suite of anti-diuretic and diuretic hormones, as comprehensively reviewed by Coast (2001; 2009), Dow and Davis (2003), and Beyenbach (2003). In this review, we present only a brief summary of diuresis in blood-feeding arthropods, based primarily on results from R. prolixus and mosquitoes.
The Malphigian tubules regulate secretion of electrolytes, organic solutes and water, and the proximal region of the Malphigian tubules, hindgut and rectum selectively absorb components still needed by the body (Beyenbach, 1995; Spring and Albarwani, 1993; O’Donnell and Maddrell, 1995; Coast, 2006; 2009). The renal activity of insects is impressive, with turnover rates peaking at nearly 200 times per day following blood feeding in Aedes aegypti (Beyenbach, 2003). This leads to an urination rate of 60 nl min−1 for A. aegypti, allowing the mosquito to quickly void excess fluid volume (Williams et al., 1983; Wheelock et al., 1988).
For many blood-feeding arthropods, including mosquitoes and tsetse flies, condensation of the bloodmeal begins during feeding, yielding an increase in concentration of nutrients in the meal (Clements, 1992). In soft ticks (Argasidae), excess water is secreted from the coxal gland, a specialized structure unique to these ticks. Within minutes of blood feeding, diuretic hormone is released in response to distension of the gut (Maddrell, 1966; Coast et al., 2005). A comprehensive list of diuretic, along with a few identified anti-diuretic, hormones are provided for R. prolixus by Coast (2009) and for Anopheles gambiae by Riehle et al. (2002). Serotonin and natriuretic hormone function as diuretic hormones for R. prolixus and mosquitoes, respectively (Coast et al., 2005; Orchard, 2006; Te Brugge et al., 2009). One recently identified hormone that seems to be particularly important for mosquitoes is Diuretic hormone 44 (Aedes aegypti CRF-like DH), which has an expression profile paralleling excretion (Jagge and Pietranonio, 2008). Diuretic hormones stimulate the Malphigian tubules, prompting removal of excess water and Na+ while preserving K+ (Coast, 2009). The peak phase of diuresis yields urine highly concentrated in Na+. Eventually, the level of Na+ declines and the level of K+ secretion increases, a phase associated with K+ release during blood cell digestion (Williams et al., 1983). After this initial phase of rapid diuresis, mosquitoes enter a stable post-peak phase of diuresis in which secretion levels of K+ and Na+ become more varied and the urine becomes hypo-osmotic, allowing the hemolymph to return to normal, post-feeding osmotic concentrations (Beyenbach, 2003). Diuresis is likely terminated by the combined effect of the end of adominal distension, a signal for secreting diuretic hormone, and the presence of anti-diuretic hormones. At this point, the vector has completed its feeding cycle and reverts to the non-feeding state.
5.2. Cuticular changes elicited by blood feeding
Blood feeding elicits responses in the cuticle as documented in mosquitoes (Marinotti et al., 2006), R. prolixus (Ianowski et al., 1998), Triatoma infestans (Melcón et al., 2005) and ticks (Yoder et al., 1997; Andersen and Roepstorff, 2005). Most changes involve rapid stretching of the cuticle during blood feeding, a process known as plasticization (Bennet-Clark, 1962; Reynolds, 1974). In R. prolixus, serotonin functions as a plasticization factor and is released from the nerve terminal to the epidermis (D’yakonova, 1986; Barrett and Orchard, 1990; Orchard, 2006). Plasticization is a consequence of the breakdown of relatively weak intermolecular bonds between proteins (Hackman, 1975; Hackman and Goldberg, 1987; Reynolds, 1975; Quesada-Allué, 1987). Additionally, the cuticle of most blood-feeding arthropods contains numerous resilin proteins that allow stretching of the cuticle. While in this distended state, water loss rates have been documented in the lone star tick, Amblyomma americanum (Yoder et al., 1997), bed bugs, Cimex lectularius, and A. aegypti (J. B. Benoit, unpublished observations), and in all three species cuticular water loss rates are nearly 3x higher than in nonfed individuals. Of particular interest is the observation that cuticular lipids increase in abundance after blood feeding, yielding insects that are more resistant to dehydration following a bloodmeal (Yoder et al., 1997). In A. aegypti, only the first bout of blood feeding increases the amount of cuticular hydrocarbons and reduces water loss; subsequent feeding cycles alter neither the amount of hydrocarbon nor the water loss rate (J. B. Benoit, unpublished observation). Thus, cuticular changes associated with blood feeding usually increase cuticular permeability, but the initial blood meal may provide a source of additional cuticular hydrocarbons that can be used to enhance dehydration resistance during the subsequent off-host phase.
5.3. Lessons from transcriptome and proteome studies during blood feeding
Recent transcriptome and proteome studies examining responses to blood feeding in mosquitoes (Sanders et al, 2003; Dana et al., 2005), sand flies (Jochim et al., 2008), ticks (Rudenko et al., 2005), tsetse flies (Lehane et al., 2003; Munks et al., 2005), and ceratopogonids (Campbell et al., 2005) offer clues about gene expression patterns that may be responsive to water stress caused by the bloodmeal. None of those studies have focused directly on issues of water balance, but Table 2 identifies genes/proteins that both increase during blood feeding and are also known to be important during water stress. Other genes with increased expression during blood feeding may also be involved in the response to excess water, but this list includes only genes previously reported to be involved in arthropod water balance (Benoit, 2010). The majority of genes included in this list contribute to the prevention of oxidative damage, an injury fairly common during dehydration, rehydration, and overhydration (França et al., 2007). Two other categories of genes that are commonly upregulated during blood feeding include chaperones, particularly heat shock proteins, and those involved in the transport of ions and fluid, particularly aquaporins. The chaperones likely repair proteins damaged during blood feeding, and transport proteins maintain cellular water and ions levels. Lastly, structural changes occur as fluid levels within the body fluctuate, and genes for both cytoskeletal proteins and membrane restructuring are notably increased during blood feeding. Table 2 thus represents a collection of genes that are likely to be involved in preventing and responding to cellular water stress during blood feeding. Future studies will be needed to directly assess functions for these genes in enabling arthropod vectors to tolerate overhydration and to process water during blood feeding.
Table 2.
Genes and/or proteins that increase during blood feeding and may be involved in responding to excess water from the bloodmeal
| Gene |
Organism |
Citation |
|---|---|---|
| Antioxidant | ||
| Catalase |
Aedes aegypti
Culicoides sonorensis Glossina morsitans Glossina morsitans Lutzomyia longipalpis |
Sanders et al., 2003
Campbell et al., 2005 Lehane et al., 2003 Munks et al., 2005 Jochim et al., 2008 |
| Cytochrome c oxidase |
Anopheles gambiae
Culicoides sonorensis Lutzomyia longipalpis |
Dana et al., 2005
Campbell et al., 2005 Jochim et al., 2008 |
| Cytochrome P450 |
Aedes aegypti
Anopheles gambiae Lutzomyia longipalpis |
Sanders et al., 2003
Dana et al., 2005 Jochim et al., 2008 |
| Ferritin |
Aedes aegypti
Culicoides sonorensis Lutzomyia longipalpis |
Sanders et al., 2003 Campbell et al., 2006 Jochim et al., 2008 |
| Iron-binding proteins Glutathione peroxidase Glutathione s-transferase |
Culicoides sonorensis
Glossina morsitans Anopheles gambiae |
Campbell et al., 2005
Munks et al., 2005 Dana et al., 2005 |
|
Ixodes ricinus
Lutzomyia longipalpis |
Rudenko et al., 2005
Jochim et al., 2008 |
|
| Peroxidase |
Culicoides sonorensis
Glossina morsitans |
Campbell et al., 2006 Lehane et al., 2003 |
| Peroxiredoxin |
Glossina morsitans
Lutzomyia longipalpis |
Munks et al., 2004 Jochim et al., 2008 |
| Superoxide dismutase |
Glossina morsitans
Lutzomyia longipalpis |
Munks et al., 2005
Jochim et al., 2008 |
| Thioredoxin reductase |
Aedes aegypti
Anopheles gambiae Culicoides sonorensis Glossina morsitans Ixodes ricinus |
Sanders et al., 2003
Dana et al., 2005 Campbell et al., 2005 Munks et al., 2005 Rudenko et al., 2005 |
| Chaperone | ||
| Hsc70 Hsc70A Hsc70B Hsp70 Hsp82 Hsp90 smHsp |
Aedes aegypti
Culicoides sonorensis Culicoides sonorensis Aedes aegypti Anopheles gambiae Aedes aegypti Anopheles gambiae |
Sanders et al., 2003 Campbell et al., 2005 Campbell et al., 2005 Benoit et al., 2009 Dana et al., 2005 Benoit et al., 2010 Dana et al., 2005 |
| Structural | ||
| Actin |
Anopheles gambiae
Ixodes ricinus Lutzomyia longipalpis |
Dana et al., 2005
Rudenko et al., 2005 Jochim et al., 2008 |
| Transport | ||
| Amino acid transporter Aquaporin Aquaporin 2 Aquaporin 4 Cl− channel Organic cation porter Na+/iodine symporter Na+/multivitamin symporter V-ATPase Water transporter |
Aedes aegypti
Lutzomyia longipalpis Aedes aegypti Aedes aegypti Aedes aegypti Aedes aegypti Anopheles gambiae Aedes aegypti Aedes aegypti Anopheles gambiae Lutzomyia longipalpis Anopheles gambiae |
Sanders et al., 2003
Jochim et al., 2008 Sanders et al., 2003 Sanders et al., 2003 Sanders et al., 2003 Sanders et al., 2003 Dana et al., 2005 Sanders et al., 2003 Sanders et al., 2003 Dana et al., 2005 Jochim et al., 2008 Dana et al., 2005 |
| Signaling | ||
| Calponin-like protein Calmodulin-beta GRP94 |
Aedes aegypti
Aedes aegypti Aedes aegypti |
Sanders et al., 2003
Sanders et al., 2003 Sanders et al., 2003 |
| Membrane restructuring | ||
| Enoyl CoA hydratase Fatty acid synthase Fatty acid desaturase Fatty-acid-conenzyme A ligase Short-branch Acyl-CoA dehydrogenase |
Aedes aegypti
Aedes aegypti Aedes aegypti Aedes aegypti Aedes aegypti |
Sanders et al., 2003
Sanders et al., 2003 Sanders et al., 2003 Sanders et al., 2003 Sanders et al., 2003 |
6. Conclusions
Many features of water balance, particularly aspects involved in the transition between blood feeding and off-host physiology, have not been fully elucidated in hematophagous arthropods. With the current wealth of molecular information on blood-feeding arthropods, establishing the underlying molecular mechanisms for changes that occur during dehydration and in response to excess water during blood feeding should now be feasible. For example, recent discoveries of aquaporins and LEA proteins (dehydrins) bring to the forefront new components of a sophisticated system of water balance, and a role for heat shock proteins in establishing water loss tolerance suggests new roles for “old proteins”. The many genes likely involved in responding to water stress portend an impressive network of genes whose interactions remain to be defined. The diuretic and anti-diuretic neuropeptides finely coordinate water balance attributes both during blood feeding and during off-host states. How does the blood feeder detect its water balance state and coordinate the transition between the two states? How are changes in cuticular plasticization and the shifts in cuticular hydrocarbon composition that accompany blood feeding initiated and executed? We currently have few answers to these interesting questions.
The vulnerability of blood-feeding disease vectors to desiccation suggests the potential to develop new methods for altering their dehydration tolerance, thus generating new strategies that could be exploited for control. Issues of desiccation vulnerability are of paramount importance in tropical regions where many of the most important disease vectors reside. Tropical dry seasons in particular pose a major seasonal challenge for water management, and understanding how vectors of malaria and other tropical diseases survive the dry season remains one of the major unanswered questions in vector biology. Moisture features of the environment offer a key indicator of habitat preference and potential distribution patterns, thus implying that an understanding of these limitations offers predictive value in defining the potential geographic spread of an invasive species, especially in a changing global environment.
7. Acknowledgements
We dedicate this review to Professor Judy Willis in gratitude for the mentoring we received as her academic son (DLD) and grandson (JBB). Research was funded by a grant from the National Institutes of Health (R01 AI058279) to DLD, and by a Mary S. Muelhaupt Endowed Presidential Fellowship to JBB from the Ohio State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- Adams TS. Hematophagy and hormone release. Annals of the Entomological Society of America. 1999;92:1–23. [Google Scholar]
- Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nature Genetics. 2002;32:402–407. doi: 10.1038/ng986. [DOI] [PubMed] [Google Scholar]
- Aksoy S, Rio RVM. Interactions among multiple genomes: tsetse, its symbionts and trypanosomes. Insect Biochemistry and Molecular Biology. 2005;35:691–698. doi: 10.1016/j.ibmb.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Andersen SO, Roepstorff P. The extensible alloscutal cuticle of the tick, Ixodes ricinus. Insect Biochemistry and Molecular Biology. 2005;35:1181–1188. doi: 10.1016/j.ibmb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Archer MA, Bradley TJ, Mueller LD, Rose MR. Using experimental evolution to study the physiological mechanisms of desiccation resistance in Drosophila melanogaster. Physiological Biochemistry and Zoology. 2007;80:386–398. doi: 10.1086/518354. [DOI] [PubMed] [Google Scholar]
- Barrett M, Orchard I. Serotonin-induced elevation of cAMP levels in the epidermis of blood sucking bug, Rhodnius prolixus. Journal of Insect Physiology. 1990;36:625–630. [Google Scholar]
- Barrozo RB, Manrique G, Lazzari CR. The role of water vapour in the orientation behaviour of the blood sucking bug Triatoma infestans (Hemiptera: Reduviidae) Journal of Insect Physiology. 2003;49:315–321. doi: 10.1016/s0022-1910(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Bayley M, Holmstrup M. Water vapor absorption in arthropods by accumulation of myoinositol and glucose. Science. 1999;285:1909–1911. doi: 10.1126/science.285.5435.1909. [DOI] [PubMed] [Google Scholar]
- Bayley M, Petersen SO, Knigge T, Köhler HR, Holmstrup M. Drought acclimation confers cold tolerance in the soil collembolan, Folsomia candida. Journal of Insect Physiology. 2001;47:1197–1204. doi: 10.1016/s0022-1910(01)00104-4. [DOI] [PubMed] [Google Scholar]
- Beard CB, Cordon-Rosales C, Durvasula RV. Bacterial symbionts of the Triatominae and their potential use in control of Chagas Disease transmission. Annual Review of Entomology. 2002;47:123–141. doi: 10.1146/annurev.ento.47.091201.145144. [DOI] [PubMed] [Google Scholar]
- Bennet-Clark HC. Active control of the mechanical properties of insect endocuticle. Journal of Insect Physiology. 1962;8:627–633. [Google Scholar]
- Benoit JB. Water management by dormant insects: comparisons between dehydration resistance during estivation and winter diapause. In: Navas C, Eduardo J, editors. Progress in Molecular and Subcellular Biology. Springer; Berlin: 2010. pp. 209–230. Vol. Estivation. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Denlinger DL. Suppression of water loss during adult diapause in the northern house mosquito, Culex pipiens. Journal of Experimental Biology. 2007;210:217–226. doi: 10.1242/jeb.02630. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Yoder JA, Rellinger EJ, Ark JT, Keeney GD. Prolonged maintenance of water balance by adult females of the American spider beetle, Mezium affine Boieldieu, in the absence of food and water resources. Journal of Insect Physiology. 2005;51:565–573. doi: 10.1016/j.jinsphys.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Yoder JA, Lopez-Martinez G, Elnitsky MA, Lee RE, Jr., Denlinger DL. Habitat requirements of the seabird tick, Ixodes uriae (Acari: Ixodidae), from the Antarctic Peninsula in relation to water balance characteristics of eggs, nonfed and engorged stages. Journal of Comparative Physiology B. 2007a;177:205–215. doi: 10.1007/s00360-006-0122-7. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Del Grosso NA, Yoder JA, Denlinger DL. Resistance to dehydration between bouts of blood feeding in the bed bug, Cimex lectularius, is enhanced by water conservation, aggregation, and quiescence. American Journal of Tropical Medicine and Hygiene. 2007b;76:987–993. [PubMed] [Google Scholar]
- Benoit JB, Lopez-Martinez G, Phillips SA, Elnitsky MA, Yoder JA, Lee RE, Jr., Denlinger DL. The seabird tick, Ixodes uriae, uses uric acid in penguin feces as a kairomone and guanine in tick feces as an assembly pheromone on the Antarctica Peninsula. Polar Biology. 2008;31:1445–1451. [Google Scholar]
- Benoit JB, Martinez G, Phillips ZP, Patrick KR, Denlinger DL. Heat shock proteins contribute to mosquito dehydration tolerance. Journal of Insect Physiology. 2010a;56:151–156. doi: 10.1016/j.jinsphys.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit JB, Patrick KR, Desai K, Hardesty JJ, Krause TB, Denlinger DL. Repeated bouts of dehydration deplete nutrient reserves and reduce egg production in the mosquito, Culex pipiens. Journal of Experimental Biology. 2010b doi: 10.1242/jeb.044883. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyenbach KW. Mechanisms and regulation of electrolyte transport in Malphigian tubules. Journal of Insect Physiology. 1995;41:197–207. [Google Scholar]
- Beyenbach KW. Transport mechanisms of diuresis in Malpighian tubules of insects. Journal of Experimental Biology. 2003;206:3845–3856. doi: 10.1242/jeb.00639. [DOI] [PubMed] [Google Scholar]
- Beyenbach KW, Petzel DH. Diuresis in mosquitoes: role of a natriuretic factor. New Physiological Science. 1987;2:171–175. [Google Scholar]
- Blomquist GJ, Nelson DR, de Renobales M. Chemistry, biochemistry and physiology of insect cuticular lipids. Archives of Insect Biochemistry and Physiology. 1987;6:227–262. [Google Scholar]
- Bowen MF. The sensory physiology of host seeking behavior in mosquitoes. Annual Review of Entomology. 1991;36:139–158. doi: 10.1146/annurev.en.36.010191.001035. [DOI] [PubMed] [Google Scholar]
- Bradley TJ. The excretory system: structure and physiology. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol. 4. Permagon Press; Oxford: 1985. pp. 421–465. [Google Scholar]
- Bradley TJ, Williams AE, Rose MR. Physiological responses to selection for desiccation resistance in Drosophila melangaster. American Zoologist. 1999;39:337–345. [Google Scholar]
- Brown MR, Klowden MJ, Crim JW, Young L, Shrouder LA, Lea AO. Endogenous regulation of mosquito host-seeking behavior by a neuropeptide. Journal of Insect Physiology. 1994;40:399–406. [Google Scholar]
- Bursell E. Spiracular control of water loss in the tsetse fly. Proceeding of the Royal Entomological Society of London. 1957;32:21–29. [Google Scholar]
- Bursell E. Loss of water by excretion and defaecation in the tsetse fly. Journal of Experimental Biology. 1960;37:689–697. [Google Scholar]
- Campbell CL, Vandyke KA, Letchworth GJ, Drolet BS, Hanekamp T, Wilson WC. Midgut and salivary gland transcriptome of the arbovirus vector Culicoides sonorensis. Insect Molecular Biology. 2005;14:121–136. doi: 10.1111/j.1365-2583.2004.00537.x. [DOI] [PubMed] [Google Scholar]
- Campbell EM, Ball A, Hoppler S, Bowman AS. Invertebrate aquaporins: a review. Journal of Comparative Physiology B. 2008;178:935–955. doi: 10.1007/s00360-008-0288-2. [DOI] [PubMed] [Google Scholar]
- Charlwood JD, Vij R, Billingsley PF. Dry season refugia of malaria-transmitting mosquitoes in a dry savannah zone of east Africa. American Journal of Tropical Medicine and Hygiene. 2000;62:726–732. doi: 10.4269/ajtmh.2000.62.726. [DOI] [PubMed] [Google Scholar]
- Chown SL. Respiratory water loss in insects. Comparative Biochemistry and Physiology B. 2002;133:791–804. doi: 10.1016/s1095-6433(02)00200-3. [DOI] [PubMed] [Google Scholar]
- Chown SL, Nicolson SW. Insect Physiological Ecology. Oxford University Press; Oxford: 2004. [Google Scholar]
- Clements AN. The Biology of Mosquitoes. Chapman and Hall; London: 1992. [Google Scholar]
- Coast GM. Insect diuretic and antidiuretic hormones. In: Kastin AJ, editor. Handboook of Biologically Active Peptides. Academic Press; London: 2006. pp. 157–162. [Google Scholar]
- Coast GM. The endocrine control of salt balance in insects. General and Comparative Endocrinology. 2007;152:332–338. doi: 10.1016/j.ygcen.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Coast GM. Neuroendocrine control of ionic homeostasis in blood-sucking insects. Journal of Experimental Biology. 2009;212:378–386. doi: 10.1242/jeb.024109. [DOI] [PubMed] [Google Scholar]
- Coast GM, Orchard I, Phillips JE, Schooley DA. Insect diuretic and antidiuretic hormones. Advances in Insect Physiology. 2002;29:279–409. [Google Scholar]
- Coast GM, Garside CS, Webster SG, Schegg KM, Schooley DA. Mosquito natriuretic peptide identified as a calcitonin-like diuretic hormone in Anopheles gambiae. Journal of Experimental Biology. 2005;208:3281–3291. doi: 10.1242/jeb.01760. [DOI] [PubMed] [Google Scholar]
- Coluzzi M, Sabatini A, della Torre A, Di Deco MA, Tetrarca V. A polytene chromosome analysis of the Anopheles gambiae species complex. Science. 2002;298:1415–1418. doi: 10.1126/science.1077769. [DOI] [PubMed] [Google Scholar]
- Crooks E, Randolph SE. Walking by Ixodes ricinus ticks: intrinsic and extrinsic factors determine attraction of moisture and host odour. Journal of Experimental Biology. 2006;209:2138–2142. doi: 10.1242/jeb.02238. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annual Review of Physiology. 1992;54:579–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- Dana AN, Hong YS, Kern MK, Hillenmeyer ME, Harker BW, Lobo NF, Hogan JR, Romans P, Collins FH. Gene expression patterns associated with blood-feeding in the malaria mosquito Anopheles gambiae. BMC Genomics. 2005;6:5. doi: 10.1186/1471-2164-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Renobales M, Nelson DR, Blomquist GJ. Cuticular lipids. In: Binnington K, Retnakaran A, editors. The Physiology of the Insect Epidermis. CSIRO; Australia: 1991. pp. 240–251. [Google Scholar]
- Djawdan M, Chippindale AK, Rose MR, Bradley TJ. Metabolic reserves and evolved stress resistance in Drosophila melanogaster. Physiological Zoology. 1998;71:584–594. doi: 10.1086/515963. [DOI] [PubMed] [Google Scholar]
- Dow JAT, Davis SA. Integrative physiology and functional genomics of epithelial function in a genetic model organism. Physiological Reviews. 2003;83:687–729. doi: 10.1152/physrev.00035.2002. [DOI] [PubMed] [Google Scholar]
- D’yakonova TL. Regulation of plastic properties of electroexcitable neuron membrane by serotonin. Neuroscience and Behavioral Physiology. 1986;16:389–394. doi: 10.1007/BF01185369. [DOI] [PubMed] [Google Scholar]
- Edney EB. Water Balance in Land Arthropods. Springer-Verlag; Berlin: 1977. [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annual Review of Physiology. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Fielden L, Krasnov B, Still KM, Khokhlova I. Water balance in two species of desert fleas, Xenopsylla ramesis and X. conformis. Journal of Medical Entomology. 2002;39:875–881. doi: 10.1603/0022-2585-39.6.875. [DOI] [PubMed] [Google Scholar]
- Folk DG, Han C, Bradley TJ. Water acquisition and portioning in Drosophila melanogaster: effects of selection for desiccation-resistance. Journal of Experimental Biology. 2001;204:3233–3331. doi: 10.1242/jeb.204.19.3323. [DOI] [PubMed] [Google Scholar]
- Folk DG, Bradley TJ. Evolved patterns and rates of water loss and ion regulation in laboratory-selected populations of Drosophila melanogaster. Journal of Experimental Biology. 2003;206:2779–2786. doi: 10.1242/jeb.00498. [DOI] [PubMed] [Google Scholar]
- França MB, Panek AD, Eleutherio ECA. Oxidative stress and its effects during dehydration. Comparative Biochemistry and Physiology A. 2007;146:621–631. doi: 10.1016/j.cbpa.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Gäde G. Regulation of intermediary metabolism and water balance of insects by neuropeptides. Annual Review Entomology. 2004;49:93–113. doi: 10.1146/annurev.ento.49.061802.123354. [DOI] [PubMed] [Google Scholar]
- Gibbs AG. Water-proofing properties of cuticular lipids. American Zoologist. 1998;38:471–482. [Google Scholar]
- Gibbs AG. Lipid melting and cuticular permeability: new insights into an old problem. Journal of Insect Physiology. 2002;48:391–400. doi: 10.1016/s0022-1910(02)00059-8. [DOI] [PubMed] [Google Scholar]
- Gibbs AG, Chippindale AK, Rose MR. Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. Journal of Experimental Biology. 1997;200:1821–1832. doi: 10.1242/jeb.200.12.1821. [DOI] [PubMed] [Google Scholar]
- Gibbs AG, Matzkin LM. Evolution of water balance in the genus Drosophila. Journal of Experimental Biology. 2001;204:2331–2338. doi: 10.1242/jeb.204.13.2331. [DOI] [PubMed] [Google Scholar]
- Gillies MT. The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): a review. Bulletin of Entomological Research. 1980;70:525–532. [Google Scholar]
- Goyal K, Wlaton LJ, Browne JA, Burnell AM, Tunnacliffe A. Molecular anhydrobiology: identifying molecules implicated in invertebrate anhydrobiosis. Integrative and Comparative Biology. 2005;45:702–709. doi: 10.1093/icb/45.5.702. [DOI] [PubMed] [Google Scholar]
- Graça-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GRC, Paes MC, Sorgine MHF, Oliveira MF, Oliveira PL. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochemsitry and Molecular Biology. 2006;36:322–335. doi: 10.1016/j.ibmb.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Graves JL, Toolson EC, Jeong LN, Vu LN, Rose MR. Desiccation, flight, glycogen, and postponed senescence in Drosophila melanogaster. Physiological Zoology. 1992;65:268–286. [Google Scholar]
- Gray EM, Bradley TJ. Physiology of desiccation resistance in Anopheles gambiae and Anopheles arabiensis. American Journal of Tropical Medicine Hygiene. 2005;73:553–559. [PubMed] [Google Scholar]
- Gray EM, Rocca KAC, Costantini C, Besansky NJ. Inversion 2La is associated with enhanced desiccation resistance in Anopheles gambiae. Malaria Journal. 2009;8:215–227. doi: 10.1186/1475-2875-8-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman RH. Expanding abdominal cuticle in the bug Rhodnius and the tick Boophilus. Journal of Insect Physiology. 1975;21:1613–1623. doi: 10.1016/0022-1910(75)90199-7. [DOI] [PubMed] [Google Scholar]
- Hackman RH, Goldberg M. Comparative study of some expanding arthropod structures and function. Journal of Insect Physiology. 1987;33:39–50. [Google Scholar]
- Hadley NF. Cuticular lipids of terrestrial plants and arthropods: a comparison of their structure, composition, and waterproofing functions. Biological Review of the Cambridge Philosophical Society. 1981;56:23–47. [Google Scholar]
- Hadley NF. Water relations of terrestrial arthropods. Academic Press; New York: 1994. [Google Scholar]
- Hafez M, El-Ziady S, Hefnawy T. Biochemical and physiological studies of certain ticks (Ixodoidea). Cuticular permeability of Hyalomma (H.) dromedarii (Ixodidae) and Ornithodoros (O.) savigny (Audouin) (Argasidae) Journal of Parasitology. 1970;56:154–168. [PubMed] [Google Scholar]
- Hahn DA, Denlinger DL. Meeting energetic demands of insect diapause: nutrient storage and utilization. Journal of Insect Physiology. 2007;53:760–773. doi: 10.1016/j.jinsphys.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Hayward SAL, Rinehart JP, Denlinger DL. Desiccation and rehydration elicit distinct heat shock protein transcript responses in flesh fly pupae. Journal of Experimental Biology. 2004;207:963–971. doi: 10.1242/jeb.00842. [DOI] [PubMed] [Google Scholar]
- Hood WG, Tschinkel WR. Desiccation resistance in arboreal and terrestrial ants. Physiological Entomology. 1990;15:23–35. [Google Scholar]
- Hyatt AD, Marshall AT. Sequestration of haemolymph sodium and potassium by the fat body in the water-stressed cockroach, Periplaneta americana. Journal of Insect Physiology. 1977;23:1437–1441. [Google Scholar]
- Hyatt AD, Marshall AT. Water and ion balance in the tissues of the dehydrated cockroach, Periplaneta Americana. Journal of Insect Physiology. 1985a;31:27–34. [Google Scholar]
- Hyatt AD, Marshall AT. X-ray analysis of the cockroach fat body in relation to ion and water regulation. Journal of Insect Physiology. 1985b;31:495–508. [Google Scholar]
- Ianowski JP, Manrique G, Núñez JA, Lazzari CR. Feeding is not necessary for triggerting plasticization of the abdominal cuticle in hematophagous bugs. Journal of Insect Physiology. 1998;44:379–384. doi: 10.1016/s0022-1910(98)00023-7. [DOI] [PubMed] [Google Scholar]
- Ignatova Z, Gierasch LM. Inhibition of protein aggregation in vitro and in vivo by a natural osmoprotectant. Proceeding of the National Academy of Sciences USA. 2006;103:13357–13361. doi: 10.1073/pnas.0603772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagge CL, Pietrantonio PV. Diuretic hormone 44 receptor in Malphigian tubules of the mosquito Aedes aegypti: evidence for transcriptional regulation paralleling urination. Insect Molecular Biology. 2008;17:413–426. doi: 10.1111/j.1365-2583.2008.00817.x. [DOI] [PubMed] [Google Scholar]
- Jochim RC, Teixeira CR, Laughinghouse A, Mu J, Oliveira F, Gomes RB, Elnaiem D-E, Valenzuela JG. The midgut transcriptome of Lutzomyia longipalpis: comparative analysis of CDNA libraries from sugar-fed, blood-fed, post-digested and Leishmania infantum chagasi-infected sand flies. BMC Genomics. 2008;9:15. doi: 10.1186/1471-2164-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann N, Mathai JC, Hill WG, Dow JAT, Zeidel ML, Brodsky JL. Developmental expression and biophysical characterization of a Drosophila melanogaster aquaporin. American Journal of Physiology, Cell Physiology. 2005;289:C397–407. doi: 10.1152/ajpcell.00612.2004. [DOI] [PubMed] [Google Scholar]
- Kessler S, Guerin PM. Reponses of Anopheles gambiae, Anopheles stephani, Aedes aegypti, and Culex pipiens mosquitoes (Diptera: Culicidae) to cool and humid refugium conditions. Journal of Vector Ecology. 2008;33:145–149. doi: 10.3376/1081-1710(2008)33[145:roagas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kikawada T, Minakawa N, Watanabe M, Okuda T. Factors inducing successful anhydrobiosis in the African chironomid Polypedilum vanderplanki: significance of the larval tubular nest. Integrative and Comparative Biology. 2005;45:710–714. doi: 10.1093/icb/45.5.710. [DOI] [PubMed] [Google Scholar]
- Kikawada T, Saito A, Kanamori Y, Fujita M, Śnigórska K, Watanabe M, Okuda T. Dehydration-inducible changes in the expression of two aquaporins in the sleeping chironomid, Polypedilum vanderplanki. Biochimica Biophysica Acta. 2008;1778:514–520. doi: 10.1016/j.bbamem.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Kim KC. Evolution and host association of Anoplura. In: Kim KC, editor. Coevolution of Parasitic Arthropods and Mammals. Wiley; New York: 1985. [Google Scholar]
- Klowden MJ. The endogenous regulation of mosquito reproductive behavior. Experientia. 1990;46:660–670. doi: 10.1007/BF01939928. [DOI] [PubMed] [Google Scholar]
- Klowden MJ. Vector behavior. In: Beaty BJ, Marquadt WC, editors. The Biology of Disease Vectors. University Press of Colorado; Colorado: 1996. pp. 34–50. [Google Scholar]
- Knülle W. Water vapour uptake in mites and insects: an ecophysiological and evolutionary perspective. In: Griffiths DA, Bowman CE, editors. Acarology VI. Ellis Horwood; Chichester: 1984. pp. 71–82. [Google Scholar]
- Lee Y, Meneses CR, Fofana A, Lanzaro GC. Desiccation resistance among subpopulations of Anopheles gambiae ss. from Selinkenyi, Mali. Journal of Medical Entomology. 2009;46:316–320. doi: 10.1603/033.046.0216. [DOI] [PubMed] [Google Scholar]
- Lees AD. Water balance in Ixodes ricinus L. and certain other species of ticks. Parasitology. 1946;37:1–20. doi: 10.1017/s0031182000013093. [DOI] [PubMed] [Google Scholar]
- Lehane MJ. Biology of Blood-Sucking Insects. First Edition Cambridge University Press; Cambridge: 1991. [Google Scholar]
- Lehane MJ. Biology of Blood-Sucking Insects. Second Edition. Cambridge University Press; Cambridge: 2005. Cambridge. [Google Scholar]
- Lehane MJ, Aksoy S, Gibson W, Kerhornou A, Berriman M, Hamilton J, Soares MB, Bonaldo MF, Lehane S, Hall N. Adult midgut expressed sequence tags from the tsetse fly Glossina morsitans and expression analysis of putative immune response genes. Genome Biology. 2003;4:R63. doi: 10.1186/gb-2003-4-10-r63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Benoit JB, Lopez-Martinez G, Elnitsky MA, Lee RE, Jr., Denlinger DL. Distinct contractile and cytoskeletal protein patterns in the Antarctic midge are elicited by desiccation and rehydration. Proteomics. 2009;9:2788–2797. doi: 10.1002/pmic.200800850. [DOI] [PubMed] [Google Scholar]
- Lighton JRB, Garrigan DA, Duncan FD, Jonson RA. Spiracular control of respiratory water loss in female alates of the harvester ant Pogonomyrmex rugosus. Journal of Experimental Biology. 1993;179:233–244. [Google Scholar]
- Lighton JRB. Discontinuous gas exchange in insects. Annual Review of Entomology. 1996;41:309–324. doi: 10.1146/annurev.en.41.010196.001521. [DOI] [PubMed] [Google Scholar]
- Lockey KH. Lipids of the insect cuticle: origin, composition, and function. Comparative Biochemistry and Physiology B. 1988;89B:595–645. [Google Scholar]
- Lopez-Martinez G, Benoit JB, Rinehart JP, Elnitsky MA, Lee RE, Jr., Denlinger DL. Dehydration, rehydration and overhydration alter patterns of gene expression in the Antarctic midge, Belgica antarctica. Journal of Comparative Physiology B. 2009;179:481–491. doi: 10.1007/s00360-008-0334-0. [DOI] [PubMed] [Google Scholar]
- Maddrell SHP. The site of release of the diuretic hormone in Rhodnius- a neurohaemal system in insects. Journal of Experimental Biology. 1966;45:499–508. [Google Scholar]
- Marinotti O, Calvo E, Nguyen QK, Dissanayake S, Ribeiro JM, James AA. Genome-wide analysis of gene expression in adult Anopheles gambiae. Insect Molecular Biology. 2005;15:1–12. doi: 10.1111/j.1365-2583.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- Marshall AG. The Ecology of Ectoparasitic Insects. Academic Press; New York: 1981. [Google Scholar]
- Mboera LE, Knols BGJ, Takken W, Huisman PWT. Olfactory responses of female Culex quinquefasciatus Say (Diptera: Culicidae) in a dual choice olfactometer. Journal of Vector Ecology. 1998;23:107–113. [PubMed] [Google Scholar]
- Melcón ML, Lazzari CR, Manrique G. Repeated plasticization and recovery of cuticular stiffness in the blood-sucking bug Triatoma infestans in the feeding context. Journal of Insect Physiology. 2005;51:989–993. doi: 10.1016/j.jinsphys.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Michaud MR, Denlinger DL. Oleic acid is elevated in cell membranes during rapid cold hardening and pupal diapause in the flesh fly, Sarcophaga crassipalpis. Journal of Insect Physiology. 2006;52:1073–1082. doi: 10.1016/j.jinsphys.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Michaud MR, Benoit JB, Lopez-Martinez G, Elnitsky MA, Lee RE, Jr., Denlinger DL. Metabolomics reveals unique and shared metabolic changes in response to heat shock, freezing, and desiccation in the Antarctic midge, Belgica antarctica. Journal of Insect Physiology. 2008;54:645–655. doi: 10.1016/j.jinsphys.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Munks RJL, Sant’Anna MRV, Grail W, Gibson W, Igglesden T, Yoshiyama M, Lehane SM, Lehane MJ. Antioxidant gene expression in the blood-feeding fly Glossina morsitans morsitans. Insect Molecular Biology. 2005;14:483–491. doi: 10.1111/j.1365-2583.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- Needham GR, Teel PD. Water balance of ticks between bloodmeals. In: Sauer JR, Hair JA, editors. Morphology, Physiology, and Behavioral Biology of Ticks. Horwood; Chichester, United Kingdom: 1986. pp. 100–151. [Google Scholar]
- Needham GR, Teel PD. Off-host physiological ecology of ixodid ticks. Annual Review of Entomology. 1991;36:659–681. doi: 10.1146/annurev.en.36.010191.003303. [DOI] [PubMed] [Google Scholar]
- Nicolson SW. Water balance and osmoregulation in Onymacris plana, a tenebrionid beetle from Namib Desert. Jounral of Insect Physiology. 1980;26:315–320. [Google Scholar]
- Noble-Nesbitt J. Cuticular permeability and its control. In: Binnington K, Retnakaran A, editors. The Physiology of the Insect Epidermis. CSIRO; Melbourne, Australia: 1991. pp. 262–283. [Google Scholar]
- O’Donnell MJ, Machin J. Water vapor absorption by terrestrial organisms. Advances in Comparative and Environmental Physiology. 1988;2:47–90. [Google Scholar]
- O’Donnell MJ, Maddrell SHP. Fluid reabsorption and ion transport by the lower Malpighian tubules of adult female Drosophila. Journal of Experimental Biology. 1995;198:1647–1653. doi: 10.1242/jeb.198.8.1647. [DOI] [PubMed] [Google Scholar]
- Orchard I. Serotonin: a coordinator of feeding-related physiological events in the blood-gorging bug, Rhodnius prolixus. Comparative Biochemistry and Physiology A. 2006;2006:316–324. doi: 10.1016/j.cbpa.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annual Review of Genetics. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Philip BN, Yi S-X, Elnitsky MA, Lee RE., Jr. Aquaporins play a role in desiccation and freeze tolerance in larvae of the goldenrod gall fly, Eurosta solidaginis. Journal of Experimental Biology. 2008;211:1114–1119. doi: 10.1242/jeb.016758. [DOI] [PubMed] [Google Scholar]
- Powell JR, Petrarca V, Della Torre A, Caccone A, Coluzzi M. Population structure, speciation, and introgression in the Anopheles gambiae complex. Parasitologia. 1999;41:101–113. [PubMed] [Google Scholar]
- Quesada-Allué L. Biochemistry of the integument. In: Brenner RR, Stoka AM, editors. Chagas’ Disease Vectors: Anatomic and Physiological Aspects. III. CRC Press; Boca Raton, FL, USA: 1987. [Google Scholar]
- Rebers JE, Willis JH. A conserved domain in arthropod cuticular proteins binds chitin. Insect Biochemistry and Molecular Biology. 2001;31:1083–1093. doi: 10.1016/s0965-1748(01)00056-x. [DOI] [PubMed] [Google Scholar]
- Reynolds SE. Pharmacological induction of plasticization in the abdominal cuticle of Rhodnius. Journal of Experimental Biology. 1974;61:705–718. doi: 10.1242/jeb.61.3.705. [DOI] [PubMed] [Google Scholar]
- Reynolds SE. The mechanism of plasticization of the abdominal cuticle of Rhodnius. Journal of Experimental Biology. 1975;62:81–98. doi: 10.1242/jeb.62.1.81. [DOI] [PubMed] [Google Scholar]
- Ribeiro JMC. Blood feeding arthropods: live syringes or invertebrate pharmacologist? Infectious Agents of Disease. 1995;4:143–152. [PubMed] [Google Scholar]
- Ribeiro JMC. Common problems of arthropod vectors of disease. In: Beaty BJ, Marquadt WC, editors. The Biology of Disease Vector. University Press of Colorado; Colorado: 1996. pp. 25–33. [Google Scholar]
- Ribeiro JMC, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annual Review of Entomology. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Garczynski SF, Crim JW, Hill CA, Brown MR. Neuropeptides and peptide hormones in Anopheles gambiae. Science. 2002;298:172–175. doi: 10.1126/science.1076827. [DOI] [PubMed] [Google Scholar]
- Romoser WS. The vector alimentary system. In: Beaty BJ, Marquadt WC, editors. The Biology of Disease Vector. University Press of Colorado; Niwot, Colorado: 1996. pp. 298–317. [Google Scholar]
- Rudenko N, Golovchenko M, Edwards MJ, Grubhoffer L. Differential expression of Ixodes ricinus tick genes induced by blood feeding or Borrelia burgdorferi infection. Journal of Medical Entomology. 2005;42:36–41. doi: 10.1093/jmedent/42.1.36. [DOI] [PubMed] [Google Scholar]
- Sanders HR, Evans AM, Ross LS, Gill SS. Blood meal induces global changes in midgut gene expression in the disease vector, Aedes aegypti. Insect Biochemistry and Molecular Biology. 2003;33:1105–1122. doi: 10.1016/s0965-1748(03)00124-3. [DOI] [PubMed] [Google Scholar]
- Simard F, Ayala D, Kamdem GC, Pombi M, Etouna J, Ose K, Fotsing J-M, Fontenille D, Besansky NJ, Constantini C. Ecological niche partitioning between Anopheles gambiae molecular forms in Cameroon: the ecological side of speciation. BMC Ecology. 2009;9:17. doi: 10.1186/1472-6785-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair BJ, Gibbs AG, Roberts SP. Gene transcription during exposure to, and recovery from, cold and desiccation stress in Drosophila melanogaster. Insect Molecular Biology. 2007;16:435–443. doi: 10.1111/j.1365-2583.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- Sláma K. Active regulation of insect respiration. Annals of the Entomological Society of America. 1999;92:916–929. [Google Scholar]
- Sonenshine DE. Biology of Ticks. Oxford University Press; New York: 1991. [Google Scholar]
- Spring JH, Albarwani SA. Excretion in the house cricket, Acheta domesticus: effect of cAMP on membrane dynamics, cell ultrastructure, and secretion. American Zoologist. 1993;40:1218–1229. [Google Scholar]
- Spring JH, Robichaux SR, Hamlin JA. The role of aquaporins in excretion in insects. Journal of Experimental Biology. 2009;212:358–362. doi: 10.1242/jeb.024794. [DOI] [PubMed] [Google Scholar]
- Stark KR, James AA. The salivary glands of disease vectors. In: Beaty BJ, Marquadt WC, editors. The Biology of Disease Vectors. University Press of Colorado; Colorado: 1996. pp. 333–348. [Google Scholar]
- Storey KB. Biochemical adaptations. In: Storey KB, editor. Functional Metabolism: Regulation and Adaptation. Wiley; Cambridge: 2004. pp. 383–413. [Google Scholar]
- Stys P, Damiel M. Lyctocoris campestris F. (Heteroptera: Anthocoridae) as a human facultative ectoparasite. Acta Societatis Entomologicae Cechosloveniae. 1957;54:1–10. [Google Scholar]
- Suemoto T, Kawai K, Imabayashi H. A comparison of desiccation tolerance among 12 species of chironomid larvae. Hydrobiologia. 2004;515:107–114. [Google Scholar]
- Tammariello SP, Rinehart JP, Denlinger DL. Desiccation elicits heat shock protein transcription in the flesh fly, Sacrophaga crassipalpis, but does not enhance tolerance to high or low temperature. Journal of Insect Physiology. 1999;45:933–938. doi: 10.1016/s0022-1910(99)00073-6. [DOI] [PubMed] [Google Scholar]
- Te Brugge V, Ianowski JP, Orchard I. Biological activity of diuretic factors on the anterior midgut of the blood-feeding bug, Rhodnius prolixus. General and Comparative Endocrinology. 2009;162:105–112. doi: 10.1016/j.ygcen.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Thiemann T, Fielden LJ, Kelrick MI. Water uptake in the cat flea, Ctenocephalides felis (Pulicidae: Siphonaptera) Journal of Insect Physiology. 2003;49:1085–1092. doi: 10.1016/s0022-1910(03)00153-7. [DOI] [PubMed] [Google Scholar]
- Touré YT, Petrarca V, Traoré SF, Coulibaly A, Maïga HM, Sankaré O, Sow M, Di Deco MA, Coluzzi M. Ecological genetic studies in the chromosomal form Mopti of Anopheles gambiae s.s. in Mali, West Africa. Genetica. 1994;94:213–223. doi: 10.1007/BF01443435. [DOI] [PubMed] [Google Scholar]
- Waage JK. The evolution of insect/vertebrate association. Biological Journal of the Linnaean Society. 1979;12:187–224. [Google Scholar]
- Watanabe M. Anhydrobiosis in invertebrates. Applied Entomology and Zoology. 2006;41:15–31. [Google Scholar]
- Wharton GW. Water balance of insects. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemsitry and Pharmacology. Vol. 4. Pergamon Press; Oxford: 1985. pp. 565–603. [Google Scholar]
- Wheelock GD, Petzel DH, Gillett JD, Beyenbach KB, Hagedorn HH. Evidence for hormonal control of diuresis after a bloodmeal in the mosquito Aedes aegypti. Archives of Insect Biochemistry and Physiology. 1988;7:75–90. [Google Scholar]
- White BJ, Hahn MW, Pombi M, Cassone BJ, Lobo NF, Simard F, Besansky NJ. Localization of candidate regions maintaining a common polymorphic inversion (2La) in Anopheles gambiae. PloS Genetics. 2007;3:217. doi: 10.1371/journal.pgen.0030217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JC, Hagedorn HH, Beyenbach KW. Dynamic changes in flow rate and composition of urine during the post-bloodmeal diuresis in Aedes aegypti (L.) Journal of Comparative Physiology A. 1983;153:257–265. [Google Scholar]
- Willmer PG. The effects of fluctuating environments on the water relations of larval Lepidoptera. Ecological Entomology. 1980;5:271–292. [Google Scholar]
- Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. Journal of Experimental Biology. 2005;208:2819–2830. doi: 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]
- Yoder JA, Denlinger DL. Water balance in the flesh fly pupae and water vapor absorption associated with diapause. Journal of Experimental Biology. 1991;157:273–286. [Google Scholar]
- Yoder JA, Spielman A. Differential capacity of larval deer ticks (Ixodes dammini) to imbibe water from subsaturated air. Journal of Insect Physiology. 1992;3:863–869. [Google Scholar]
- Yoder JA, Denlinger DL, Wolda H. Aggregation promotes water conservation during diapause in the tropical fungus beetle, Stenotarsus rotundus. Entomologia Expermentalis et Applicata. 1993;63:203–205. [Google Scholar]
- Yoder JA, Selim ME, Needham GR. Impact of feeding, molting, and relative humidity on cuticular wax deposition and water loss in the lone star tick, Amblyomma americanum. Journal of Insect Physiology. 1997;43:547–551. doi: 10.1016/s0022-1910(97)00006-1. [DOI] [PubMed] [Google Scholar]
- Yoder JA, Benoit JB, Rellinger EJ, Murray SA, Zettler LW. Water balance of a tick-fungus relationship, featuring the life cycle of the fungus Scopulariopsis brevicaulis (Sacc.) Bainier (Deuteromycota) in a tick host. International Journal of Acarology. 2004;30:93–101. [Google Scholar]
- Yoder JA, Buchan DR, Ferrari NF, Tank JT. Dehydration tolerance of the Rocky Mountain wood tick, Dermacentor andersoni Stiles (Acari: Ixodidae), matches preference for a dry environment. International Journal of Acarology. 2007;33:173–180. [Google Scholar]
- Yoder JA, Christensen BS, Croxall TJ, Schumaker LK, Tank JL. Moisture requirements for activity/survival of the gulf coast tick, Amblyomma maculatum (Acari:Ixodidae) based on a water balance study of all life cycle stages. International Journal of Acarology. 2009;35:13–20. [Google Scholar]
- Yoder JA, Benoit JB, Rellinger EJ, Tank JT. Developmental profiles in tick water balance with a focus on the Rocky Mountain spotted fever vector, Rhipicephalus sanguineus. Medical and Veterinary Entomology. 2006a;20:365–372. doi: 10.1111/j.1365-2915.2006.00642.x. [DOI] [PubMed] [Google Scholar]
- Yoder JA, Benoit JB, Denlinger DL, Rivers DB. Stress-induced accumulation of glycerol in the flesh fly, Sarcophaga bullata: Evidence indicating anti-desiccant and cryoprotectant functions of this polyol and a role for the brain in coordinating the response. Journal of Insect Physiology. 2006b;52:202–214. doi: 10.1016/j.jinsphys.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Yoder JA, Ark JT, Benoit JB, Rellinger EJ, Tank JT. Inability of the lone star tick, Amblyomma americanum (L.) to resist desiccation and maintain water balance following application of the entomopathogenic fungus Metarhizium anisopliae var. anisopliae (Deuteromycota) International Journal of Acarology. 2006c;32:211–218. [Google Scholar]
- Zachariassen KE, Einarson S. Regulation of body fluid compartments during dehydration of the tenebrionid beetle, Rhytinota praelonga. Journal of Experimental Biology. 1993;182:283–289. [Google Scholar]
- Zachariassen KE, Pedersen SA. Volume regulation during dehydration of desert beetles. Comparative Biochemistry and Physiology A. 2002;133:805–811. doi: 10.1016/s1095-6433(02)00193-9. [DOI] [PubMed] [Google Scholar]
- Zhang J, Goyer C, Pelletier Y. Environmental stresses induce the expression of putative glycine-rich insect cuticular protein genes in adult Leptinotarsa decemlineata (Say) Insect Molecular Biology. 2008;17:209–216. doi: 10.1111/j.1365-2583.2008.00796.x. [DOI] [PubMed] [Google Scholar]