Abstract
Based on the potential of resveratrol as a colon cancer chemopreventive agent, a set of 26 stilbenes were synthesized and tested against the colon cancer cell lines HT-29 and Caco-2. (Z)-4-(3,5-dimethoxystyryl)aniline (4), (Z)-methyl 4-(3,5-dimethoxystyryl)benzoate (6), and (Z)-1,3-dimethoxy-5-(4-methoxystyryl)benzene (10) showed strong inhibitory activity in vitro. In vivo studies using HT-29 xenografts in immunodeficient mice were conducted with 4, 6 and 10, together with their corresponding trans isomers (3, 5, and 9, respectively), at the dose of 10 mg/kg body weight. Tumor volume was significantly lowered in 3-, 4-, and 9-treated groups. The cis- and trans-amino analogs (4 and 3, respectively) had similar effect on tumor growth, a 40% decrease compared to the control. Analysis of the serum revealed that 4 isomerized to 3, which may explain their similar effects in SCID mice. Stilbenes 5, 6, 9, and 10 retained their configurations in the serum. Stilbenes 6 and 10 lacked tumor suppressive effect in SCID mice; the serum levels of these analogs were low (18.8 and 15.5 ng/mL, respectively). Stilbene 9, while weakly active in vitro demonstrated good activity in vivo; 9 was found at higher levels in the serum (69.9 ng/mL) compared to 10. The anti-tumorigenic activity of these stilbene analogs may be partly linked to their effects on proteins involved in cell proliferation, as observed by lowered expression of proliferating cell nuclear antigen and upregulation of the cyclin dependent kinase inhibitor, p27, in the tumor tissues. Overall, identification of the anti-tumorigenic potential of these compounds provides opportunities for their use against colorectal cancer.
Keywords: Stilbenes; 4-(3,5-dimethoxystyryl)aniline; HT-29 xenograft; colon cancer
1. Introduction
Resveratrol and pterostilbene are natural phenolic stilbenes found in foods and drink such as grapes, berries, peanuts, and wine. Stilbenes are defense compounds produced by some plants in response to pathogen attack and other stresses. In spite of being known for several years, resveratrol is still the subject of a number of studies and render scientists numerous publications every year. In the year 2008 alone, more than 900 papers have been published on resveratrol, which report a wide range of novel discoveries, such as new extraction methods [1,2] and new applications [3,4,5]. Among the most significant activities of resveratrol are its antioxidant [6] and anticarcinogenic [7] properties. Resveratrol is associated with the French Paradox, i.e., wine-drinking population with high intake of saturated fat shows low incidence of cardiovascular diseases. This effect has been attributed to resveratrol’s presence in wine, which has been reported to inhibit LDL oxidation in human [8], in addition to its blocking of platelet aggregation [9] and vasorelaxing activities [10]. In yeast assays, resveratrol was also found to significantly mimic calorie restriction by stimulating Sir2 and extended lifespan by 70% [11].
The chemopreventive property of resveratrol proposed by Jang et al. [12] was based on the inhibition of tumor initiation, promotion and progression. Analogs of resveratrol inhibit TNF-α induced activation of nuclear factor κB (NF-κB) [13]. Inhibition of activation of NF-κB is an approach used to treat diseases that have inflammatory component, such as cancer [14,15,16]. The anti-proliferative activity of resveratrol has been observed in several human cancer cell lines, mainly by disturbing progression through the S and G2 phases of the cell cycle [17]. Cell cycle regulation and induction of apoptosis are key check points in attempts to control tumorigenesis. The naturally-occurring stilbenes resveratrol, piceatannol, and pterostilbene have been identified to elicit these effects in several human cancer cells via a number of mechanisms which include G1, S phase arrests in cell cycle, modulation of levels of cyclins and the cyclin dependent kinases, and increase the cyclin dependent kinase inhibitor proteins of the Cip-Kip family [18–21].
Resveratrol exhibits proapoptotic activity in several cell lines including human leukemia [22] and breast cancer [23]. Resveratrol also inhibited proliferation of colon cancer cells ls174t [24] and significantly suppressed colon crypts in azoxymethane-induced aberrant colon crypt model [25]. Methoxylation has been suggested to significantly improve the anti-tumor potential of compounds. The greater the number of methoxy groups that are added, the better the anti-tumor activity of the compound becomes [26]. In an earlier study by our group we showed that pterostilbene, a dimethylether analog of resveratrol, suppressed the formation of colonic aberrant crypt foci in rats [27]. The synthetic stilbene analog trans-3,4,5,4′-tetramethoxystilbene (DMU-212) exhibited superior availability than resveratrol in the colon [28] and effectively reduced adenoma load in ApcMin/+ mice [29]. 3,5,4′-Trimethoxystilbene also presented greater anti-tumor activity than the parent compound, resveratrol, in COLO 205 tumor xenografts [21]. These reports provided impetus for us to explore the potential of other resveratrol analogs as colon cancer inhibitory agents or chemopreventive agents.
A comprehensive study was undertaken to evaluate the activity of a wide range of stilbenes, including analogs with different substituents at the C-4′ position such as amino, nitro, methoxy, hydroxyl, or halogens. The cis- isomers were compared alongside their trans- diastereomer. The stilbenes were initially tested for their potency against human HT-29 and Caco-2 colon cancer cell lines. With the exception of pterostilbene, resveratrol, and 3,5,4′-trimethoxystilbene, the compounds have not been tested for this activity. In the light of the data obtained from the in vitro assays, a few analogues were selected to determine their effects on HT-29 xenograft tumor growth in immunodeficient mice. Possible mechanism(s) for the observed tumor growth inhibition were also studied and reported here.
2. Results
2.1. In vitro activity against HT-29 and Caco-2 colon cancer cells
While most studies on stilbenes have focused on the trans isomers, it was interesting that in the present study the cis isomers were, in general, the most active in vitro (Table 1). The stilbenes showed similar activity against HT-29 and Caco-2 cells except for 6, which was very active against HT-29 cells (IC50 = 0.2 μM) but was weakly active against Caco-2 cells (IC50 = 14.71 μM). The cis trimethoxy stilbene derivative 10, which has been reported as a naturally-occurring compound [30], was the most inhibitory among all the stilbenes tested (IC50 = 0.04 and 0.08 μM in HT-29 and Caco-2 cells, respectively). The majority of the compounds showed better activity than resveratrol (17) and pterostilbene (16), both of which have been reported to prevent colon cancer development in animals [27,31]. With the exception of the carboxylic acids 7 and 8, desoxyrhapontigenins 13 and 14, and fluorides 21 and 22, where both isomers showed weak activity, the cis analogs of the diastereomeric pairs (1 and 2, 3 and 4, 5 and 6, 9 and 10, 19 and 20, 23 and 24, 25 and 26) had greater activity than the trans analogs. The compounds with dimethoxy substitution at C-3 and C-5 (9, 10 and 16) had better inhibitory activity than the corresponding analogs with hydroxyl substitution at the same positions (13, 14 and 17, respectively). Notably, while dimethoxy substituted analog 10 was very potent against HT-29 and Caco-2 cells the hydroxylated analog 14 had relatively weak activity.
Table 1.
IC50 values for stilbenes in HT-29 and Caco-2 colon cancer cell growth inhibition assay.
| Compound | IC50 (μM) against HT-29 cells | IC50 (μM) against Caco-2 cells |
|---|---|---|
| 1 | >30 | 22.87 ± 0.0 |
| 2 | 10.1 ± 0.0 | 18.43 ± 5.5 |
| 3 | 12.7 ± 2.0 | 19.57 ± 4.8 |
| 4 | 3.63 ± 0.8 | 2.61 ± 1.0 |
| 5 | 33.4 ± 4.6 | 42.64 ± 5.8 |
| 6 | 0.2 ± 0.05 | 14.71 ± 1.2 |
| 7 | >30 | >30 |
| 8 | >30 | >30 |
| 9 | 16.1 ± 5.0 | 11.95 ± 2.9 |
| 10 | 0.04 ± 0.01 | 0.08 ± 0.02 |
| 11 | 33.5 ± 2.4 | 19.21 ± 2.9 |
| 12 | >30 | >30 |
| 13 | 24.5 ± 5.5 | >30 |
| 14 | >30 | >30 |
| 15 | >30 | 19.59 ± 3.2 |
| 16 | 23.8 ± 3.1 | 14.46 ± 1.2 |
| 17 | 45.3 ± 4.4 | 24.35 ± 0.2 |
| 18 | >10 | >10 |
| 19 | >10 | >10 |
| 20 | 2.2 ± 1.0 | 7.1 ± 0.2 |
| 21 | >10 | >10 |
| 22 | >10 | >10 |
| 23 | >10 | >10 |
| 24 | 0.6 ± 0.01 | 0.59 ± 0.01 |
| 25 | >10 | >10 |
| 26 | 3.2 ± 0.7 | 2.95 ± 0.7 |
| 27 | >10 | >10 |
2.2. Anti-tumorigenic activity of the compounds against HT-29 xenograft tumor growth
Based on results from the in vitro assays 4, 6, and 10 were selected for in vivo testing against HT-29 xenograft tumor growth in severe combined immunodeficiency (SCID) mice. Furthermore, in our interest to determine whether cis-trans isomerization occurs in vivo, the corresponding trans isomers (3, 5, and 9, respectively) were also tested in SCID mice. The amino derivatives 3 and 4 showed the best activity, resulting in mice with the lowest tumor weight and tumor volume. Both compounds decreased tumor weight by 40% after 3 weeks (Table 2). The ester derivatives 5 and 6 did not demonstrate antitumor effects against HT-29 xenografts. Compound 9 had better tumor inhibitory effect than its cis isomer 10. Tumor weight was 21% lower and tumor volume was 45% lower in animals treated with 9 compared to the control. Tumor weight of animals treated with 10 was 15% lower than the control, but this effect was found to be not statistically significant.
Table 2.
Tumor growth inhibitory effect of selected stilbene analogs against HT-29 xenograft in SCID mice.
| Treatment* | No. of animals | Body weight at autopsy (grams) (mean ± S.E.) | Tumor weight (grams) (mean ± S.E.) | Tumor volume (mm3) (mean ± S.E.) |
|---|---|---|---|---|
| Control | 10 | 20.4 ± 0.4 | 0.72 ± 0.1 | 1087.0 ± 138.9 |
| 3 | 8 | 19.4 ± 0.6 | 0.43 ± 0.0 (p=0.011)‡ | 661.4 ± 76.0 (p=0.020)‡ |
| 4 | 8 | 20.0 ± 0.5 | 0.44 ± 0.1 (p=0.015)‡ | 592.7 ± 84.5 (p=0.010)‡ |
| 5 | 4 | 19.5 ± 0.7 | 0.78 ± 0.1 | 1321.5 ± 275.9 |
| 6 | 8 | 20.0 ± 0.8 | 0.76 ± 0.1 | 1148.9 ± 95.5 |
| 9 | 8 | 19.5 ± 0.7 | 0.57 ± 0.1 | 590.3 ± 87.2 (p=0.014)‡ |
| 10 | 8 | 19.8 ± 0.3 | 0.61 ± 0.1 | 1023.6 ± 199.4 |
HT-29 colon cancer cells were suspended in DMEM at a density of 107 cells/ml in a 1:1 (vol:vol) mix of DMEM Matrigel. Female SCID mice (7–8 week old) were inoculated subcutaneously in the hind flank with 0.1 ml (106 cells/animal) of cell suspension. Beginning the day after injection of the cells, the mice were treated with stilbenes intraperitoneally once daily for 3 weeks. The size of palpable lesions was measured twice a week with calipers. Tumors at autopsy were measured and weighed.
p is the value for the comparison of tumor weight and tumor volume in mice treated with stilbene analogs to the tumor measurements in control mice.
2.3. Analysis of stilbenes in the serum
GC-MS analysis of the serum from mice treated with the cis-amino analog 4 revealed this analog isomerized to 3, based on the retention time (10.0 min) and mass spectrum (m/z 255, [M]+) of the peak displayed, which was the same as that for the trans-amino analog 3. The serum levels of 4 and 3 were also similar (Table 3). Stilbenes 5, 6, 9, and 10 retained their configurations in the serum, as determined from their retention times and mass spectra, compared to standards. Stilbenes 6 and 10 demonstrated strong activity in vitro; however, both compounds did not demonstrate activity in SCID mice. This may be due to low levels of 6 and 10 in the serum (18.8 and 15.5 ng/mL, respectively). Stilbene 9 was found at a higher level in the serum (69.9 ng/mL). Thus, while only moderately active in vitro, this level may have been a sufficient concentration to provide tumor growth inhibition in SCID mice. Stilbene 5, while found in moderate levels in the serum, did not inhibit tumor growth. This observation is consistent with in vitro results, which showed only weak inhibition of the cells. A higher serum concentration of 5 may be needed for an effective dose.
Table 3.
Serum levels of stilbenes in SCID micea.
| Compound name (no.) | Serum levels (ng/mL) means ± std |
|---|---|
| Control | 0 |
| (E)-4-(3,5-dimethoxystyryl)aniline (3) | 99.0 ± 7.8 |
| (Z)-4-(3,5-dimethoxystyryl)aniline (4) | 109.1 ± 11.6b |
| (E)-methyl 4-(3,5-dimethoxystyryl)benzoate (5) | 54.6 ± 2.4 |
| (Z)-methyl 4-(3,5-dimethoxystyryl)benzoate (6) | 18.8 ± 5.3 |
| (E)-1,3-dimethoxy-5-(4-methoxystyryl)benzene (9) | 69.9 ± 0.3 |
| (Z)-1,3-dimethoxy-5-(4-methoxystyryl)benzene (10) | 15.5 ± 3.4 |
n = 4 for 4, 5, 6, 9, and 10; n = 5 for 3; n = 8 for control.
Value represents that of the trans isomer (3). No cis isomer (4) was detected in the serum.
2.4. Effects of the stilbenes on cell proliferation and cell cycle proteins
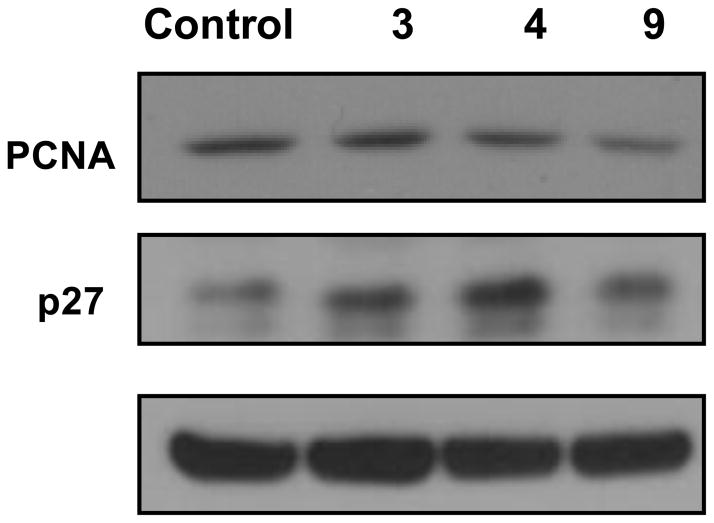
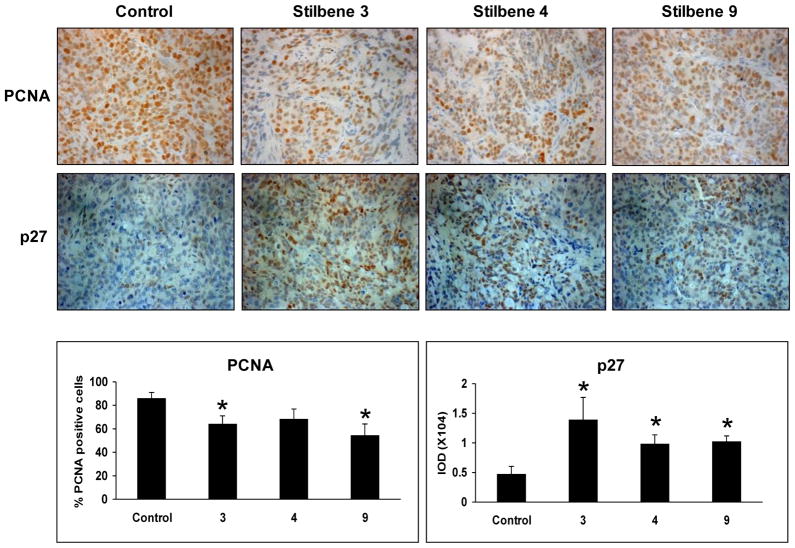
Analysis of the tumors was conducted for the stilbenes that demonstrated reduction in tumor growth (i.e., 3, 4 and 9). Figures 2 and 3 represent the effects of these stilbenes on the markers of cell proliferation by western blotting and immunohistochemistry, respectively. Proliferating cell nuclear antigen (PCNA) levels were lowered by 3, 4, and 9. p27 expression levels were upregulated by all the treatments as shown by western and immunohistochemical analysis and their presence was predominant in the nucleus. However, markers of apoptosis such as caspase-3 and PARP were unaltered by the compounds (data not shown).
Figure 2. Effect of stilbenes on molecular markers in HT-29 tumors by western blotting.
Tissue homogenates from individual mice in each treatment group were pooled together and analyzed by western blotting for PCNA and p27 as detailed under Experimental section. β-actin was used as the loading control.
Figure 3. Immunohistochemical staining of tumors and quantification.
A representative section of tumor tissue from the control group or groups treated with 3, 4, and 9 and observed for positive staining either in the nucleus or cytoplasm. PCNA: Positive cells in the nucleus (brown) and PCNA-negative cells (blue). Quantification to obtain PCNA labeling index (PI) was done as described under Experimental section. Data are represented as ± SE. p27: Positive brown staining in the nucleus. Staining intensity p27 was quantified based on the IOD values.
3. Discussion
The present study was able to identify a few stilbenes that possess superior growth inhibition than either resveratrol or pterostilbene of HT-29 and Caco-2 colon cancer cells in vitro. Barring a few exceptions, the cis analogs demonstrated greater activity than the trans isomers, as indicated by the IC50 values. Compounds with amino, ester, and methoxy substitutions at the 4′ position of the B ring exhibited good anti-proliferative effects in vitro. However, the tumor growth inhibitory effect in vivo was different from in vitro observations for the six compounds tested in SCID mice. The cis-amino analog 4, which showed only moderate activity in vitro, had the same effect as its trans-isomer 3 in vivo. This was because 4 isomerized to 3, as shown from the analysis of the serum of mice administered with 4. Compounds 3 and 4 were also detected at high levels in the serum, thus for these analogs, cis-trans isomerization and bioavailability appeared to be important factors for in vivo activity. Stilbenes 5, 6, 9, and 10 retained their configuration in the serum.
It is known that trans- and cis-stilbenes easily isomerize under the influence of light. However, substituents on the phenyl rings affect isomerization. 3,5-Dimethoxyl substitution in one of the phenyl rings has been shown to interfere with delocalization of π-electrons because of steric repulsion, thus isomerization is restricted [32, 33]. This could explain why compounds 5, 6, 9, and 10 kept their configurations. In the case of 4 ((Z)-4-(3,5-dimethoxystyryl)aniline), delocalization of the lone pair electron of the amino substituent in the other phenyl ring increases the single bond character of the vinyl double bond and twisting of the rings is induced [32, 34], which could explain the isomerization of 4 to 3 ((E)-4-(3,5-dimethoxystyryl)aniline). Irreversible cis-to-trans conversion is not unusual, this has been observed with some olefins of the series ArCH=CHt-Bu. Similar to stilbenes, the cis-trans isomerization depends on ethylenic substituents [35].
The cis isomers 6 and 10 demonstrated strong activity in vitro, but did not inhibit tumor growth in vivo. The lack of activity may be due to low bioavailability, thus low levels of 6 and 10 in the serum. The serum levels of 9 (69.9 ng/mL) were higher than that of its cis isomer 10; thus while 9 was only moderately active in vitro, it demonstrated good activity in vivo. Stilbene 5, although it was found in relatively high levels in the serum, did not show in vivo activity, consistent with the weak effects observed in vitro. A higher serum level of 5 may be needed for in vivo activity. These data suggest that for 5, 6, 9, and 10, bioavailability of the compounds may have influenced their in vivo activity. While there is a discrepancy between in vivo and in vitro results, it must be noted that our in vitro study first provided leads for the selection of the compounds to be investigated in vivo. The in vivo studies have discovered 3 and 4 with better anticancer activity than 9, which has been previously reported to have activity in vivo using COLO 205 tumor xenograft [21]. It is also worth noting that Pan et al. [21] used 50 mg/kg dose, while we showed that 9 reduced tumor growth at the dose of 10 mg/kg body weight. Moreover, although 3 and 4 have been evaluated in vitro against leukemia cell lines HL-60 [22] and nasopharyngeal carcinoma cell lines CNE-1 and CNE-2 [36], this is the first study to report the in vitro and in vivo activity of 3 and 4 against cancer of the colon.
Tumor analysis by Western blotting and immunohistochemistry suggests that the anti-tumor activity of 3, 4 and 9 may be predominantly associated with the effects on cell proliferation, as noted by lower expression of PCNA, with significant increase in p27 protein (Fig. 2). PCNA, being an auxiliary protein of DNA polymerase δ required for DNA synthesis during S-phase, is a useful marker in reporting cell proliferation [37]. These three compounds decreased the % of PCNA labeled cells. The stilbene analogs proved to be effective in lowering cell proliferation, although treatment with 4 did not show statistical significance.
p27, also known as kinase inhibitory protein (Kip 1) and related CDK inhibitor, p21 (CDK2 inhibitory protein 1 or CIP 1), regulates the G0-S transition in cell cycle [38]. These regulatory proteins when upregulated under certain conditions block the activation of CDKs by cyclins or can promote the assembly and nuclear import of cyclin D- CDK complexes [39]. Figures 2 and 3 show that treatment with 3, 4, and 9 increased the levels of these cell cycle inhibitors, and in particular very pronounced effects were observed with p27 expression. Immunohistochemistry showed nuclear localization and the compounds significantly increased the % of nuclei stained with p27. One of the earlier studies has identified p27 as an independent prognostic marker, particularly in stage 2 tumors [40]. Survival amongst patients with colorectal adenocarcinoma was up by approximately 60% for p27/Kip 1 positive patients compared to those who were negative [41]. The combined results from effects of 3, 4, and 9 on PCNA and p27 lead us to interpret that these compounds may induce G1 cell cycle arrest in HT-29 xenografts.
Overall, in the present study we were able to screen various stilbene analogs for their anti-proliferation effects against two different colon cancer cell lines. The in vivo administration of a few of these in immunodeficient mice with HT-29 xenograft further identified the more potent ones (3, 4, and 9). Interestingly, we also observed difference in efficacies between in vitro and in vivo systems, and notably that 4 isomerized to 3 in vivo. The significant lowering of the molecular markers involved in cell proliferation by the treatment with active stilbenoids underscore the relevance of further research of these interesting compounds and consideration for treatment of human colorectal cancer.
4. Experimental
4.1. Chemistry
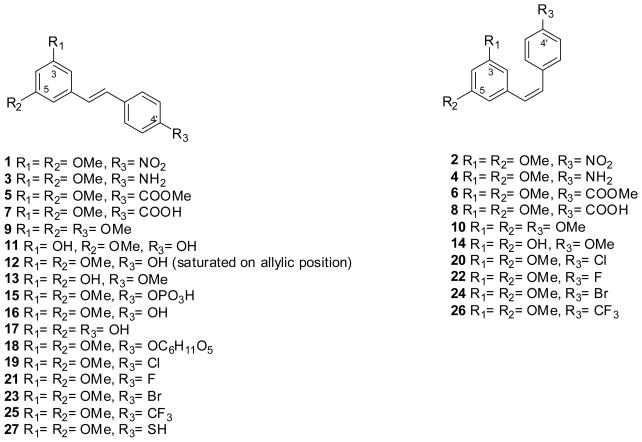
1H and 13C NMR were recorded on a Variant Oxford AS400 MHz spectrometer (operating at 400MHz for 1H and 100 MHz for 13C). Chemical shifts are reported as part per million (ppm) and the multiplicities are as follow: s (singlet), bs (broad singlet) d (doublet), t (triplet), q (quartet), m (multiplet). Melting points were recorded on a SRS Optimelt and were uncorrected. High resolution mass spectra were performed on a JEOL AccuTOF JMS-T100LC. Elemental analyses were performed with a Perkin Elmer series II CHNS/O analyzer 2400. HPLC chromatograms were obtained on an Agilent Technologies 1200series. Stilbenes 1–15 and 18–27 (Fig. 1) were synthesized via Wittig reaction of different phosphonium salts and aromatic aldehydes, as previously reported [42, 43]. Pterostilbene (16) was synthesized following published procedures [44]. Resveratrol (17) was a commercial sample (Sigma-Aldrich, Milwaukee, WI). For the in vitro assays, the stilbenes were dissolved in dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO), and the final concentration of DMSO in the assay system was 0.1% or less. The controls (DMSO alone) were included in all experiments.
Figure 1.
Chemical structures of stilbenes 1–27.
4.1.1 General procedure for stilbene synthesis
To a cold solution (−78°C) of phosphonium salt (1.0 equiv) in THF was added n-butyllithium [45] (1.6 mol in hexanes, 1.0 equiv) and the resulting solution was stirred under inert atmosphere for 2h. A solution of aldehyde (1.0 equiv) in THF was added dropwise, and the mixture was stirred for 12 h at room temperature. The resulting suspension was poured into water and extracted with dichloromethane. The organic phase was combined and dried over MgSO4 and concentrated under reduced pressure. The crude product was purified through automated flash purification eluting with hexanes/ethyl acetate (97:3). The cis isomer eluted first followed by the trans isomer.
4.1.1.1. (E)-4-(3,5-dimethoxystyryl)aniline (3)
Yellow solid (36% yield). Mp 96–98 °C. 1H NMR (CDCl3, 400 MHz): δ 3.82 (s, 6H); 6.37 (s, 1H); 6.65–6.68 (m, 4H); 6.86 (d, 1H, J = 16 Hz); 7.01 (d, 1H, J = 16 Hz); 7.33 (d, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ 55.5 (2C), 99.6, 104.4 (2C), 115.5 (2C), 125.2, 128, 128.1 (2C), 129.5, 140.2, 146.4, 161.2 (2C). HRMS: calcd for [M+H] C16H18NO2 256.13375, found 256.14321. Anal. for C16H17NO2, MW (255.31). Calcd.: % C, 75.27; H, 6.71; N, 5.49. Found: % C, 74.57; H, 6.52; N, 5.35.
4.1.1.2. (Z)-4-(3,5-dimethoxystyryl)aniline (4)
Yellow solid (47% yield). Mp 51–54 °C. 1H NMR (CDCl3, 400 MHz): δ 3.68 (s, 6H); 6.32 (s, 1H); 6.39 (s, 1H); 6.47 (m, 3H); 6.54 (d, 2H, J = 8 Hz); 7.11 (d, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ 55.4 (2C), 99.7, 106.8 (2C), 114.8 (2C), 127.6, 127.7, 130.4 (2C), 130.8, 140.1, 145.7, 160.7 (2C). HRMS: calcd for [M+H] C16H18NO2 256.13375, found 256.10757. Anal. for C16H17NO2, MW (255.31). Calcd.: %C, 75.27; H, 6.71; N, 5.49. Found: %C, 74.44; H, 5.82; N, 5.25.
4.1.1.3. (E)-Methyl-4-(3,5-dimethoxystyryl)benzoate (5)
White solid (27% yield). Mp 117–121 °C. 1H NMR (CDCl3, 400 MHz): δ 3.83 (s, 6H); 3.92 (s, 3H); 6.42 (s, 1H); 6.68 (s, 2H); 7.07–7.16 (m, 2H); 7.55 (d, 2H, J = 8 Hz); 8.02 (d, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ 52.3, 55.5 (2C), 100.7, 105.0 (2C), 126.6 (2C), 128.2, 129.1, 130.2 (2C), 131.4, 138.9, 141.8, 161.2 (2C), 167. HRMS: calcd for [2M+Na] C36H36NaO8 619.23079, found 619.22594. Anal. for C18H18O4, MW (298.33). Calcd.: % C, 72.47; H, 6.08. Found: % C, 72.28; H, 5.59.
4.1.1.4. (Z)- Methyl 4-(3,5-dimethoxystyryl)benzoate (6)
Viscous liquid (52% yield). 1H NMR (CDCl3, 400 MHz): δ 3.63 (s, 6H), 3.88 (s, 3H), 6.33 9s, 1H), 6.36 (s, 2H), 6.57 (q, 2H, J1,2 = 12 Hz), 7.32 (d, 2H, J = 8 Hz), 7.90 (d, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ 52.0, 55.1 (2C), 100.0, 106.7 (2C), 128.6, 128.9 (2C), 129.4 (2C), 129.5, 132.1, 138.4, 142.0, 160.6 (2C), 166.8. HRMS. calcd for [2M+Na] C36H36NaO8 619.23079, found 619.22729. Anal. for C18H18O4, MW (298.33). Calcd.: % C, 72.47; H, 6.08. Found: % C, 72.54; H, 5.96.
4.1.1.5. (E)-1,3-dimethoxy-5-(4-methoxystyryl)benzene (9)
White solid (9% yield). Mp 57–58 °C. 1H NMR (CDCl3, 400 MHz): δ 3.83 (s, 9H); 6.39 (s, 1H); 6.67 (s, 2H); 6.89 (s, 2H); 6.92 (d, 1H, J = 8 Hz); 7.02 (d, 1H, J = 8 Hz); 7.45 (d, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ 55.3 (3C), 99.6, 104.3 (2C), 114.1 (2C), 126.5, 127.8 (2C), 128.7, 129.9, 139.7, 159.4, 161.0 (2C). HRMS: calcd for [2M+Na] C34H36NaO6 563.24096, found 563.24419. Anal. for C17H18O3, MW (270.32). Calcd.: % C, 75.53; H, 6.71. Found: % C, 75.67; H, 6.84.
4.1.1.6. (Z)-1,3-dimethoxy-5-(4-methoxystyryl)benzene (10)
Viscous liquid (35% yield). 1H NMR (CDCl3, 400 MHz): δ 3.69 (s, 6H); 3.78 (s, 3H); 6.37 (s, 1H); 6.46–6.49 (m, 3H); 6.55 (d, 1H, J = 12 Hz); 6.80 (d, 2H, J = 8 Hz); 7.26 (d, 2H, J = 8 Hz). 13C NMR (CDCl3, 100 MHz): δ 55.1 (2C), 99.6, 106.6 (2C), 113.5 (2C), 128.7, 129.5, 130.2 (2C), 130.3 (2C), 139.5, 158.8, 160.6 (2C). HRMS: calcd for [2M+Na] C34H36NaO6 563.24096, found 563.24396. Anal. for C17H18O3, MW (270.32). Calcd.: %C, 75.53; H, 6.71. Found: %C, 75.37; H, 5.99.
4.2. Cell culture
Human colon carcinoma cell lines HT-29 and Caco-2 cells were obtained from the American Type Culture Collection (Manassas, VA). The cells were maintained in Dulbecco’s modification of Eagle’s medium (DMEM) supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C and 5% CO2.
4.3. Measurement of cell proliferation by [3H]thymidine incorporation
HT-29 cells or Caco-2 cells were plated at a density of 20,000 cells/well in a 24-well plate and treated with varying concentrations of stilbenes for 3 days at 37°C. Before harvest, the cells were incubated with 1 μCi [3H]thymidine for 4 h at 37°C and were washed with phosphate buffered saline. The cells were precipitated with cold 10% trichloroacetic acid for 10 min and solubilized with 0.5 mL solubilization buffer (0.2 M NaOH, 40 μg/L salmon sperm DNA) for 1 h at room temperature. The lysate was transferred to 5 mL Ecolume and the [3H]thymidine incorporated into the DNA of the cells was determined using a scintillation spectrometer (Beckman Coulter, Fullerton, CA).
4.4. In vivo assay using HT-29 xenograft tumor growth in severe combined immunodeficiency (SCID) mice
HT-29 colon cancer cells were trypsinized and suspended in DMEM at a density of 107 cells/mL in a 1:1 volume/volume mix of DMEM Matrigel (BD Biosciences, Bedford, MA). Seven to eight week old female severe combined immunodeficiency (SCID) mice were inoculated subcutaneously in the hind flank with 0.1 mL (106 cells/animal) of cell suspension. Beginning one day after injection of the cells, the mice were treated with vehicle control (0.1 mL cremophor: PBS [1:8] mixture) or stilbene analogs (10 mg/kg body weight in 0.1 mL vehicle) intraperitoneally once daily for 3 weeks. The size of palpable lesions was measured twice a week with calipers. Tumors at autopsy were measured and weighed. All animal studies were performed in accordance with an institutionally approved protocol.
4.5. Analysis of stilbenes in mice serum
Blood was collected into microvette (Sarstedt, Germany) at autopsy. The tubes were allowed to sit for 1 h at room temperature, and then centrifuged for 10 min at 2000 rcf (4000 rpm) in Eppendorf 5417 Microfuge to obtain serum. Mice sera were stored at −20°C until assayed. Sera were thawed in ice prior to analysis. To 50 μL of thawed serum was added 50 μL of sodium acetate trihydrate (1M, pH 5), then heated for 60 min at 37°C in water bath. Serum mixture was cooled to room temperature then partitioned with ethyl acetate (3x, 100 μL each). The combined ethyl acetate extracts was dried under a stream of nitrogen, and redissolved in 50 μL MeOH:CHCl (1:1) for analysis by gas chromatography-mass spectrometry (GC-MS).
GC-MS analysis was performed using a JEOL GCMate II System (JEOL USA Inc., Peabody, MA) with a J&W DB-5 capillary column (0.25 mm internal diameter, 0.25 μm film thickness, 30 m length; Agilent Technologies, Foster City, CA). The GC temperature program was as follows: initial 190°C, raised to 240°C at a rate of 20°C/min, then raised to 280°C at the rate of 2°C/min, then finally raised to 300°C at the rate of 10°C/min. The carrier gas was ultra high purity helium at a flow rate of 1.0 mL/min. The inlet (splitless), GC interface, and ion chamber temperatures were 250, 300, and 300°C, respectively. The sample injection volume used was 2.0 μL. The retention times for the stilbenes are: 3, 10.0 min; 4, 6.1 min; 5, 11.2 min; 6, 6.7 min; 9, 8.2 min; and 10, 5.1 min. Quantitative analyses (Table 3) were performed from calibration curves of the individual stilbene, as external standards, monitored at the m/z 255 for 3 and 4, m/z 298 for 5 and 6, and m/z 270 for 9 and 10.
4.6. Western Blot Analysis
The tumor samples were homogenized in lysis buffer and the samples from each group were pooled together, as previously described [46]. Equal protein was loaded into and resolved on 4–15% SDS-PAGE gels (Biorad, Hercules, CA), transferred to a polyvinylidene fluoride (PVDF) membrane. Membranes was blocked with 5% nonfat dry milk in Tris buffer for 1 h and then incubated with primary antibody solutions overnight at 4°C. The membranes were incubated with horseradish peroxidase conjugated secondary antibody solutions for 1 h at room temperature and washing with Tris buffer in between and after incubation with antibodies. The protein bands were visualized using a chemiluminescence based kit from Amersham Biosciences (Buckinghamshire, UK). The primary antibodies against PCNA (BD PharMingen, San Diego, CA), p27 (Santa Cruz Biotechnology, Santa Cruz, CA), and actin (Sigma, St. Louis, MO) and secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were used.
4.7. Immunohistochemistry
The procedures were done as described previously [27]. The tumor samples were embedded in paraffin, microtomed into 4 μm thick sections followed by the steps for hydration, antigen retrieval and blocking of endogenous peroxidase. The sections were incubated overnight at room temperature with the primary antibodies against proliferating cell nuclear antigen, PCNA (1:1000 diluted), and p27 (1:50 diluted). The slides were incubated with the appropriate biotinylated secondary antibody, and then with avidin/biotinylated peroxidase complex for 30 min at room temperature (Vector Labs, Burlingame, CA). Incubation of the slides with with 3′-diaminobenzamine substrate was performed followed by counterstaining with modified Harris hematoxylin. The images were taken randomly at 400x using Zeiss AxioCam HRc camera fitted to a Zeiss Axioskope 2 Plus microscope. Nuclear staining was observed with PCNA and p27.
4.8. Quantification of immunohistochemical staining
Image-pro plus 6.2 software (Media Cybernetics, Inc., Bethesda, MD) was used for quantifying the images obtained after immunohistochemistry of the tumor samples. Positive red staining or negative blue counterstaining was assigned a unique color using the hue, saturation, intensity (HSI) model under the segmentation feature of the software. Threshold values enable to filter the dark positive staining from the background light staining. Five random fields per slide were selected at 400X magnification. PCNA staining was analyzed based on the cell counts, and reported as the PCNA labeling index (PI) which is [(number of positive cells)/(total number of cells)] × 100 for each field, which was averaged to get the PI for each tumor sample. Integrated optical density (IOD) calculated by the software for each field which was averaged to obtain the IOD for each tumor sample and finally for each treatment group, was used to report the staining intensity for and p27.
4.9. Statistical analysis
The statistical significance for each treatment compared to the control group was measured using Student’s t-test. p<0.05 was considered as significant in all the cases.
Supplementary Material
Acknowledgments
This work was supported in part by grant from the Trustees Research Fellowship Program at Rutgers, The State University of New Jersey.
We would like to thank Gloria Hervey for her excellent technical help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xiang H-y, Dai K-j, Luo Q-z, Xuebao Zhongnan Daxue. Ziran Kexueban. 2008;39:700–704. [Google Scholar]
- 2.Zheng K, Zheng X, Wu Q, Wenli Baoji, Xuebao Xueyuan. Ziran Kexueban. 2008;28:110–115. [Google Scholar]
- 3.Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, Kim S, Xu X, Zheng Y, Chilton B, Jia R, Zheng ZM, Appella E, Wang XW, Ried T, Deng CX. Cancer Cell. 2008;14:312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha-Gonzalez HI, Ambriz-Tututi M, Granados-Soto V. CNS Neuro Ther. 2008;14:234–247. doi: 10.1111/j.1755-5949.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vieira de Almeida LM, Pineiro CC, Leite Marina MC, Brolese G, Leal RB, Gottfried C, Goncalves C-A. Neurochem Res. 2008;33:8–15. doi: 10.1007/s11064-007-9399-5. [DOI] [PubMed] [Google Scholar]
- 6.Stivala LA, Savio M, Carafoli F, Perucca P, Bianchi L, Maga G, Forti L, Pagnoni UM, Albini A, Prosperi E, Vannini V. J Biol Chem. 2001;276:22586–22594. doi: 10.1074/jbc.M101846200. [DOI] [PubMed] [Google Scholar]
- 7.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CWW, Fong HHS, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Science (Washington, D C) 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 8.Arichi H, Kimura Y, Okuda H, Baba K, Kozawa M, Arichi S. Chem Pharm Bull (Tokyo) 1982;30:1766–1770. doi: 10.1248/cpb.30.1766. [DOI] [PubMed] [Google Scholar]
- 9.Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. Clin Chim Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- 10.Chen CK, Pace-Asciak CR. Gen Pharmacol. 1996;27:363–366. doi: 10.1016/0306-3623(95)02001-2. [DOI] [PubMed] [Google Scholar]
- 11.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang L-L, Scherer B, Sinclair DA. Nature (London, United Kingdom) 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 12.Jang M, Pezzuto JM. Drug Exp Clin Res. 1999;25:65–77. [PubMed] [Google Scholar]
- 13.Heynekamp JJ, Weber WM, Hunsaker LA, Gonzales AM, Orlando RA, Deck LM, Vander Jagt DL. J Med Chem. 2006;49:7182–7189. doi: 10.1021/jm060630x. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto Y, Gaynor RB. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HJ, Hawke N, Baldwin AS. Cell Death Differ. 2006;13:738–747. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- 16.Viatour P, Merville MP, Bours V, Chariot A. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh TC, Wu JM. Exp Cell Res. 1999;249:109–115. doi: 10.1006/excr.1999.4471. [DOI] [PubMed] [Google Scholar]
- 18.Kundu JK, Surh Y-Y. Cancer Lett (Shannon, Irel) 2008;269:243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 19.Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB. Clin Cancer Res. 2002;8:893–903. [PubMed] [Google Scholar]
- 20.Wolter F, Clausnitzer A, Akoglu B, Stein J. J Nutr. 2002;132:298–302. doi: 10.1093/jn/132.2.298. [DOI] [PubMed] [Google Scholar]
- 21.Pan MH, Gao J-H, Lai CS, Wang YJ, Chen WM, Lo CY, Wang M, Dushenkov S, Ho C-T. Mol Carcinog. 2008;47:184–196. doi: 10.1002/mc.20352. [DOI] [PubMed] [Google Scholar]
- 22.Roberti M, Pizzirani D, Simoni D, Rondanin R, Baruchello R, Bonora C, Buscemi F, Grimaudo S, Tolomeo M. J Med Chem. 2003;46:3546–3554. doi: 10.1021/jm030785u. [DOI] [PubMed] [Google Scholar]
- 23.Minutolo F, Sala G, Bagnacani A, Bertini S, Carboni I, Placanica G, Prota G, Rapposelli S, Sacchi N, Macchia M, Ghidoni R. J Med Chem. 2005;48:6783–6786. doi: 10.1021/jm050528k. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Dong X-s, Guo X-s. Chem Res Chin Univ. 2008;24:756–761. [Google Scholar]
- 25.Steele VE, Wargovich MJ, McKee K, Sharma S, Wilkinson BP, Wyatt GP, Gao P, Kelloff GJ. Pharm Bio (Lisse, Netherlands) 1998;36:62–68. [Google Scholar]
- 26.Gosslau A, Chen M, Ho CT, Chen KY. Br J Cancer. 2005;92:513–521. doi: 10.1038/sj.bjc.6602300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh N, Paul S, Hao X, Simi B, Xiao H, Rimando AM, Reddy BS. Clin Cancer Res. 2007;13:350–355. doi: 10.1158/1078-0432.CCR-06-1528. [DOI] [PubMed] [Google Scholar]
- 28.Sale S, Verschoyle RD, Boocock D, Jones DJ, Wilsher N, Ruparelia KC, Potter GA, Farmer PB, Steward WP, Gescher AJ. Br J Cancer. 2004;90:736–744. doi: 10.1038/sj.bjc.6601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sale S, Tunstall RG, Ruparelia KC, Potter GA, Steward WP, Gescher AJ. Int J Cancer. 2005;115:194–201. doi: 10.1002/ijc.20884. [DOI] [PubMed] [Google Scholar]
- 30.MacRae WD, Towers GHN. Phytochemistry. 1985;24:561–566. [Google Scholar]
- 31.Kineman BD, Au A, Paiva NL, Kaiser MS, Brummer EC, Birt DF. Nut Cancer. 2007;58:66–74. doi: 10.1080/01635580701308208. [DOI] [PubMed] [Google Scholar]
- 32.Majima T, Tojo S, Ishida A, Takamuku S. J Phys Chem. 1996;100:13615–13623. [Google Scholar]
- 33.Tojo S, Morishima K, Ishida A, Majima T, Takamuku S. J Org Chem. 1995;60:4684–4685. [Google Scholar]
- 34.Lewis FD, Weigel W, Zuo X. J Phys Chem A. 2001;105:4691–4696. [Google Scholar]
- 35.Arai T, Karatsu T, Misawa H, Kuriyama Y, Okamotoa H, Hiresaki T, Furuuchi H, Zeng H, Sakuragi H, Tokumaru K. Pure & Appl Chem. 1988;60:989–998. [Google Scholar]
- 36.Liu H, Dong A, Gao C, Tan C, Liu H, Zu X, Jiang Y. Bioorg Med Chem. 2008;16:10013–10021. doi: 10.1016/j.bmc.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 37.Shimazaki N, Yazaki T, Kubota T, Sato A, Nakamura A, Kurei S, Toji S, Tamai K, Koiwai O. Genes Cells. 2005;10:705–715. doi: 10.1111/j.1365-2443.2005.00868.x. [DOI] [PubMed] [Google Scholar]
- 38.Chu IM, Hengst L, Slingerland JM. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 39.Sherr CJ, Roberts JM. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 40.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 41.Noguchi T, Kikuchi R, Ono K, Takeno S, Moriyama H, Uchida Y. Oncol Rep. 2003;10:827–831. [PubMed] [Google Scholar]
- 42.Mizuno CS, Ma G, Khan S, Patny A, Avery MA, Rimando AM. Bioorg M ed Chem. 2008;16:3800–3808. doi: 10.1016/j.bmc.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 43.Mizuno CS, Schrader KK, Rimando AM. J Ag Food Chem. 2008;56:9140–9145. doi: 10.1021/jf801988p. [DOI] [PubMed] [Google Scholar]
- 44.Joseph JA, Fisher DR, Cheng V, Rimando AM, Shukitt-Hale B. J Agric Food Chem. 2008;56:10544–10551. doi: 10.1021/jf802279h. [DOI] [PubMed] [Google Scholar]
- 45.Pettit GR, Grealish MP, Jung MK, Hamel E, Pettit RK, Chapuis JC, Schmidt JM. J Med Chem. 2002;45:2534–2542. doi: 10.1021/jm010119y. [DOI] [PubMed] [Google Scholar]
- 46.Lee HJ, Ju J, Paul S, So JY, DeCastro A, Smolarek A, Lee MJ, Yang CS, Newmark HL, Suh N. Clin Cancer Res. 2009;15:4242–4249. doi: 10.1158/1078-0432.CCR-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.