Abstract
Postnatal lung growth and development has primarily been evaluated from a very limited number of autopsied lungs; however, it still remains unclear whether alveolarization of the lung is complete during infancy and whether the conducting airways grow proportionately. The purpose of our study was to evaluate lung growth and development in vivo in infants and toddlers using multi-slice computed tomography.
Thirty-eight subjects (14 male, 24 female) aged 24–142 weeks had low-dose volumetric HRCT imaging at an inflation pressure of 20 cmH2O during an induced respiratory pause. Lung volume and weight were determined, as well as airway dimensions (inner and outer area, and wall area) for the trachea and next 3–4 generations. Lung volume, air volume, and tissue volume increased linearly with body length. The air and tissue components of the lung parenchyma increased at a constant rate with each other. In addition, airway caliber decreased with increasing generation from the trachea into each lobe. Airway caliber also correlated with body length; however, there was no interaction effect between airway generation and body length on transformed airway size. Our in vivo assessment suggests that growth of the lung parenchyma in infants and toddlers occurred with a constant relationship between air volume and lung tissue, which is consistent with lung growth occurring primarily by the addition of alveoli, rather than expansion of alveoli. In addition, the central conducting airways grow proportionately in infants and toddlers. This information may be important for evaluating subjects with arrested lung development.
INTRODUCTION
The lung undergoes significant growth and development early in life with increases in lung volume and airway size. Increases in lung volume with somatic growth can occur by alveolarization or by expansion in size of existing alveoli. The former should produce a relatively constant relationship between volume of air and parenchymal tissue, while the later should produce a greater increase in volume relative to parenchymal tissue. Most of our knowledge about lung structure for infants and toddlers has been derived from a very limited number of morphometric studies of autopsied lungs, which has provided conflicting results as to whether alveolarization of the lung is complete during infancy (1–6). In addition, morphometric studies have provided relatively limited data on growth of the conducting airways early in life(7–8). In contrast to assessing lung structure from autopsied lungs, in adults and cooperative older children, lung structure has been evaluated in vivo using high resolution computed tomography (HRCT), which can provide a quantitative assessment of the lung parenchyma, as well as airway dimensions(9–13). Lung structure is dependent upon the lung volume at which the measurements are obtained and thus needs to be standardized to interpret the results. Several studies have used multi-slice computed tomography to obtain quantitative measurements of lung structure in healthy infants and toddlers; however, these studies often had varying limitations: imaging obtained during tidal breathing, images for subjects not obtained at the same lung volume, only a few lung slices or airways imaged, and the evaluation of relatively few subjects less than 3 years old(14–16). The purpose of our study was to assess post-natal lung growth in infants and toddlers using volumetric scans obtained at a standardized inflation pressure during an induced respiratory pause. We hypothesized that if lung growth in infants and toddlers occurs primarily by alveolarization, then the volume of air in the lung will increase linearly with the increase in the amount of parenchymal tissue. In addition, we evaluated whether the size of the central conducting airways increase proportionate to each other during this period of rapid lung growth. Lastly, the data obtained would provide normative reference values for evaluating infants and toddlers with lung disease. This information may be important for future studies evaluating lung growth in subjects with arrested lung development, such as chronic lung disease of infancy, as well as outcomes for clinical research studies evaluating mechanisms that stimulate lung growth.
METHODS
Subjects
Subjects less than 3 years of age who were scheduled to obtain a CT scan under sedation, but not of the chest, were recruited from the Department of Radiology, to obtain an additional non-clinical CT scan of the chest; the medical diagnosis for the clinically scheduled non-chest CT are summarized in Table 1. Subjects were excluded if born < 37 weeks gestation, had congenital cardio-respiratory abnormalities, history of wheezing, hospitalization for respiratory illness, or use of asthma medications. The study was approved our Institutional Review Board in 2005 and signed consent was obtained from the parents.
Table 1.
| Subject # | Length (cm) | Weight (Kg) | Age (wks) | Gender | Race | Reason for CT scan |
|---|---|---|---|---|---|---|
| 1 | 64.8 | 5 | 17 | F | Non Caucasian | Sarcoma |
| 2 | 66 | 7.3 | 24 | F | Non Caucasian | Hearing loss |
| 3 | 67.5 | 7.8 | 26 | M | Caucasian | Scoliosis |
| 4 | 68 | 7.3 | 27 | M | Caucasian | Abnormal Skull Shape |
| 5 | 66.5 | 6.4 | 29 | M | Caucasian | Microtia |
| 6 | 66.8 | 7 | 30 | F | Caucasian | Hearing loss |
| 7 | 64.5 | 8.6 | 31 | F | Non Caucasian | Hearing loss |
| 8 | 68.25 | 8.4 | 32 | M | Non Caucasian | Macrocephalus |
| 9 | 72 | 8.2 | 40 | M | Caucasian | Hearing loss |
| 10 | 75.5 | 12.1 | 43 | M | Caucasian | Dermoid cyst |
| 11 | 70.6 | 9.09 | 43 | F | Caucasian | Hearing loss |
| 12 | 68 | 10 | 45 | M | Caucasian | Craniosynostosis |
| 13 | 72.5 | 9.5 | 46 | M | Non Caucasian | Sarcoma |
| 14 | 75.8 | 9.6 | 47 | M | Non Caucasian | Hearing loss |
| 15 | 75 | 9.5 | 49 | M | Caucasian | Hearing loss |
| 16 | 75 | 10.9 | 50 | M | Caucasian | Hepatoma |
| 17 | 78.2 | 10.9 | 51 | M | Non Caucasian | Abnormal Skull Shape |
| 18 | 75.6 | 10 | 54 | F | Caucasian | Torticollis |
| 19 | 72.5 | 9 | 61 | F | Caucasian | Preauditory tag |
| 20 | 83 | 10.9 | 66 | F | Caucasian | Histiocytosis |
| 21 | 84 | 11.8 | 73 | F | Non Caucasian | Dermoid cyst |
| 22 | 78.5 | 8.2 | 78 | F | Caucasian | Sarcoma |
| 23 | 81.5 | 10.9 | 79 | F | Non Caucasian | Synostosis |
| 24 | 69.7 | 7.7 | 79 | F | Caucasian | Hearing loss |
| 25 | 81.3 | 11.8 | 80 | F | Caucasian | Thrombocytopenia |
| 26 | 80.5 | 11.8 | 86 | M | Non Caucasian | Plagiocephaly |
| 27 | 88 | 12.7 | 87 | F | Caucasian | Retinoblastoma |
| 28 | 86 | 14.1 | 91 | F | Caucasian | Neuroblastoma |
| 29 | 83.6 | 11.3 | 93 | F | Non Caucasian | Hearing loss |
| 30 | 84.3 | 15.1 | 99 | F | Non Caucasian | Neuroblastoma |
| 31 | 83.8 | 14.6 | 100 | F | Caucasian | Neuroblastoma |
| 32 | 87.6 | 10.7 | 103 | F | Caucasian | Microtia |
| 33 | 86 | 11.3 | 105 | F | Non Caucasian | Rhabdomyosarcoma |
| 34 | 90.5 | 12.5 | 113 | F | Caucasian | Hearing loss |
| 35 | 90 | 13.2 | 120 | F | Non Caucasian | Hearing loss |
| 36 | 91.7 | 12.5 | 124 | F | Non Caucasian | Lymphangioma |
| 37 | 88.5 | 12.3 | 125 | F | Non Caucasian | Hearing loss |
| 38 | 92.25 | 14.5 | 142 | M | Caucasian | Club feet |
High Resolution imaging
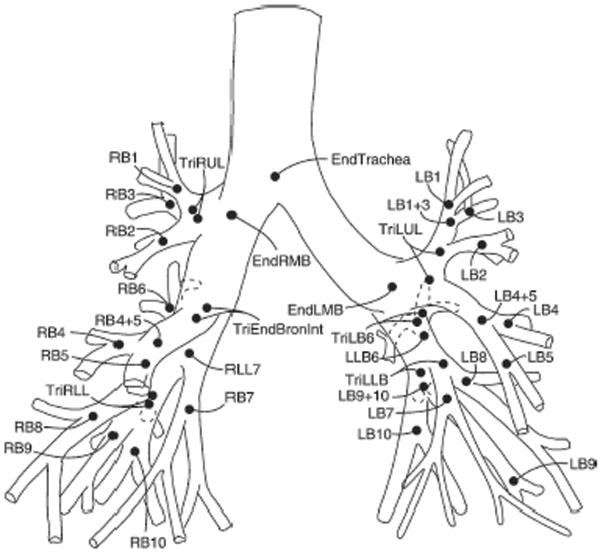
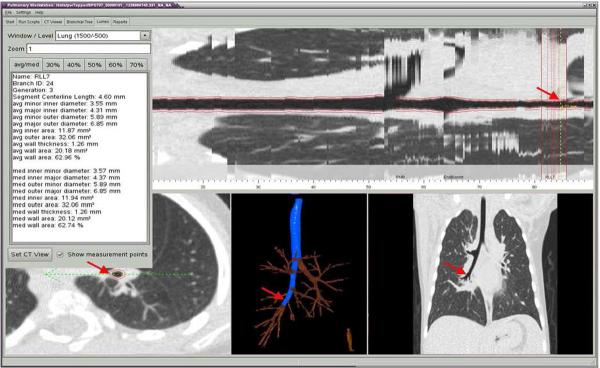
Multi-slice volumetric acquisition of images were obtained during an induced respiratory pause at an elevated lung volume defined by an airway pressure of 20 cmH2O(17–18) using a light speed Ultra 16 scanner (GE Healthcare; Milwaukee) with settings of 120 KV, 20 MAS, 0.625mm collimation thickness, pitch 1.2, and rotation speed 0.5 second. Images were reconstructed using both a high resolution and a standard algorithm (Sharp and normal kernels) with slice thickness of 0.625 mm. All patients had bismuth shielding to decrease dose to the breast and thyroid. Radiation exposure from chest CT imaging was estimated as 4.8 mGy. Patient imaging data were de-identified before scans were analyzed using automated, quantitative software, which segmented the airway tree for each lobe and the segments labeled as illustrated in Figure 1 (VIDA diagnostics: Iowa City, IA)(19–20). Each airway segment could be localized, and the inner and outer areas were measured and expressed as the average over the middle third of the segment, as indicated in Figure 2. Wall area was calculated as the difference between the inner and outer areas. Measurements were obtained for the trachea and the next 3–4 segments for each lobe. The lung parenchyma was segmented from the chest wall and the hilar structures using attenuation values between −910 to −200 Hounsfield units (HU). Lung volume was calculated by summing the voxels within the lung, while lung density was calculated from the X-ray attenuation values and lung tissue weight was calculated by multiplying the lung volume by lung density. Tissue volume was calculated as lung or tissue weight divided by tissue density, which was assumed to be 1.065 gm/ml; air volume was obtained as lung volume minus tissue volume.
Figure 1.
Airway tree and nomenclature used for airway segments(20).
Figure 2.
Illustration of different views of Vida software for localizing airway segment and measurements of inner and outer airway dimensions.
Statistical Analysis
Demographic and patients characteristics were summarized by mean +/− SD for continuous variables and frequency tables for discrete variables. Simple linear regression models was used to evaluate the association between airway size (measured by average inner area, average outer area, average wall area, and average area ratio) and body length. Bonferroni's method was used to adjust for the multiple comparisons of tests on the four measurements of each airway(21). Similar analysis was performed to evaluate whether body weight, or age were better covariates than body length; however, body length was the best predictor of airway size (result not shown). To evaluate whether the somatic size effect of airway growth is different (disproportional) across airway generation, we fit a multiple linear regression model using natural log transformed airway size of all generation. The interaction terms between body length and generations represent the disproportional body length effect among generations. This analysis was done for each lobe, separately. Simple linear regression models were used to evaluate association between body length and each of the total lung volume, air volume, and total lung weight. SAS 9.1.3 software (SAS Institute, Inc, Cary, NC) was used to conduct all of the analysis.
RESULTS
Subjects
Thirty-eight subjects (14 males and 24 females) between 17 weeks and 142 weeks of age were evaluated between 2007 and 2009. Individual demographics and the indications for the clinically scheduled CT scans are summarized in Table 1.
Lung Parenchyma
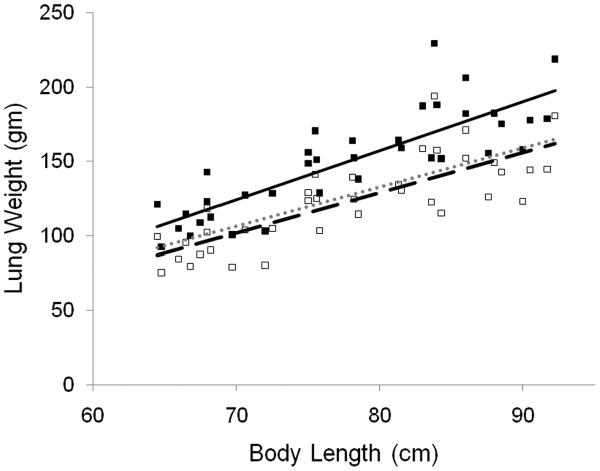
Total lung volume, air volume, and tissue volume increased with increasing body length, as illustrated in Figure 3 and the regressions are summarized in Table 2. After adjusting for body length, gender and race were not significant. Figure 4 illustrates the individual values for lung weight calculated from the CT scan, the regression line for this in vivo data, as well as a regression line generated from published data for weights of autopsied lungs(22). The in vivo values for lung weight calculated from the CT scans averaged 17% greater than published values from autopsied lungs. Each subjects pulmonary capillary blood volume was estimated by extrapolating published data in children adjusting for body surface area(23). Subtracting the estimated pulmonary capillary blood volume yielded in vivo lung tissue weights from CT scans that were very similar to values for autopsied lungs (Figure 3).
Figure 3.
Total parenchymal lung volume (ml), parenchymal air volume (ml), and parenchymal tissue volume (ml) versus body length (cm). All three parameters increased significantly with increasing body length (Table 3).
Table 2.
| Outcome | Intercept | Std Err Intercept | Length (cm) | p value | R2 | N |
|---|---|---|---|---|---|---|
| Total Lung Volume (cm3) | −718.62 | 151.47 | 17.07 | <.0001 | 0.68 | 38 |
| Air Volume (cm3) | −613.40 | 133.23 | 13.90 | <.0001 | 0.65 | 38 |
| Tissue Volume (cm3) | −105.22 | 29.11 | 3.17 | <.0001 | 0.67 | 38 |
| Lung Tissue weight (gram) | −112.05 | 31.01 | 3.38 | <.0001 | 0.67 | 38 |
| Hounsfield units | −651.94 | 45.49 | −1.32 | 0.0289 | 0.13 | 38 |
Figure 4.
Lung tissue weight versus body length (cm).
(■) lung weights calculated from CT images and linear regression ( ); (□) lung weights calculated from CT adjusted for pulmonary blood volume, and linear regression (
); (□) lung weights calculated from CT adjusted for pulmonary blood volume, and linear regression ( ); linear regression for weight from autopsied lungs (……).
); linear regression for weight from autopsied lungs (……).
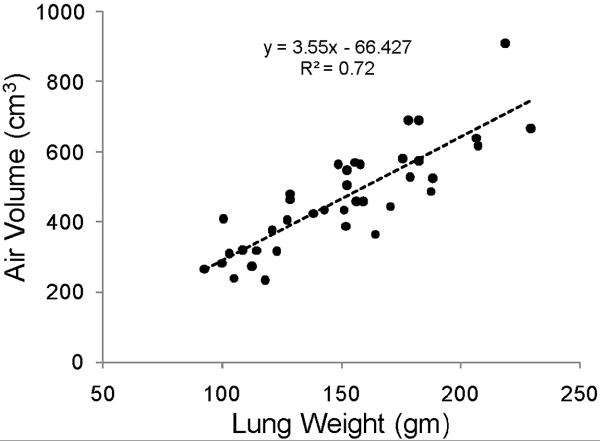
As the air and parenchymal tissue may increase at different rates with lung growth and development, we analyzed the relationship between these two components of the lung parenchyma. Figure 5 illustrates a linear increase in air volume and parenchymal tissue weight with a slope of 3.6 cm3 of air/gm tissue, which has been referred to as lung expansion, the inverse of lung density.
Figure 5.
Parenchymal air volume (ml) increased linearly with parenchymal tissue weight (gm). The slope of the linear regression, lung expansion, was 3.6 ml of air/gm tissue.
Airways
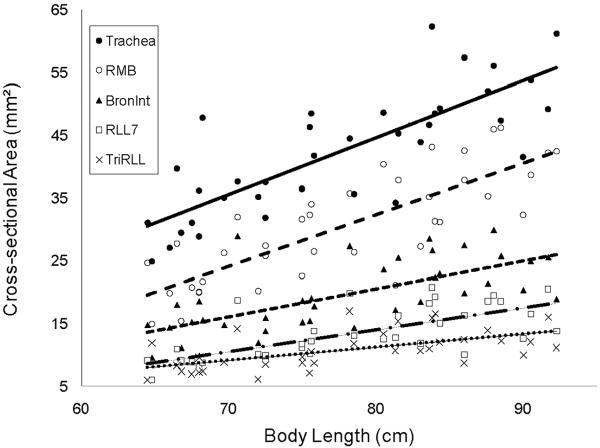
The lumen cross-sectional areas for the trachea and the next four airway segments into the right lower lobe, which is the pathway evaluated with the largest diameters, are illustrated in Figure 6. The maximal and minimal lumen diameters calculated from the cross-sectional areas ranged from 8.9 mm to 2.7 mm. Trachea (gen 0), Right main bronchus (RMB) (gen 1), Bronchus intermedius (Bronint) (gen 2), RLL7 (gen 3), and TriRLL (gen 4) increase with increasing body length and the regression equations for these airways are summarized in Table 3. Measurements of outer cross-sectional area and wall area also demonstrated significant correlations with body length (Table 3). Gender and race were not significant determinants of airway size after adjusting for body length. The data for the pathways from the trachea into the other lobes demonstrated similar findings and is summarized in the on-line supplemental material.
Figure 6.
Lumen cross-sectional area (mm2) versus body length (cm) for airway generations. Individual data points are plotted, as well as the linear regression equations, which demonstrated significant increases in cross-sectional area with increasing body length (Table 2). Trachea (•;  ), Right main bronchus (RMB) (○;
), Right main bronchus (RMB) (○;  ), Bronchus intermedius (Bronint) (▲;
), Bronchus intermedius (Bronint) (▲;  ), RLL7 (□;
), RLL7 (□;  ), and TriRLL (x;
), and TriRLL (x;  ).
).
Table 3.
| Airway | Outcome | intercept | SE intercept | Length | p value Length | RSquare | N |
|---|---|---|---|---|---|---|---|
| TRACHEA | Inner Area mm2 | −28.10 | 9.01 | 0.91 | <.0001 | 0.63 | 38 |
| Outer Area mm2 | −27.17 | 15.36 | 1.34 | <.0001 | 0.56 | 38 | |
| Wall Area mm2 | 0.92 | 8.67 | 0.43 | 0.0004 | 0.29 | 38 | |
| Outer/Inner Area | 0.39 | 0.06 | 0.00 | 0.007 | 0.19 | 38 | |
| RMB | Inner Area mm2 | −33.32 | 7.77 | 0.82 | <.0001 | 0.65 | 38 |
| Outer Area mm2 | −48.80 | 13.24 | 1.39 | <.0001 | 0.65 | 38 | |
| Wall Area mm2 | −15.48 | 6.59 | 0.57 | <.0001 | 0.56 | 38 | |
| Outer/Inner Area | 0.38 | 0.05 | 0.00 | 0.0098 | 0.17 | 38 | |
| BRONINT | Inner Area mm2 | −14.80 | 6.34 | 0.44 | <.0001 | 0.45 | 38 |
| Outer Area mm2 | −20.75 | 11.14 | 0.81 | <.0001 | 0.47 | 38 | |
| Wall Area mm2 | −5.95 | 5.53 | 0.36 | <.0001 | 0.42 | 38 | |
| Outer/Inner Area | 0.33 | 0.05 | 0.00 | 0.013 | 0.16 | 38 | |
| RLL7 | Inner Area mm2 | −14.03 | 4.62 | 0.35 | <.0001 | 0.5 | 37 |
| Outer Area mm2 | −19.61 | 8.43 | 0.65 | <.0001 | 0.51 | 37 | |
| Wall Area mm2 | −5.58 | 4.41 | 0.30 | <.0001 | 0.46 | 37 | |
| Outer/Inner Area | 0.24 | 0.05 | 0.00 | 0.0014 | 0.26 | 37 | |
| RLL | Inner Area mm2 | −5.49 | 4.06 | 0.21 | 0.0003 | 0.33 | 35 |
| Outer Area mm2 | −7.82 | 7.56 | 0.45 | <.0001 | 0.4 | 35 | |
| Wall Area mm2 | −2.33 | 4.06 | 0.24 | <.0001 | 0.39 | 35 | |
| Outer/Inner Area | 0.30 | 0.06 | 0.00 | 0.1378 | 0.07 | 35 |
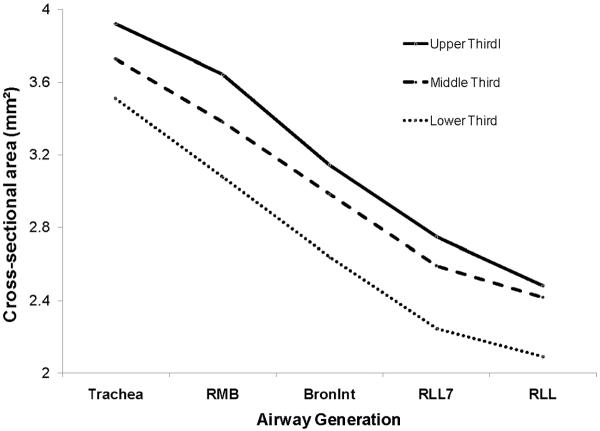
We evaluated whether the decrease in airway size with somatic growth is proportional among airway generation. Log transformation of cross-sectional area (CSA) decreased linearly with airway generation and increased with increasing body length; however, there was no significant interaction term between airway generation and body length. These relationships are illustrated in Figure 7, where log transformed cross-sectional area is plotted versus airway generation for the subjects divided into three groups based upon body length. Log transformed CSA decreased linearly with airway generation, the intercepts increased with increasing body size, and the regressions for the 3 different groups based upon body length paralleled each other. Similar results were obtained when lung volume was substituted for body length.
Figure 7.
Log transformed lumen cross-sectional area (mm2) versus airway generation for subjects grouped by body length (cm) into upper, middle and lower thirds. Cross-sectional area decreased with increasing airway generation for each of the three height groups; there was no significant interaction between generation and body length.
DISCUSSIONS
Using HRCT imaging, we found that lung volume was highly correlated with body length in infants and toddlers, while the air and the tissue components of the lung parenchyma increased at a constant rate. This later finding is consistent with morphometric and physiologic data that for infants and toddlers, the lung volume increases primarily by alveolarization, rather than expansion of existing alveoli. In addition, we found that the size of the central conducting airways correlated with body length in infants and toddlers and these airways appear to grow proportionately in this age range.
We found that lung tissue weight estimated from in vivo scans increased with increasing body length. These estimated weights will include both the parenchymal tissue and the pulmonary capillary blood volume, which we are not able to separate without contrast. However, when we subtracted estimates of pulmonary capillary blood volume the CT lung tissue weights were very similar to predicted values from autopsied lungs of infants and toddlers.
When the relationship between air volume and parenchymal lung tissue weight was evaluated, these two components of the lung parenchyma increased linearly with a slope of 3.6 cc of air/gm of tissue. This slope reflects lung expansion with growth, the increase of air volume relative to parenchymal lung tissue. Similar slopes were obtained with or without correcting the estimated tissue weight for pulmonary capillary blood volume. This constant relationship between air and tissue over a range of somatic growth when lung volume more than doubles suggests that lung growth occurred primarily by the addition of equal amounts of air and tissue, rather than the expansion of existing alveoli. Our in vivo value for lung expansion (3.6 cc air/gm tissue) is similar to the average value of 3.5 cc air/gm tissue reported by Stigol and coworkers, who obtained in vitro measurements of air volume and tissue weight in isolated autopsied lobes or lungs for subjects less than 90 cm body length (< 3 years of age)(24). The data from the autopsied specimens in these very young subjects suggested that lung expansion increased with body length, although lung expansion was clearly less in these very young subjects compared to values of 8–9 cc air/gm tissue obtained from the lung tissues of older children and adults. Using in vivo HRCT images, De Jong and coworkers initially reported that lung expansion decreased in the first few years of life; however measurements were obtained during tidal breathing and not at an elevated lung volume and without respiratory motion(14). A subsequent study by these investigators reported values for lung expansion in infants and toddlers similar to those of Stigol and that lung expansion also increased with body length(15). We believe that the relationship between the amount of air and tissue is best presented as air volume versus tissue weight, rather than a transformed variable, such as lung expansion versus body length. The increase in lung expansion with increasing body length presented by Stigol (2) and De Jong(15) most likely reflects the non-zero, negative intercept in the linear regression of air volume and lung tissue (Figure 5). When this relationship is transposed by dividing air volume by lung weight (lung expansion) and then plotted versus body length, which is highly correlated with lung volume, there will be a positive slope suggesting that lung expansion increases with body length in this age range. Our in vivo data of a constant relationship between air volume and tissue weight obtained with HRCT imaging is also consistent with our recent in vivo physiologic data(25). In infants and toddlers, pulmonary diffusing capacity, an indirect estimate of surface area increased linearly with alveolar volume. Cumulatively, the HRCT and physiologic data suggest that in infants and toddlers lung volume increases primarily by alveolarization that produces comparable increases in air and lung tissue, rather than by expansion of existing alveoli.
We also demonstrated with in vivo imaging that airway caliber decreased from the trachea into each of the lobes, and that the caliber of each airway segment increased with somatic size. These relationships appear the same during this period of rapid lung growth and thus suggest that in this age range the central conducting airways grow proportionately. Using a low dose protocol, which limited radiation exposure, also limited imaging quality and resolution; quantification of airway dimensions were limited to the trachea and 3–4 generations; therefore, our interpretation of growth of the conducting airways cannot be extended to more peripheral airway generations, which may have different growth patterns than the more central airways. However, our in vitro findings are consistent with observation by Hislop, who compared isolated airways from a few autopsied lungs from infants and adults(7). Although we were not able to assess more peripheral conducting airways, our previous physiologic results that density dependence of forced expiratory flows did not change early in life and were similar to values in older children and adults would support the concept of isotropic growth of the central and peripheral airways(26). We did not find gender differences in airway caliber in this age group, which is consistent with morphometric data in this age group and previous HRCT studies(15). As males have lower forced expiratory flows than females, the lower flows in males in this age group may be secondary to gender differences in more peripheral airways than evaluate by HRCT imaging, the dynamic properties of the airways, or the elastic recoil of the lung parenchyma(27).
Our study has several limitations. First, our subjects cannot be characterized as completely normal, as they were scheduled for a CT scan for non-respiratory medical problems. However, we excluded subjects with current or prior history of respiratory problems and their lung scans were determined to be normal by a pediatric radiologist. Therefore, we believe that our subjects are representative of those with healthy lungs and should not have affected the interpretation of our results. Second, lung volume (cc air) and thus lung expansion (cc air/mg tissue) depends upon inflation pressure. We used an inflation pressure of 20 cmH2O and not total lung capacity, which would require at least 40 cmH2O(28); this inflation pressure has only been used in vivo for infants and toddlers who were intubated and mechanically ventilated(28). Previously published data in this age group has demonstrated that an inflation pressure of 20 cmH2O remains on the linear portion of the pressure volume curve of the respiratory system(29)(30). Therefore, the methodology should provide a standardized approach to assess lung growth in this age group. With respect to assessing airway caliber, previous studies have demonstrated that adults, as well as immature and mature rabbits, reach a plateau in airway caliber at a transpulmonary pressure of 10 cmH2O(31–32), which will be obtained at an inflation pressure of 20 cmH2O. Lastly, we assessed lung growth from cross-sectional data. Although longitudinal data would be preferable, repeated HRCT imaging that also provides adequate imaging of the airways is not possible secondary to radiation exposure.
In summary, our results suggest that lung growth in the first few years of life occurs primarily by the addition of alveoli, and that the central conducting airways grow isotropically. In addition, this normative data for in vivo assessment of lung structure using multi-slice computed tomography in the first few years of life can be used to assess abnormalities in lung structure related to congenital anomalies and lung diseases that develop early in life.
Acknowledgements
The authors want to thank members of the radiology department for their assistance in recruiting patients and performing CT scans: Erv Herman, Marie Holder, Brian Towell, Pamela Monroe, Kathy Steriwalt, Mary Beth miller and Shelley Skinner.
Supported by NIH grant #HL054062
Appendix
Right Upper Lobe
| Airway | Outcome | intercept | SE intercept | Length (cm) | p value | R2 | N |
|---|---|---|---|---|---|---|---|
| Trachea | Inner Area (mm2) | −28.10 | 9.01 | 0.91 | <.0001 | 0.63 | 38 |
| Outer Area (mm2) | −27.17 | 15.36 | 1.34 | <.0001 | 0.56 | 38 | |
| Wall Area (mm2) | 0.92 | 8.67 | 0.43 | 0.0004 | 0.29 | 38 | |
| Outer/Inner Areas | 0.39 | 0.06 | 0.00 | 0.007 | 0.19 | 38 | |
| RMB (Gen1) | Inner Area (mm2) | −33.32 | 7.77 | 0.82 | <.0001 | 0.65 | 38 |
| Outer Area (mm2) | −48.80 | 13.24 | 1.39 | <.0001 | 0.65 | 38 | |
| Wall Area (mm2) | −15.48 | 6.59 | 0.57 | <.0001 | 0.56 | 38 | |
| Outer/Inner Areas | 0.38 | 0.05 | 0.00 | 0.0098 | 0.17 | 38 | |
| RUL (Gen 2) | Inner Area (mm2) | −20.23 | 5.56 | 0.45 | <.0001 | 0.53 | 38 |
| Outer Area (mm2) | −34.57 | 10.64 | 0.88 | <.0001 | 0.54 | 38 | |
| Wall Area (mm2) | −14.33 | 5.35 | 0.43 | <.0001 | 0.52 | 38 | |
| Outer/Inner Areas | 0.29 | 0.04 | 0.00 | 0.0021 | 0.23 | 38 | |
| RB1 (Gen 3) | Inner Area (mm2) | −8.71 | 3.57 | 0.18 | 0.0004 | 0.31 | 37 |
| Outer Area (mm2) | −13.22 | 6.87 | 0.37 | 0.0001 | 0.34 | 37 | |
| Wall Area (mm2) | −4.51 | 3.64 | 0.19 | 0.0002 | 0.33 | 37 | |
| Outer/Inner Areas | 0.06 | 0.06 | 0.00 | 0.0002 | 0.33 | 37 |
| Right Middle Lobe | |||||||
|---|---|---|---|---|---|---|---|
| Airway | Outcome | intercept | SE intercept | Length (cm) | pvalue | R2 | N |
| Trachea | Inner Area (mm2) | −28.10 | 9.01 | 0.91 | <.0001 | 0.63 | 38 |
| Outer Area (mm2) | −27.17 | 15.36 | 1.34 | <.0001 | 0.56 | 38 | |
| Wall Area (mm2) | 0.92 | 8.67 | 0.43 | 0.0004 | 0.29 | 38 | |
| Outer/Inner Areas | 0.39 | 0.06 | 0.00 | 0.007 | 0.19 | 38 | |
| RMB (Gen 1) | Inner Area (mm2) | −33.32 | 7.77 | 0.82 | <.0001 | 0.65 | 38 |
| Outer Area (mm2) | −48.80 | 13.24 | 1.39 | <.0001 | 0.65 | 38 | |
| Wall Area (mm2) | −15.48 | 6.59 | 0.57 | <.0001 | 0.56 | 38 | |
| Outer/Inner Areas | 0.38 | 0.05 | 0.00 | 0.0098 | 0.17 | 38 | |
| Bron lnt (Gen 2) | Inner Area (mm2) | −14.80 | 6.34 | 0.44 | <.0001 | 0.45 | 38 |
| Outer Area (mm2) | −20.75 | 11.14 | 0.81 | <.0001 | 0.47 | 38 | |
| Wall Area (mm2) | −5.95 | 5.53 | 0.36 | <.0001 | 0.42 | 38 | |
| Outer/Inner Areas | 0.33 | 0.05 | 0.00 | 0.013 | 0.16 | 38 | |
| RB4_5 (Gen 3) | Inner Area (mm2) | −6.91 | 2.65 | 0.20 | <.0001 | 0.5 | 38 |
| Outer Area (mm2) | −10.26 | 5.77 | 0.43 | <.0001 | 0.49 | 38 | |
| Wall Area (mm2) | −3.35 | 3.49 | 0.23 | <.0001 | 0.42 | 38 | |
| Outer/Inner Areas | 0.26 | 0.05 | 0.00 | 0.0113 | 0.17 | 38 | |
| RB5 (Gen 4) | Inner Area (mm2) | −2.53 | 1.83 | 0.10 | 0.0002 | 0.35 | 34 |
| Outer Area (mm2) | −2.45 | 4.85 | 0.22 | 0.001 | 0.29 | 34 | |
| Wall Area (mm2) | 0.08 | 3.21 | 0.13 | 0.0044 | 0.23 | 34 | |
| Outer/Inner Areas | 0.26 | 0.05 | 0.00 | 0.1153 | 0.08 | 34 | |
| Left Upper Lobe | |||||||
|---|---|---|---|---|---|---|---|
| Airway | Outcome | intercept | SE intercept | Length (cm) | p value | R2 | N |
| Trachea | Inner Area (mm2) | −28.10 | 9.01 | 0.91 | <.0001 | 0.63 | 38 |
| Outer Area (mm2) | −27.17 | 15.36 | 1.34 | <.0001 | 0.56 | 38 | |
| Wall Area (mm2) | 0.92 | 8.67 | 0.43 | 0.0004 | 0.29 | 38 | |
| Outer/Inner Areas | 0.39 | 0.06 | 0.00 | 0.007 | 0.19 | 38 | |
| LMB (Gen 1) | Inner Area (mm2) | −14.97 | 4.44 | 0.45 | −.0001 | 0.63 | 38 |
| Outer Area (mm2) | −20.32 | 8.86 | 0.81 | <.0001 | 0.59 | 38 | |
| Wall Area (mm2) | −5.36 | 5.09 | 0.36 | <.0001 | 0.46 | 38 | |
| Outer/Inner Areas | 0.33 | 0.04 | 0.00 | 0.0019 | 0.24 | 38 | |
| LUL (Gen 2) | Inner Area (mm2) | −9.37 | 6.52 | 0.31 | 0.0006 | 0.28 | 38 |
| Outer Area (mm2) | −13.02 | 12.47 | 0.62 | 0.0005 | 0.29 | 38 | |
| Wall Area (mm2) | −3.65 | 6.33 | 0.30 | 0.0006 | 0.28 | 38 | |
| Outer/Inner Areas | 0.30 | 0.06 | 0.00 | 0.0456 | 0.11 | 38 | |
| LB1_2 (Gen 3) | Inner Area (mm2) | −0.92 | 2.27 | 0.09 | 0.0052 | 0.22 | 34 |
| Outer Area (mm2) | −0.36 | 5.44 | 0.23 | 0.0026 | 0.25 | 34 | |
| Wall Area (mm2) | 0.57 | 3.49 | 0.14 | 0.0035 | 0.24 | 34 | |
| Outer/Inner Areas | 0.28 | 0.05 | 0.00 | 0.2703 | 0.04 | 34 | |
| LB1 (Gen 4) | Inner Area (mm2) | 0.92 | 1.84 | 0.04 | 0.0994 | 0.09 | 33 |
| Outer Area (mm2) | 5.45 | 4.75 | 0.10 | 0.0994 | 0.09 | 33 | |
| Wall Area (mm2) | 4.53 | 3.07 | 0.06 | 0.1177 | 0.08 | 33 | |
| Outer/Inner Areas | 0.24 | 0.05 | 0.00 | 0.2729 | 0.04 | 33 | |
| Left Lower lobe | |||||||
|---|---|---|---|---|---|---|---|
| Airway | Outcome | intercept | SE intercept | Length (cm) | p value | R2 | N |
| Trachea | Inner Area (mm2) | −28.10 | 9.01 | 0.91 | <.0001 | 0.63 | 38 |
| Outer Area (mm2) | −27.17 | 15.36 | 1.34 | <.0001 | 0.56 | 38 | |
| Wall Area (mm2) | 0.92 | 8.67 | 0.43 | 0.0004 | 0.29 | 38 | |
| Outer/Inner Areas | 0.39 | 0.06 | 0.00 | 0.007 | 0.19 | 38 | |
| LMB (Gen 1) | Inner Area (mm2) | −14.97 | 4.44 | 0.45 | <.0001 | 0.63 | 38 |
| Outer Area (mm2) | −20.32 | 8.86 | 0.81 | <.0001 | 0.59 | 38 | |
| Wall Area (mm2) | −5.36 | 5.09 | 0.36 | <.0001 | 0.46 | 38 | |
| Outer/Inner Areas | 0.33 | 0.04 | 0.00 | 0.0019 | 0.24 | 38 | |
| LLB6 (Gen 2) | Inner Area (mm2) | −15.15 | 8.53 | 0.43 | 0.0003 | 0.3 | 38 |
| Outer Area (mm2) | −20.79 | 14.17 | 0.79 | 0.0001 | 0.34 | 38 | |
| Wall Area (mm2) | −5.64 | 6.23 | 0.35 | <.0001 | 0.35 | 38 | |
| Outer/Inner Areas | 0.29 | 0.06 | 0.00 | 0.0134 | 0.16 | 38 | |
| LLB (Gen 3) | Inner Area (mm2) | −19.79 | 4.48 | 0.43 | <.0001 | 0.61 | 38 |
| Outer Area (mm2) | −29.80 | 7.48 | 0.79 | <.0001 | 0.66 | 38 | |
| Wall Area (mm2) | −10.00 | 3.73 | 0.36 | <.0001 | 0.62 | 38 | |
| Outer/Inner Areas | 0.19 | 0.06 | 0.00 | 0.0005 | 0.29 | 38 | |
| LB10 (Gen 4) | Inner Area (mm2) | −10.07 | 3.29 | 0.22 | <.0001 | 0.43 | 38 |
| Outer Area (mm2) | −15.83 | 6.77 | 0.45 | <.0001 | 0.43 | 38 | |
| Wall Area (mm2) | −5.76 | 3.89 | 0.23 | <.0001 | 0.38 | 38 | |
| Outer/Inner Areas | 0.17 | 0.06 | 0.00 | 0.0017 | 0.24 | 38 | |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hislop AA, Wigglesworth JS, Desai R. Alveolar development in the human fetus and infant. Early Human Development. 1986;13(1):1–11. doi: 10.1016/0378-3782(86)90092-7. [DOI] [PubMed] [Google Scholar]
- 2.Thurlbeck WM. Post natal growth and development of the lung. American Review of Respiratory Disease. 1975;111:803–44. doi: 10.1164/arrd.1975.111.6.803. [DOI] [PubMed] [Google Scholar]
- 3.Zeltner TB, Burri PH. The postnatal development and growth of the human lung. II. Morphology. Respiration Physiology. 1987;67(3):269–82. doi: 10.1016/0034-5687(87)90058-2. [DOI] [PubMed] [Google Scholar]
- 4.Zeltner TB, Caduff JH, Gehr P, Pfenninger J, Burri PH. The postnatal development and growth of the human lung. I. Morphometry. Respiration Physiology. 1987;67(3):247–67. doi: 10.1016/0034-5687(87)90057-0. [DOI] [PubMed] [Google Scholar]
- 5.Davies G, Reid L. Growth of the alveoli and pulmonary arteries in childhood. Thorax. 1970;25(6):669–81. doi: 10.1136/thx.25.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunnill MS. Postnatal growth of the lung. Thorax. 1962;17:329–33. [Google Scholar]
- 7.Hislop A, Muir DCF, Jacobsen M, Simon G, Reid L. Postnatal growth and function of the pre-acinar airways. Thorax. 1972;27:265–74. doi: 10.1136/thx.27.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horsfield K, Gordon WI, Kemp W, Phillips S. Growth of the Bronchial Tree in Man. Thorax. 1987;42(5):383–8. doi: 10.1136/thx.42.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Washko GR, Criner GJ, Mohsenifar Z, Sciurba FC, Sharafkhaneh A, Make BJ, et al. Computed tomographic-based quantification of emphysema and correlation to pulmonary function and mechanics. Copd. 2008;5(3):177–86. doi: 10.1080/15412550802093025. [DOI] [PubMed] [Google Scholar]
- 10.de Blic J, Tillie-Leblond I, Emond S, Mahut B, ng Duy TL, Scheinmann P. High-resolution computed tomography scan and airway remodeling in children with severe asthma. Journal of Allergy and Clinical Immunology. 2005;116(4):750–4. doi: 10.1016/j.jaci.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Nakano Y, Muller NL, King GG, Niimi A, Kalloger SE, Mishima M, et al. Quantitative assessment of airway remodeling using high-resolution CT. Chest. 2002;122(6:Suppl):Suppl-275S. [PubMed] [Google Scholar]
- 12.de Jong PA, Nakano Y, Hop WC, Long FR, Coxson HO, Pare PD, et al. Changes in Airway Dimensions on Computed Tomography Scans of Children With Cystic Fibrosis. American Journal of Respiratory and Critical Care Medicine. 2005:200410–1311OC. doi: 10.1164/rccm.200410-1311OC. [DOI] [PubMed] [Google Scholar]
- 13.Hislop AA. Lung growth and computed teomography. European Respiratory Journal. 2003;22:195–6. doi: 10.1183/09031936.03.00040603. [DOI] [PubMed] [Google Scholar]
- 14.de Jong PA, Nakano Y, Lequin MH, Merkus PJ, Tiddens HA, Hogg JC, et al. Estimation of lung growth using computed tomograph. European Respiratory Journal. 2003;22(2):235–8. doi: 10.1183/09031936.03.00089702. [DOI] [PubMed] [Google Scholar]
- 15.de Jong PA, Long FR, Wong JC, Merkus PJ, Tiddens HA, Hogg JC, et al. Computed tomographic estimation of lung dimensions throughout the growth period. European Respiratory Journal. 2006;27(2):261–7. doi: 10.1183/09031936.06.00070805. [DOI] [PubMed] [Google Scholar]
- 16.Long FR, Williams RS, Castile RG. Inspiratory and expiratory CT lung density in infants and young children. Pediatric Radiology. 2005;35(7):677–83. doi: 10.1007/s00247-005-1450-6. [DOI] [PubMed] [Google Scholar]
- 17.Long FR. High-resolution CT of the lungs in infants and young children. Journal of Thoracic Imaging. 2001;16(4):251–8. doi: 10.1097/00005382-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Martinez TM, Llapur CJ, Williams TH, Coates C, Gunderman R, Cohen MD, et al. High-resolution computed tomography imaging of airway disease in infants with cystic fibrosis. American Journal of Respiratory & Critical Care Medicine. 2005;172(9):1133–8. doi: 10.1164/rccm.200412-1665OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tschirren J, Pal gyi K, Reinhardt JM, Hoffman EA, Sonka M, Kikinis R. Segmentation, Skeletonization, and Branchpoint Matching - A Fully Automated Quantitative Evaluation of Human Intrathoracic Airway Trees. Springer-Verlag; Berlin Heidelberg: 2002. pp. 12–9. [Google Scholar]
- 20.Tschirren J, McLennan G, Palagyi K, Hoffman EA, Sonka M. Matching and anatomical labeling of human airway tree. IEEE Trans Med Imaging. 2005;24(12):1540–7. doi: 10.1109/TMI.2005.857653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagano M, Gauvereau . Principles of Biostatistics. 2nd Edt. Duxbury: 2000. [Google Scholar]
- 22.Coppoletta JM, Wolbach SB. Body Length and Organ Weights of Infants and Children: A Study of the Body Length and Normal Weights of the More Important Vital Organs of the Body between Birth and Twelve Years of Age. Am J Pathol. 1933;9(1):55–70. [PMC free article] [PubMed] [Google Scholar]
- 23.Bucci G, Cook CD, Barrie H. Studies of Respiratory Physiology in Children: Total Lung Diffusion, diffusing capacity of pulmonary membrane, and pulmonary capillary blood volume in normal subjects from 7 to 40 years of age. Journal of Pediatrics. 1961:820–8. doi: 10.1172/JCI104374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stigol LC, Vawter GF, Mead J. Studies on elastic recoil of the lung in a pediatric population. Am Rev Respir Dis. 1972;105(4):552–63. doi: 10.1164/arrd.1972.105.4.552. [DOI] [PubMed] [Google Scholar]
- 25.Balinotti JE, Tiller CJ, Llapur CJ, Jones MH, Kimmel RN, Coates CE, et al. Growth of the lung parenchyma early in life. American Journal of Respiratory & Critical Care Medicine. 2009;179(2):134–7. doi: 10.1164/rccm.200808-1224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis S, Jones M, Kisling J, Castile R, Tepper RS. Density dependence of forced expiratory flows in healthy infants and toddlers. Journal of Applied Physiology. 1999;87(5):1796–801. doi: 10.1152/jappl.1999.87.5.1796. [DOI] [PubMed] [Google Scholar]
- 27.Jones M, Castile R, Davis S, Kisling J, Filbrun D, Flucke R, et al. Forced expiratory flows and volumes in infants. Normative data and lung growth. American Journal of Respiratory and Critical Care Medicine. 2000;161(2 Pt 1):353–9. doi: 10.1164/ajrccm.161.2.9903026. [DOI] [PubMed] [Google Scholar]
- 28.Hammer J, Numa A, Newth CJ. Total lung capacity by N2 washout from high and low lung volumes in ventilated infants and children. American Journal of Respiratory & Critical Care Medicine. 1998;158(2):526–31. doi: 10.1164/ajrccm.158.2.9710096. [DOI] [PubMed] [Google Scholar]
- 29.Tepper RS, Hiatt PW, Eigen H, Smith J. Total respiratory system compliance in asymptomatic infants with cystic fibrosis. Am Rev Respir Dis. 1987;135(5):1075–9. doi: 10.1164/arrd.1987.135.5.1075. [DOI] [PubMed] [Google Scholar]
- 30.Tepper RS, Williams T, Kisling J, Castile R. Static compliance of the respiratory system in healthy infants. American Journal of Respiratory and Critical Care Medicine. 2001;163(1):91–4. doi: 10.1164/ajrccm.163.1.2002130. [DOI] [PubMed] [Google Scholar]
- 31.Brown RH, Mitzner W, Wagner E, Permutt S, Togias A. Airway distension with lung inflation measured by HRCT1. Academic Radiology. 2003;10(10):1097–103. doi: 10.1016/s1076-6332(03)00333-7. [DOI] [PubMed] [Google Scholar]
- 32.Shen X, Ramchandani R, Dunn B, Lambert R, Gunst S, Tepper R. Effect of transpulmonary pressure on airway diameter and airway responsiveness of immature and mature rabbits. Journal of Applied Physiology. 2000;89:1584–90. doi: 10.1152/jappl.2000.89.4.1584. [DOI] [PubMed] [Google Scholar]