Abstract
The question of whether storage of red blood cells (RBCs) alters their capacity to deliver oxygen and affects patient outcomes remains in a state of clinical equipoise. Studies of the changes which occur while RBC are stored have led to several physiologically plausible hypotheses that these changes impair RBC function when the units are transfused. Although there is some evidence of this effect in vivo from animal model experiments, the results of several largely retrospective patient studies have not been consistent. Some studies have shown an association between worse clinical outcomes and transfusion of RBC which have been stored for longer periods of time, while others have found no effect. Three multicenter, randomized, controlled trials have been developed to address this important, but currently unanswered, question. Two clinical trials, one in low birth weight neonates and the other in intensive care unit patients, are enrolling subjects in Canada (the Age of Red Blood Cells in Premature Infants; the Age of Blood Study). The third trial, which is being developed in the United States, is the Red Cell Storage Duration Study (RECESS). This is a multicenter, randomized, controlled trial in which patients undergoing complex cardiac surgical procedures who are likely to require RBC transfusion will be randomized to receive RBC units stored for either 10 or fewer days or 21 or more days. Randomization will only occur if the blood bank has enough units of RBC of both storage times to meet the crossmatch request; hence, subjects randomized to the ≥ 21 day arm will receive RBC of the same storage time as they would have following standard inventory practice of “oldest units out first”. The primary outcome is the change in the Multiple Organ Dysfunction Score (MODS), a composite measure of multiorgan dysfunction, by day 7. Secondary outcomes include the change in the MODS by day 28, all-cause mortality, and several composite and single measures of specific organ system function. The estimated total sample size required will be 1434 evaluable subjects (717 per arm). The RECESS trial is registered through the US National Institutes of Health (clinicaltrials.gov) as NCT00991341.
Introduction
Even though red blood cell (RBC) transfusion has been a standard therapy for at least 50 years, there is still much that we do not understand about the delivery of oxygen and its utilization in the tissues of the body. Furthermore, the efficacy of RBC transfusion has not been rigorously established through testing in robust clinical trials. It is only in the past 20 years that information from well designed clinical studies has been available to support an evidence-based approach to transfusion practices. This gap in medical information is nowhere more apparent than in the enduring discussions of the effects of RBC storage on patient outcomes.[1]
As summarized in the preceding review by J. Hess in this issue of Transfusion and Apheresis Science [2], the changes that occur in RBCs under standard blood banking conditions and as they senesce in vivo have been recognized for decades, and have been described at increasing levels of sophistication. These changes have generated the physiologically plausible hypothesis that stored RBCs might not deliver oxygen as well as native RBCs. Examination of the behavior and effects of stored RBCs in the microcirculation [3] and in animal model studies [4] have yielded data supporting this hypothesis, although the results are not always uniform among studies. This is perhaps not surprising given the use of different animals and experimental interventions among these studies. These considerations have prompted a number of studies in human subjects. In the last decade, several new technologies, and adaptations of existing technologies, have been developed to assess the effects of RBC transfusion on the microcirculation and tissue oxygenation in humans, as reviewed in the section by Y. Sakr in this issue. [5] In addition, several observational, largely retrospective, clinical studies seeking associations between the duration of RBC storage and patient outcomes have been carried out. As described in the review by D. Triulzi and M. Yazer, [6] the results from this heterogeneous group of studies have not been consistent. Some studies report an association between poor clinical outcomes, such as increased mortality and length of stay, and the administration of RBCs stored for a longer period of time, while others do not. A smaller number of randomized trials have also been conducted, but most were not powered to detect differences in clinical endpoints. Some were not expected to have sufficient power because they were intended as pilot studies.[7,8] A meta-analysis of several observational and small randomized interventional studies did not find an association between the transfusion of RBCs stored for a study-defined longer period of time and either in-house mortality or postoperative pneumonia.[9] The issue of whether the changes that occur to the RBC during storage have a significant effect on clinical outcomes remains in a state of equipoise.[1,6] We simply cannot answer this question with the data available. Fortunately, three randomized, controlled clinical trials have been designed to address this issue. Two trials being conducted in Canada, one in low birth weight neonates (Age of Red Blood Cells in Premature Infants;ARIPI) [10] and another in intensive care unit (ICU) patients, (Age of Blood Study; ABLE),[11] were described in the preceding review.
A third trial being conducted in the United States, the Red Cell Storage Duration Study (RECESS), is designed to compare clinical outcomes in cardiac surgery patients randomized to receive RBCs stored for either a longer or shorter period of time. This multicenter clinical trial underwent a rigorous peer development and review process by the Transfusion Medicine and Hemostasis Clinical Trials Network (TMHCTN)[12] over several years and is funded by the National Heart, Lung, and Blood Institute (NHLBI). The Data Coordinating Center is New England Research Institutes (NERI).[13] An independent Data and Safety Monitoring Board is responsible for review and approval of the protocol and consent form, and for monitoring adverse events among trial subjects. The purpose of this article is to describe the RECESS protocol and review some of the methodological, scientific and medical considerations that went into its design.
Methods and Trial Design
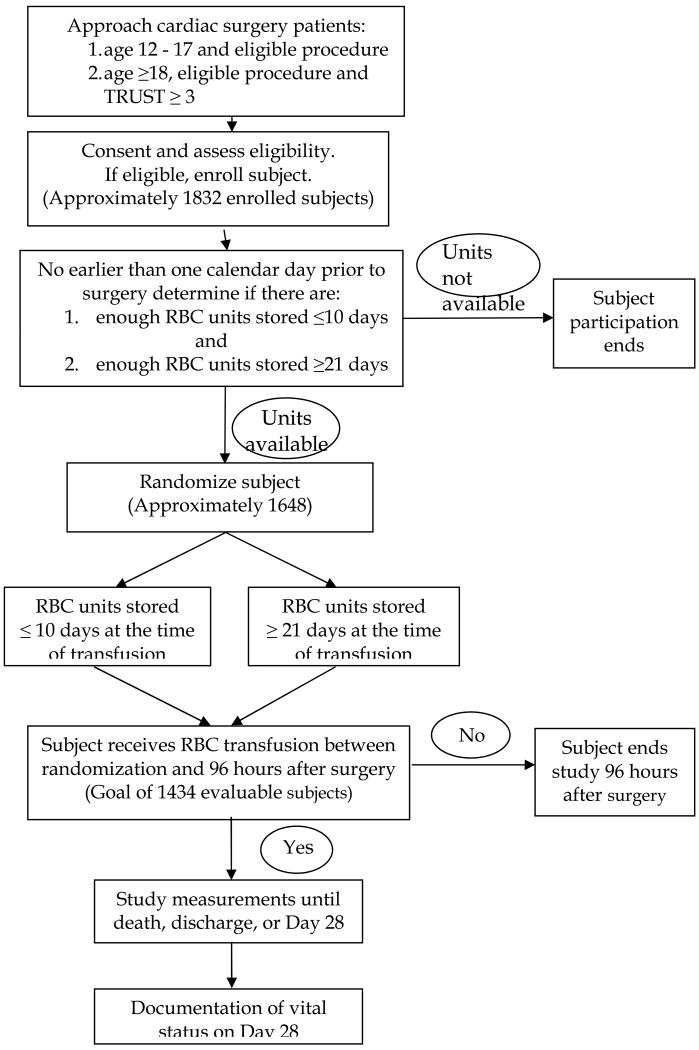
RECESS is a multicenter, partially blinded, randomized clinical trial evaluating the effects of RBCs stored 10 or fewer days or 21 or more days at the time of transfusion to patients undergoing complex cardiac surgery or repeat cardiac surgery who are likely to require RBC transfusion (Figure 1). Participating centers were identified from within the TMHCTN if: 1) they had cardiac surgery programs that performed complex operations or repeat surgeries, and 2) their blood banks expect to be able to support the randomization process and have sufficient inventory to provide appropriately stored RBCs from the time of the patient's preoperative randomization throughout the hospitalization up to postoperative day 28. Additional centers were invited to participate based on their experience with transfusion medicine clinical trials along with the criteria above. Currently, 20 to 25 centers plan to participate in RECESS.
Figure 1.
Study Schema
Study Population
The cardiac surgery population was chosen as the study group for RECESS for several reasons. Patients undergoing complex or repeat cardiac operations commonly require multiple RBC transfusions, and hence would be more likely to manifest any possible adverse consequences of exposure to banked RBCs. In particular, cardiac surgery patients exposed to the cardiopulmonary bypass circuit are in a proinflammatory state and may be particularly vulnerable to any deleterious effects of RBC transfusion.[14] The cardiac surgery group constitutes a large population of patients who commonly receive RBC transfusions and accounts for a significant proportion of blood component utilization.[15] Also, this is a particularly important group to study since there are conflicting data from several retrospective studies attempting to evaluate the association of RBC storage time and clinical outcomes in cardiac surgery patients.[6] In addition, these patients often undergo invasive cardiopulmonary monitoring for a period of time postoperatively, making data on oxygen consumption and delivery, as well as other physiologic parameters, readily available for assessing RBC transfusion effects. Lastly, RECESS complements the ABLE study, which is enrolling ICU patients and does not include cardiac surgery patients.[11]
In order to maximize the ability to detect a clinical effect of RBC storage duration, RECESS seeks to enroll subjects who will have a high likelihood of being transfused. Potential subjects at least 18 years old are screened using a preoperative transfusion risk assessment tool as described in the screening section below. This tool has not been validated in children, hence subjects between 12 and 18 years of age are eligible if they are scheduled to undergo cardiac surgical procedures which generally require RBC transfusion.
The age range for subjects was chosen to include the widest possible group of subjects, without introducing an undue degree of clinical heterogeneity. RECESS will enroll subjects who are at least 12 years old and weigh at least 40 kg. Cardiac surgeons at the participating institutions were polled regarding the youngest patient age at which they would allow randomization; all centers would enroll patients who were at least 12 years old. Many centers would not enroll younger patients, often because they have specific RBC transfusion protocols for them. While the Multiple Organ Dysfunction Score (MODS), the primary outcome measure, has been validated only in adults [16], the normal ranges of the measurements used to determine the MODS are essentially the same in adolescents and adults, so it is reasonable to use this assessment of organ dysfunction for subjects between 12 and 18 years old.
Patients with clinically significant RBC alloantibodies or a history of transfusion reactions or who require washed RBCs, volume reduced RBCs, or removal of the additive solution are not eligible for the study, primarily to avoid confounding the study data through the introduction of these variables.
Design
Considerable discussion focused on the choice of storage duration for the two arms of the RECESS study. The choice of treatment arms was made to balance the competing demands of:
Availability of sufficient blood inventory for the study, to minimize protocol violations;
Widely separate distributions of RBC storage durations between the two arms, to maximize the likelihood of detecting a difference if storage duration does affect clinical outcomes; and,
Storage durations not markedly different from those often seen with standard inventory practice.
There is no consensus as to what defines “young” or “fresh” RBC. The kinetics of the various changes that occur in stored RBCs, and are hypothesized to affect in vivo function, are highly variable ranging from hours for loss of nitric oxide, to a few days for 2,3-diphosphoglycerate depletion, to many days for reducing membrane constituents such as Band 3 (anion exchange protein).[2] A storage duration of 10 or fewer days was chosen as the shortest time which still made it feasible to meet inventory demands for the study, particularly as banked RBC distributed to hospital transfusion services are seldom less than three days old. This storage duration was also comparable to that used in other studies in cardiac surgery patients which used cutoffs of eight days,[7] 14 days,[17] and 18 days.[18]
A duration of 21 or more days was chosen for the longer storage arm. This strategy creates a minimum difference of 11 days in the storage time of the RBC given to patients in the two arms of the study. A storage time minimum of 21 days is comparable to that of the “longer storage” arm in several published studies, some of which demonstrated a difference in outcomes[17] but some of which did not.[7,18,19] This longer storage time is also similar to that of RBCs commonly provided by the standard inventory practice of issuing “oldest units out first”, a practice intended to minimize the outdating of banked RBC. A review of all RBC released to cardiac surgery patients over one week in 2007 was conducted at three TMH centers, and showed that 26-44% of RBC released had been stored ≥ 21 days. The median storage time for RBC issued to cardiac surgery patients at one of the TMH centers over the course of one year was 21 days. (Data not shown.) A recent analysis of a full year of data from a single non-TMH center showed that the median time in storage of RBCs at the point of transfusion ranged from 14.9 to 36.3 days depending on ABO/Rh blood group.[20] Since subjects are only randomized if there is a minimum number of RBC units within both study-defined storage ranges, subjects randomized to receive RBCs stored for 21 or more days arm receive units of the same age distribution as they would if they were not in the study.
An alternate strategy would have been to compare the shorter storage duration arm to a “standard inventory practice” arm, in which RBC would be provided to patients according to the usual blood bank inventory management procedure of issuing “oldest units out first”, with no specified minimum storage time for units in the “standard practice” arm. The storage times of the standard inventory practice units would vary, depending on the ABO and Rh type and the vagaries of the blood supply, and the storage times of the units for patients in the two arms would be likely to overlap with one another. For example, in a pilot study in ICU patients comparing RBCs stored eight days or less to standard inventory practice, the expectation was that the patients in the latter arm would receive units that had been stored for at least 15 days. However, while 91% of the patients in the group assigned to receive RBC stored 8 days or less actually received at least 90% of their RBC units as assigned, only 59% of patients in the standard inventory practice group received at least 90% of their RBC units stored 15 days or more.[7] Similarly, the first phase of a pilot study in cardiac surgery patients who were randomized to standard inventory practice vs. transfusion with no RBCs stored for more than 21 days found considerable overlap between the two arms.[8]
There are significant drawbacks to using this alternate approach. First, it does not answer the primary scientific question of what are the clinical consequences of receiving RBC stored for a shorter or longer period of time. Second, the median storage times under standard practice differ between sites, and even within the same site during different times of the year, thus complicating statistical analysis of pooled data. Third, the smaller difference in the median storage times of the RBCs used for the two arms, and the presence of an overlap in the storage duration distributions, might obscure any small but real difference in outcomes between subjects receiving units stored for shorter and longer periods.
Hence, storage durations of 10 or fewer days and 21 or more days were chosen for the two arms to maintain feasibility with respect to blood bank inventory, achieve a wide separation in RBC storage times between the two arms, enhance the ability to detect differences in clinical outcomes between shorter and longer storage RBC (if in fact such differences exist), and achieve minimal deviation from storage duration of RBC that might be provided under standard inventory practice.
The RECESS study does not specify a RBC transfusion threshold or “trigger”. There have been no studies to date that have conclusively identified either an efficacious or a safe RBC transfusion threshold for patients with coronary artery or other cardiac disease,[21,22] so it would be difficult to justify one transfusion guideline over another. To make the results generalizable and clinically relevant, patient management should, insofar as possible, reflect current practice, which does not impose a uniform approach to RBC transfusion. In order to minimize the effect of variation between hospitals with respect to RBC transfusion indications, as well asd anesthesia, surgical and critical care practices, patient randomization for RECESS will be balanced by center, ensuring that approximately half the subjects in each hospital will be randomized to each treatment arm.
Inventory availability is a crucial component of trial success in studies such as RECESS. For example, the second phase of a recent pilot study showed that considerable planning was necessary to adhere to the storage duration assignment, as 100% of the subjects randomized to receive RBC stored 3–11 days did so, while only 50% of the patients in the 17 – 25 day storage arm were transfused with RBCs of the appropriate storage time.[8]
The study protocol mandates that the blood bank must have enough units of ABO compatible RBC of both storage durations to fulfill the crossmatch request before a subject may be randomized. Randomization must occur no earlier than one day prior to surgery which is the time frame within which units of RBC are typically crossmatched for surgery.
The requirement that the transfusion service must confirm that it can support the subject with RBCs from either treatment arm before the subject can be randomized is intended to reduce the likelihood of protocol violations. This may be especially important for group O patients. The average storage time for RBCs varies among ABO and Rh blood groups[20] and may be affected by local transfusion practices. For example, the disproportionate use of group O RBCs by a large trauma service or due to an institutional policy of issuing group O RBCs to new patients whose ABO/Rh types have only been determined once, may result in higher turnover of group O RBC units and shorter average storage times than for other blood groups. Since the levels of von Willebrand's protein are higher in non-group O patients and correlate with increased risk of various thrombotic events, [23] it will be important to minimize protocol violations in both treatment arms and in subjects of all ABO blood groups, so as not to introduce this confounding variable. A second reason for not randomizing patients unless there are enough units of RBCs of both storage durations to meet the crossmatch request is to ensure that subjects in the arm receiving RBCs stored 21 or more days would receive units with the same distribution of storage times as they would receive if they were not in the study.
Screening, Recruitment, Consent and Randomization
Patients who are at least 18 years old undergoing complex cardiac surgery will be identified through scheduling procedures in advance of their operations.
These patients will also be screened to determine the likelihood that they will require perioperative RBC transfusion using the Transfusion Risk Understanding Screening Tool (TRUST).[24] This tool uses basic features of the patient medical and surgical histories (i.e. age, gender, weight, creatinine, hemoglobin, previous cardiac surgery and type of surgery) to generate a score which has been shown to correlate with the likelihood the patient will require RBC transfusion during or within 96 h after surgery. (See Table 1.) Patients are eligible if the TRUST score is 3 or greater. A TRUST score of 3 predicts a 60%-79% probability of RBC transfusion. (See Table 2.) This system was validated at 4 TMH centers which retrospectively calculated the TRUST scores for all patients undergoing cardiac surgery at their institutions for one year (2006). From a total of 2861 patients, 52% had a TRUST score of 3 or more, and 87% of those patients received one or more units of RBCs intra- or post-operatively, consistent with the TRUST prediction.
Table 1.
Components of TRUST score
| Parameter | Finding | Points |
|---|---|---|
| Age of patient in years | ≤ 65 yr | 0 |
| > 65 yr | 1 | |
| Gender | Male | 0 |
| Female | 1 | |
| Hemoglobin | ≥ 13.5 gm/dl | 0 |
| < 13.5 gm/dl | 1 | |
| Body weight in kilograms | ≥ 77 kg | 0 |
| < 77 kg | 1 | |
| Serum creatinine | ≤ 1.36 mg/dL (120 μmol/L) | 0 |
| > 1.36 mg/dL (120 μmol/L) | 1 | |
| Elective Surgery | Yes | 0 |
| No | 1 | |
| History of previous cardiac surgery | No | 0 |
| Yes | 1 | |
| Surgical task(s) | Isolated | 0 |
| Multiple | 1 |
Adapted from Alghamdi AA, et al.[24]
Table 2.
Likelihood of receiving transfusion based on TRUST score
| Total TRUST Score | Probability of Transfusion |
|---|---|
| 0 | < 20% |
| 1 | 20-39% |
| 2 | 40-59% |
| 3 | 60-79% |
| ≥4 | 80-100% |
Adapted from Alghamdi AA, et al.[24]
Patients 12-17 years old weighing at least 40 kilograms who are scheduled to undergo one of the cardiac surgical procedures specified in the RECESS protocol will be identified in the same manner as adult patients. The TRUST screening tool will not be used because it has not been validated in patients who are less than 18 years old. The protocol-specified list of eligible surgical procedures includes those which have historically been shown to have a high likelihood of requiring RBC transfusions in this age group.
Potentially eligible patients, as described in the previous paragraphs, will be approached for study consent prior to their operation. Individual center scheduling practices will influence how this contact is arranged. Patients already hospitalized may be included.
In order to randomize a patient, the study coordinator contacts the institution's blood bank within one calendar day of surgery to notify the staff that an eligible RECESS subject has been identified, the planned date of surgery, and the subject's ABO and Rh blood type. Blood bank staff determine whether enough units of ABO compatible RBCs of both storage times are available to fulfill the crossmatch request. If the blood bank does not have enough RBCs of both the longer and shorter storage time, then the subject will not be randomized.
Randomization is stratified by subject age (at least 18 years old or less than 18 years old) and by whether the subject had been admitted to an ICU before surgery to equally distribute pre-operative patient acuity and differences in pediatric and adult patient practice patterns between treatment arms. The randomization scheme uses permuted blocks within each of the strata. To ensure that approximately half of the subjects at each hospital are assigned to each of the two treatment groups, institutional balancing [25] is used.
If a subject is randomized, and the planned surgery is not performed within 30 days, the subject will be withdrawn from the study.
Intervention
Study subjects are randomized by the blood bank coordinators to receive RBCs stored for either 10 or fewer days or 21 or more days. If a subject does not receive any RBC transfusions between randomization and 96 hours following the end of the surgery, the subject will be withdrawn from the study. Subjects who do receive RBC transfusions during this time period are transfused according to their randomized treatment arm up to 28 days after surgery, or until hospital discharge or death, whichever occurs first.
All study RBC transfusions will be of the assigned storage time as often as feasible without compromising patient care. If there are not enough units of the assigned storage time to meet all of the patient's needs, the blood bank will provide units as close as possible to the randomized treatment assignment.
Subjects may only receive RBC which have been prestorage leukoreduced and stored in AS1, AS3 or AS5. Irradiated units may be used but irradiation should occur within 12 hours before issue. Units washed in the blood bank or the operating room will be considered protocol violations. Institutional standard procedures for compatibility testing and issuing of blood components are maintained for all study subjects. In addition, transfusions are administered as ordered by the medical team for patient care needs according to local practice and following all the usual institutional policy and safety standards.
Blinding of Treatment Allocation
Only blood bank staff with the appropriate security level in the data management system (DMS) are able to access the treatment arm assignment directly. Access to case report forms containing information about the collection, processing, and expiration dates of the RBC units issued for study subjects is also restricted to the appropriate blood bank staff at each site. Clinical staff caring for the subject, and study personnel collecting and reporting data in the DMS, are not informed of the study arm assignment and do not have access in the DMS to this information, or to data about the collection, processing, and expiration dates of the RBC units transfused.
However, no alteration is made to the labels on the RBC units. The expiration date, collection date, and any processing dates (e.g. date of irradiation) are not obscured. Therefore any patient care personnel handling those units could calculate the storage duration and infer the study arm assignment. Personnel providing care to study subjects are instructed not to seek to identify the storage time of the RBCs the patients are receiving. They are also instructed not to disclose the randomization assignments, should they become aware of them, to the patients themselves or to other health care providers. The informed consent document indicates that subjects will not be informed of their randomization assignment and should not seek to identify the storage time of the units they are receiving.
A blinded pilot study of 20 red cell units was performed at one of the TMH centers to determine whether the storage time of the red cell unit could be predicted based on its appearance or color. The study demonstrated that there was no reliable correlation between the appearance of the units and the amount of time they had been stored. (Data not shown.)
Although the study is only partially blinded, the components of the MODS, the primary endpoint, are objective physiologic measurements, as are most of the secondary outcome measures. Thus, inadvertent unblinding of the randomization assignment would have little effect, since the primary outcome data are minimally subject to observer bias and the unblinding will not compromise the validity of the study.
Primary Outcome
The primary endpoint of the RECESS study is the change in clinical outcome assessed using the Multiple Organ Dysfunction Score (MODS), which is a composite endpoint of multisystem organ dysfunction.[16]
Mortality was considered as a primary outcome for this proposed study in cardiac surgery patients. However, mortality for this group of patients is only expected to be in the range of 7-8%. To have 85% power to detect a reduction in mortality from 8% to 6%, approximately 3000 evaluable patients per arm would be required, and the study would be prohibitively expensive at $18-21 million. Therefore, a morbidity endpoint, which encompasses mortality and has been shown to correlate with mortality, was chosen as the primary endpoint.
The MODS was chosen for use in RECESS from multiple scoring systems that have been developed to measure organ dysfunction. The MODS is easily calculated from readily available, objective clinical data. Scores are assigned from 0-4 for graduated levels of dysfunction in each of six organ systems (respiratory, renal, hepatic, cardiac, hematologic and central nervous system) and can be summed as a daily score, totaled using the worst individual organ system score over an interval, and/or compared to a baseline score. (See Table 3.) The MODS incorporates mortality as the extreme manifestation of multiple organ dysfunction, with a maximum score of 24 assigned to death. In validation studies, increasing MODS correlated well with increasing risk of mortality, as well as hospital and ICU length of stay.[16] MODS has since been used in many clinical studies to standardize assessment of cumulative organ dysfunction, including in the Transfusion Requirements in Critical Care (TRICC) trial and the subgroup analysis of patients with cardiac disease, to compare the clinical effects of liberal vs. restrictive RBC transfusion practice.[22, 26]
Table 3.
Clinical Components of the Multiple Organ Dysfunction Score
| Organ system | Points | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Respiratory (PaO2/FiO2) |
> 300 | 226-300 | 151-225 | 76-150 | ≤75 |
| Renal (creatinine uM/L) |
≤100 | 101-200 | 201-350 | 351-500 | > 500 |
| Hepatic (bilirubin uM/L) |
≤ 20 | 21-60 | 61-120 | 121-240 | > 240 |
| CV (PAR) (HR × CVP/MAP) |
≤ 10.0 | 10.1-15.0 | 15.1-20.0 | 20.1-30.0 | > 30.0 |
| Heme (plt # ct/mlx10-3) |
> 120 | 81-120 | 51-80 | 21-50 | ≤ 20 |
| Neuro (Glasgow Coma Score) | 15 | 13-14 | 10-12 | 7-9 | ≤6 |
Modified from Marshall JC, et al.[16]
The MODS calculated immediately prior to surgery is considered the baseline value from which change in MODS is calculated. The 7-day cumulative MODS is calculated as the sum of the worst individual component scores for each of the systems occurring from surgery through postoperative Day 7, hospital discharge, or death, whichever occurs first. This measure incorporates the maximum dysfunction in each organ system over the 7-day time period, even if these maxima occur on different days. It is not unusual for organs to develop dysfunction at different times after surgery. If dysfunction occurs in one organ system, it may be treated and perhaps resolved before dysfunction occurs in another organ system. Subjects who die within 7 days after surgery are assigned the maximum possible 7-day MODS of 24 points. The change in the MODS value (ΔMODS) from baseline to 7 days will be compared between subjects who were randomized to receive RBCs which were stored 10 or fewer days vs. those randomized to receive RBCs which were stored 21 or more days. Analysis will be restricted to subjects who undergo cardiac surgery and receive at least one RBC unit between randomization and 96 hours following surgery.
Other scoring systems such as the Acute Physiology And Chronic Health Evaluation (APACHE) also define and tabulate organ failures as risk factors contributing to morbidity and mortality.[27] Yet other scoring systems assign mortality risk by including the intensity of clinical interventions. These include the Sequential Organ FAilure (SOFA) score,[28] which includes inotrope administration, and the more recently developed CArdiac SUrgery Score (CASUS), which includes the use of vascular assist devices and dialysis in its risk assignment.[29] However, clinical studies employing scoring systems which are based in part on clinical interventions should standardize patient management to minimize the effects of observer bias and confounding.
Outcomes comparing leukoreduced vs. non-leukoreduced RBC transfusions given to heart valve surgery patients defined multiple organ dysfunction as two or more systems failing and found that a high incidence of failure of two or more systems was strongly associated with the number of transfusions given.[30] Several series of cardiac surgical patients demonstrated the SOFA score and its changes over time correlated well with morbidity and mortality.[28,31,32] However, those studies did not require standardized management.
Several pediatric-specific scoring systems have also been developed and validated [33, 34, 35, 36], including Leteutre's pediatric adaptation of Marshall's MODS, named the PEdiatric Logistic Organ Dysfunction (PELOD) score, which includes mechanical ventilation interventions.[37] Change in PELOD score was a secondary endpoint in the recent Transfusion Strategies for Patients in Pediatric Intensive Care Units (TRIPICU) study evaluating liberal vs. standard transfusion practices in pediatric ICU patients.[38]
No scoring system for multiorgan dysfunction has been validated in both adults and children. In order to include both adolescents and adults in the RECESS analysis, a common scoring system must be used. Older children with a weight of at least 40 kg have similar physiology to adults, and the normal ranges of the parameters used to calculate MODS are very similar in adolescents and adults. Therefore, MODS is considered to have a similar interpretation in both age groups.
In summary, the change in MODS was chosen as the primary endpoint for RECESS since: it has been validated; it correlates with mortality and other measures of morbidity such as length of stay; its components are based on objective measurements; it reflects organ dysfunction as well as failure; it incorporates death; and it has been used in many studies including studies of cardiac surgery patients.
Secondary Outcomes
Many individual clinical events, such as myocardial infarction, pulmonary embolus, and cardiac arrest, are relatively infrequent in the weeks following cardiac surgery. Therefore, representatives of the NHLBI-supported Cardiothoracic Surgery Trials Clinical Trials Network provided input to help define composite endpoints that clinicians would find relevant. Their assistance was also incorporated in selecting other secondary endpoints.
Secondary endpoints that will be assessed in RECESS include:
All-cause mortality through 28 days;
ΔMODS through post-operative day 28, hospital discharge, or death, whichever comes first;
Composite of major in-hospital postoperative complications through postoperative Day 7, hospital discharge, or death, whichever occurs first (death, stroke, myocardial infarction, renal failure, or culture-proven sepsis/septic shock);
Composite of major cardiac events through postoperative Day 7, hospital discharge, or death, whichever occurs first (death, myocardial infarction, low cardiac output, ventricular tachycardia, or ventricular fibrillation);
Composite of major pulmonary events through postoperative Day 7, hospital discharge, or death, whichever occurs first (pulmonary embolism, or any mechanical ventilation more than 48 hours after surgery);
Ventilation duration through postoperative day 28, hospital discharge, or death, whichever comes first;
Any mechanical ventilation from 48 hours postoperative to Day 28, hospital discharge, or death, whichever occurs first;
-
Changes in the following laboratory parameters, from the preoperative baseline to the worst recorded post-operative value through postoperative Day 7, hospital discharge, or death, whichever occurs first:
Serum creatinine;
Troponin-I;
Lactate; and,
Liver function tests (bilirubin, and for children also alanine aminotransferase);
Days to first bowel movement through post-operative day 28, hospital discharge, or death, whichever comes first; and,
Days to first solid food through post-operative day 28, hospital discharge, or death, whichever comes first.
Sample Size and Statistical Considerations
Discussion of prior clinical trials suggests a difference in ΔMODS between study arms of 1 point is not likely to be clinically significant. In the cardiac patient subset of TRICC, the treatment arm difference in ΔMODS was also only 1.3 points (SD 7.3).[22] Similarly, discussion of the results of the ABLE pilot stated, “Absolute differences in major outcomes such as mortality, organ failure and infections less than 3-4% between RBC (of different storage times) [corresponding to a 1-2 point difference in ΔMODS] may not be worth pursuing”.[7] Small differences in outcomes would have to be weighed against the effect that changes in standard RBC banking practices might have, particularly if such changes had a deleterious impact on the availability of RBCs. Reduced RBC availability could result in under-transfusion, or cause delays in surgery or treatment, which could also have adverse clinical effects.
To determine the sample size for a trial designed to demonstrate superiority of one treatment strategy over another, investigators must pre-specify the treatment difference the study should have good statistical power to detect. To determine the sample size for a non-inferiority or equivalence study, the investigators must pre-specify the smallest treatment difference that would be considered clinically important. The smallest treatment group difference in ΔMODS that would justify a change in practice is a matter of debate, given the impact a change might have on blood availability. Therefore, rather than power RECESS to detect a particular treatment difference, the sample size was guided by the desired precision of the 95% confidence interval that will be calculated around the observed treatment difference. A treatment difference of ± 0.8 points or narrower in the ΔMODS between the study arms would correspond to a limited difference in a single organ system (e.g., a drop in the platelet count from 100,000/μL to 65,000/μL) and the confidence interval will provide a precise estimate of any changes in organ function due to exposure to RBC stored for different amounts of time.
The sample size for RECESS was calculated using the following assumptions:
Two-sided 95% confidence interval with a precision of ± 0.8 points or narrower for the treatment group difference in 7-Day ΔMODS, after adjustment for baseline value;
Equal sample size in each treatment group;
Baseline MODS are integer values between 0 and 24 with a mean of 7.8 and a standard deviation of 3.9. ΔMODS are also integer values with a range that depends on the baseline MODS value (because the 7-Day MODS must also be an integer between 0 and 24), and a standard deviation of 7.3.[22]; and,
No correlation between baseline MODS and ΔMODS. (If these are correlated, the confidence interval will be narrower.)
Based on these assumptions, it was calculated that the study will need 717 patients per arm who are evaluable for the primary endpoint. The adequacy of this sample size was confirmed through simulations. Evaluable patients are those who undergo cardiac surgery and receive at least one study transfusion, and have data available to calculate baseline MODS and MODS through Day 7, hospital discharge, or death, whichever occurs first. Based on data from a survey of several participating hospitals, 87% of patients with TRUST of 3 or more will receive transfusions, so approximately 1648 subjects must be randomized to obtain 1434 evaluable subjects. Assuming that 10% of the time the blood bank will not be able to provide enough units of RBC of one or both of the storage times, then approximately 1832 patients must be enrolled. Based on other transfusion studies, 20 - 25% of eligible subjects are expected to consent to participate in RECESS, so sites must identify approximately 8142 subjects (with TRUST of 3 or more for adults) to approach for consent and further eligibility assessment. As 52% of cardiac surgery patients at participating centers are expected to have TRUST scores of 3 or more, approximately 15,700 cardiac surgery patients have to be screened for RECESS.
Trial Management
RECESS was developed through, and will be conducted mainly within, the TMHCTN which is a multicenter clinical trials network including 17 main core clinical centers across the United States, and several affiliated sites. TMHCTN was originally funded by the National Heart, Lung, and Blood Institute (Transfusion Medicine and Cellular Therapeutics Branch) of the National Institutes of Health in 2002 and renewed in 2007.[12]
The Data Coordinating Center for RECESS and other TMHCTN studies is New England Research Institutes (NERI).[13] Established more than 20 years ago, NERI has served as the data coordinating center for numerous, large, multicenter clinical trials. NERI has extensive experience in coordinating transfusion studies, including TMHCTN's platelet dose study [40], the multi-center Viral Activation by Transfusion Study (VATS) [41], and several studies of transfusion in sickle cell patients [42,43]. NHLBI has established an independent Data and Safety Monitoring Board which has oversight for all of the clinical trials being carried out by the TMHCTN.
Conclusion
RBC transfusion has become a mainstay in managing patients in intensive care units, undergoing surgery, or being treated for malignancy. Despite the widespread use of RBCs, the data guiding transfusion practices are limited, and our understanding of the impact on patient outcomes modest. The question of whether storage of RBCs under standard blood bank conditions has clinically meaningful effects on patient well-being remains, after several decades of discussion and investigation, in a state of clinical equipoise. Along with clinical trials in neonates and intensive care patients, the RECESS study is positioned to provide data with which we will be able answer this question.
Acknowledgments
The authors would like to acknowledge the contributions of all of the individuals who contributed to the development of the RECESS Protocol: S. F. Assmann, Ph.D., E. Bennett-Guerrero, M.D., M. Blajchman, M.D., FRCPC, V. Butler, E. Gerstenberger, M.S., M. Delaney, D.O., P. D'Andrea, R.N., S. Glynn M.D., MPH, S. Granger, M.S., E. Jett, B.S., CCRA, J.H. Levy, M.D., J. Miller, MPH, T. Mondoro, Ph.D., G. Nemo, Ph.D., S. Pulkrabek, MT (ASCP) CCRC, S. R. Sloan, M.D., Ph.D., M. E. Steiner, M.D., M.S., C. P. Stowell, M.D., Ph.D., D. Triulzi, M.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steiner M, Stowell C. Does red blood cell storage affect clinical outcome? When in doubt, do the experiment. Transfusion. 2009;49:1286–90. doi: 10.1111/j.1537-2995.2009.02265.x. [DOI] [PubMed] [Google Scholar]
- 2.Hess J. Red cell changes during storage. Transfus Aph Sci. 2010;43 doi: 10.1016/j.transci.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Somani A, Hebbell R. The microcirculation and RBC transfusion. Transfus Aph Sci. 2010;43 [Google Scholar]
- 4.Tsai AG, Hofmann A, Cabrales P, Intaglietta M. Perfusion vs. oxygen delivery as targets for blood transfusion: the experimental evidence. Transfus Aph Sci. 2010;43 doi: 10.1016/j.transci.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakr Y. Techniques to assess tissue oxygenation in the clinical setting. Transfus Aph Sci. 2010;43 doi: 10.1016/j.transci.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Triulzi D, Yazer M. Clinical Studies of the effect of blood storage on patient outcomes. Transfus Aph Sci. 2010;43 doi: 10.1016/j.transci.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Hébert PC, Chin-Yee I, Fergusson D, et al. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg. 2005;100:1433–8. doi: 10.1213/01.ANE.0000148690.48803.27. [DOI] [PubMed] [Google Scholar]
- 8.Bennett-Guerrero E, Stafford-Smith M, Waweru PM, Bredehoeft SJ, Campbell ML, Haley NR, Phillips-Bute B, Newman MF, Bandarenko N. A prospective, double-blind, randomized clinical feasibility trial of controlling the storage age of red blood cells for transfusion in cardiac surgical patients. Transfusion. 2009;49:1375–83. doi: 10.1111/j.1537-2995.2009.02152.x. [DOI] [PubMed] [Google Scholar]
- 9.Vamvakas EC. Meta-analysis of clinical studies of the purported deleterious effects of “old” (versus “fresh”) red blood cells: are we at equipoise? Transfusion. 2010;50:600–10. doi: 10.1111/j.1537-2995.2009.02465.x. [DOI] [PubMed] [Google Scholar]
- 10.Fergusson D, Hutton B, Hogan DL, et al. The age of red blood cells in premature infants (ARIPI) randomized, controlled trial: study design. Transfus Med Rev. 2009;23:55–61. doi: 10.1016/j.tmrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 11.The Age of Blood (ABLE) Study. [2/2/10]; http://www.controlled-trials.com/ISRCTN44878718/
- 12.Transfusion Medicine and Hemostasis Clinical Trials Network (TMHCTN) [2/2/10]; http://www.tmhnetwork.org/
- 13.New England Research Institutes. [2/2/10]; http://neriscience.com/
- 14.Elahi MM, Yii M, Matata BM. Significance of oxidants and inflammatory mediators in blood of patients undergoing cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia. 2008;22:455–67. doi: 10.1053/j.jvca.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Johnson RG, Thurer RL, Kruskall MS, et al. Comparison of two transfusion strategies after elective operations for myocardial revascularization. J Thorac Cardiovasc Surg. 1992;104:307–14. [PubMed] [Google Scholar]
- 16.Marshall JC, Cook DJ, Cristou NV, et al. Multiple Organ Dysfunction Score: A reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. NEJM. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 18.Van de Watering L, Lorinser J, Versteegh M, Westendord R, Brand A. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion. 2006;46:1712–8. doi: 10.1111/j.1537-2995.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- 19.Yap CH, Lau L, Krishnaswamy M, Gaskell M, Yii M. Age of transfused red cells and early outcomes after cardiac surgery. Ann Thoracic Surg. 2008;86:554–9. doi: 10.1016/j.athoracsur.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 20.Cheng CK, Trethewey D, Sadek I. Comprehensive survey of red blood cell unit life cycle at a large teaching institution in eastern Canada. Transfusion. 2010;50:160–165. doi: 10.1111/j.1537-2995.2009.02375.x. [DOI] [PubMed] [Google Scholar]
- 21.Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–60. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- 22.Hébert PC, Yetisir E, Martin C, et al. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med. 2001;29:227–234. doi: 10.1097/00003246-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Wu O, Bayoumi N, Vickers MA, Clark P. ABO(H) blood groups and vascular disease: a systematic review and meta-analysis. J Thromb Haemost. 2008;6:62–9. doi: 10.1111/j.1538-7836.2007.02818.x. [DOI] [PubMed] [Google Scholar]
- 24.Alghamdi AA, Davis A, Brister S, Corey P, Logan A. Development and validation of Transfusion Risk Understanding Scoring Tool (TRUST) to stratify cardiac surgery patients according to their blood transfusion needs. Transfusion. 2006;46:1120–1129. doi: 10.1111/j.1537-2995.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- 25.Zelen M. The randomization and stratification of patients to clinical trials. J Chron Dis. 1974;27:365–75. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 26.Hébert PC, Wells G, Blachman MJ, Marshall J, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. New Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 27.Knaus WA, Wagner DP. Multiple system organ failure: epidemiology and prognosis. Crit Care Med. 1989;5:221–232. [PubMed] [Google Scholar]
- 28.Ceriani R, Mazzoni M, Bortone F, Gandini S, et al. Application of the sequential organ failure assessment score to cardiac surgical patients. Chest. 2003;123:1229–1239. doi: 10.1378/chest.123.4.1229. [DOI] [PubMed] [Google Scholar]
- 29.Hekmat K, Kroener A, Stuetzer H, Schwinger RH, et al. Daily assessment of organ dysfunction and survival in intensive care unit cardiac surgical patients. Annals Thorac Surg. 2005;79:1555–1562. doi: 10.1016/j.athoracsur.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Bilgin YM, van de Watering L, Eijsman L, Versteegh MIM, et al. Double-blind randomized controlled trial on the effect of leukocyte-depleted erythrocyte transfusions in cardiac valve surgery. Circulation. 2004;109:2755–2760. doi: 10.1161/01.CIR.0000130162.11925.21. [DOI] [PubMed] [Google Scholar]
- 31.Patila T, Kukkonen S, Vento A, et al. Relation of the sequential organ failue assessment score to morbidity and mortality after cardiac surgery. Ann Thorac Surg. 2006;82:2072–2079. doi: 10.1016/j.athoracsur.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 32.Mazzoni M, De Maria M, Bortone F, et al. Long term outcome of survivors of prolonged intensive care treatment after cardiac surgery. Ann Thorac Surg. 2006;82:2080–2088. doi: 10.1016/j.athoracsur.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson JD, Pollack MM, Ruttimann UE, et al. Outcome of pediatric patients with multiple organ system failure. Crit Care Med. 1986;14:271–274. doi: 10.1097/00003246-198604000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Proulx F, Fayon M, Farrell CA, et al. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996;109:1033–1037. doi: 10.1378/chest.109.4.1033. [DOI] [PubMed] [Google Scholar]
- 35.Pollack MM, Patel KM, Ruttiman UE. PRISM III: an updated Pediatric Risk of Mortality Score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Graciano AL, Balko JA, Rahn DS, Ahmad N, Giroir BP. The Pediatric Multiple Organ Dysfunction Score (P-MODS): development and validation of an objective scale to measure the severity of multiple organ dysfunction in critically ill children. Crit Care Med. 2005;33:1484–1491. doi: 10.1097/01.ccm.0000170943.23633.47. [DOI] [PubMed] [Google Scholar]
- 37.Leteurtre S, Martinot A, Duhamel A, Proulx F, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational multicentre study. Lancet. 2003;362:192–197.38. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 38.Lacroix J, Hébert PC, Hutchison JS, Hume HA, et al. Transfusion strategies for patients in pediatric intensive care units. New Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 39.Shime N, Kageyama K, Ashida H, Tanaka Y. Application of modified sequential organ failure assessment score in children after cardiac surgery. J Cardiothorac Vasc Anesthesia. 2001;15:463–468. doi: 10.1053/jcan.2001.24983. [DOI] [PubMed] [Google Scholar]
- 40.Slichter SJ, Kaufman RM, Assmann SF, McCullough J, et al. Dose of prophylactic platelet pransfusions and prevention of hemorrhage. New Eng J Med. 2010;362:600–613. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collier AC, Kalish LA, Busch MP, Gernsheimer T, et al. Leukocyte-reduced red blood cell transfusions in patients with anemia and human immunodeficiency virus infection: The Viral Activation Transfusion Study: a randomized controlled trial. J Amer Med Assoc. 2001;285:1592–1601. doi: 10.1001/jama.285.12.1592. [DOI] [PubMed] [Google Scholar]
- 42.Lee MT, Piomelli S, Granger S, Miller ST, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): Extended follow-up and final results. Blood. 2006;108:847–852. doi: 10.1182/blood-2005-10-009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams RJ, Brambilla D, Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) Trial Investigators Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. New Engl J Med. 2005;353:2769–2778. doi: 10.1056/NEJMoa050460. [DOI] [PubMed] [Google Scholar]