Abstract
The selectively bred alcohol-preferring (P) and -nonpreferring (NP) lines were developed from Wistar rats to model high and low voluntary alcohol consumption and have been demonstrated to exhibit many of the characteristics of human alcohol dependence. Electrophysiological studies have shown P rats exhibit more electroencephalographic fast frequency activity and reduced P3 amplitude in the parietal cortex than alcohol-nonpreferring (NP) rats, findings that are more common in alcohol dependent individuals. Event-related oscillations (EROs) have been suggested to be good endophenotypes associated with ethanol dependence in clinical studies. Recently EROs have also been demonstrated to occur in rodents in response to stimuli that are similar to that used in human clinical studies. The objective of the present study was to characterize EROs in adult P and NP rats. A time-frequency representation method was used to determine delta, theta and alpha/beta ERO energy and the degree of phase variation in the parietal cortex of adult P and NP rats. The present results suggest that the decrease in P3 amplitudes previously shown in P rats were not associated with changes in ERO energy but were significantly associated with decreases in evoked delta and alpha/beta phase locking. These studies demonstrate ERO measures may also be good endophenotypes in animal models of alcoholism.
Keywords: Alcohol-preferring rats, Event-related oscillations, Ethanol, Parietal cortex, P300, Event-related potentials, Electroencephalogram
Introduction
Genetic selection studies have resulted in a number of high drinking lines of mice and rats (see Bell et al., 2006; Green and Grahame, 2008). The alcohol-preferring (P) and -nonpreferring (NP) rat lines are one of the most extensively examined animal models of alcoholism (Li et al., 1993). These rats were developed for differences in home cage ethanol consumption in order to study ethanol drinking behaviors and their consequences (Bell et al., 2006; Lumeng et al., 1977). Selectively bred P rats have been shown to voluntarily consume levels of 10% ethanol of >5 g/kg/day, with an ethanol preference ratio (vs. water) of greater than 2:1. The NP rats generally consume levels of 10% ethanol of <1.5 g/kg/day, with an ethanol preference ration of less than 0.2:1. These lines have been the focus of intensive study of the behavioral, neurobiological, and neurophysiological factors that contribute to ethanol consumption (for reviews, see Bell et al., 2006; Li et al., 1993; McBride and Li, 1998).
Electrophysiological differences between the rat lines have also been demonstrated (Breen and Morzorati, 1996; Ehlers et al., 1991, 1992, 1999; Morzorati et al., 1994; Robledo et al., 1993, 1994). The study of neurophysiological endophenotypes in P and NP rats with well-differentiated ethanol-related phenotypes could be an additional tool for identifying susceptibility genes for ethanol dependence. We have previously characterized the electrophysiological profile of P and NP rats and found that P rats have significantly lower amplitude of the P3 component of the event-related potential (ERP), when compared to NP rats (Ehlers et al., 1999). This finding is similar to what has been reported for human subjects at differing risk for ethanol dependence (for review, see Porjesz et al., 2005).
There is evidence to suggest that ERPs may originate from an additive, evoked activation of neural assemblies independent of ongoing EEG as well as by the phase resetting of ongoing EEG oscillations in response to sensory input (for review, see Rangaswamy and Porjesz, 2008; Sauseng et al., 2007). Considerable efforts have been made in understanding how these models may potentially explain the generation of human ERPs. For instance, it has been proposed that the P3 component arises from a series of superimposed event-related oscillations (EROs) that are induced by sensory or cognitive processes that influence the dynamics of EEG rhythms (e.g. Demiralp et al., 2001; Karakas et al., 2000; Yordanova and Kolev, 1996). EROs are estimated by performing a decomposition of the EEG signal into phase and magnitude information over a range of frequencies and then the changes in those frequencies are characterized over a millisecond time scale with respect to task events. EROs have been demonstrated to be sensitive measures of both normal and abnormal cognitive functioning in humans (see Basar et al., 1999, 2001; Gevins et al., 1998; Klimesch et al., 1997; Schurmann et al., 2001). Additionally, these oscillations have been linked to several relevant genes associated with ethanol dependence phenotypes (Begleiter and Porjesz, 2006; Edenberg et al., 2004; Jones et al., 2004).
Studies have further demonstrated that delta and theta EROs are the primary contributors to the human P3 ERP component (Basar et al., 1999; Basar-Eroglu et al., 1992; Demiralp et al., 2001; Karakas et al., 2000; Schurmann et al., 2001). Reductions in P3 amplitude have been related to decreased cortical ERO energy and also to higher phase variability and weaker phase locking. For instance, it has been shown that the reduction of P3 amplitude during retrieval of a working memory task is associated with a decrease in delta ERO power and an increase in phase variability (Schack and Klimesch, 2002). In addition to their role in generating the P3 ERP component, evoked oscillations have also been shown to play a role in the generation of other ERP components, including the P1-N1 complex. There is evidence to suggest that alpha and theta evoked power and phase locking (PL) plays an important role in the generation of the P1-N1 complex (Klimesch et al., 2004). However, whether differences in ERO energy and phase variability play a role in the different neurophysiological profiles identified in animal models has been less studied
In view of the evidence from human studies of the link between alcohol dependence and ERO energy (Porjesz et al., 2005), characterizing the relationship between an ethanol preference phenotype and changes in EROs, translating this information to an animal model may provide valuable information on the mechanisms underlying these measures. The present study extended our initial analyses of neurophysiological endophenotypes in P and NP rats to EROs generated in cortical sites in response to an auditory oddball paradigm (standard, rare and noise tones). In this study, we investigated oscillatory activity in the delta, theta, and alpha/beta frequency ranges in the parietal cortex of P and NP rats within the temporal window of the P3 ERP response. ERO and PL analyses were accomplished from the same datasets that were used to generate the ERP data reported in a previous publication (Ehlers et al., 1999). We hypothesize that differences in hippocampal P3 amplitudes previously reported in P and NP rats (Ehlers et al., 1999) are associated to differences in delta and theta ERO oscillatory activity.
Materials and methods
Animals
Twenty-five (25) rats, 14 P and 11 NP male rats, of the 42nd generation, bred at Indiana University were received at The Scripps Research Institute weighing 281–410 g. All rats had ad libitum access to food and water. A detailed description of the environmental conditions of rats can be found elsewhere (Ehlers et al., 1999). The work described herein adheres to the guidelines stipulated in the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 80–23, revised 1996) and was reviewed and approved by The Scripps Research Institute’s Institutional Animal Care and Use Committee.
Surgical and electrophysiological recording procedures
Surgical and electrophysiological recording procedures performed in this study were previously described (Ehlers et al., 1999). In brief, P and NP rats were deeply anesthetized with Nembutal (50 mg/kg, intraperitoneally) and surgically prepared with recording electrodes at least two weeks prior to the experimental procedures. Stainless steel single-wire electrodes were implanted into the dorsal hippocampus (AP: −3.0 mm, ML: ±3.0 mm, DV: −3.0 mm) and amygdala (AP: 1.0 mm, ML: ±5.3 mm, DV: −8.5 mm) (Pellegrino et al., 1979). A 23-gauge stainless steel guide cannula was also aimed at the lateral ventricle (AP: −1.0 mm, ML: ±1.5 mm, DV: −4.6 mm). Screw electrodes were placed in the skull overlying the frontal (AP: 3.0 mm, ML: ±3.0 mm) and parietal (AP: −3.0 mm, ML: ±4.0 mm) cortices. A midline screw “reference” electrode was placed 3 mm posterior to lambda in the skull overlying the cerebellum. Electrode connections were made to a multipin Amphenol connector and the assembly was anchored to the skull with dental acrylic and anchor screws. EEG signals were recorded with a band pass of 0.5–70 Hz with a 60-Hz notch filter in. ERP trials were digitized at a rate of 256 Hz. Potential artifacts identified by computer software were excluded only after visual analysis of raw EEG. Only neurophysiological data recorded from the parietal cortex in animals treated with saline was included in the present study. The effects of intracerebroventricular administration of neurotensin on EEG and ERP were reported in a previous publication (Ehlers et al., 1999).
General procedures
Electrophysiological recordings were collected as previously described (Ehlers et al., 1999). In brief, a three-tone auditory ‘oddball’ paradigm originally developed to directly model studies employed in humans was used (Ehlers and Chaplin, 1992; Ehlers et al., 1991, 1994). The auditory ERP session consisted of 312 individual tone presentations. Three tone types were presented: standard tones (1000 Hz square wave, 70 dB, 84% probability), rare tones (2000 Hz square wave, 85 dB, 10% probability), and noise tones (white noise, 100 dB, 6% probability). Individual trials were 1000 ms in duration (100 ms pre-stimulus + 900 ms post-stimulus) and were separated by variable intervals ranging from 500 to 1000 ms. Rare tones were interspersed with standards such that no two rare tones occurred successively. The noise tone occurred every 16th trial. Further details about the auditory ERP sessions were described previously (Ehlers et al., 1999).
ERO and PLI analyses
ERO and phase-locking index (PLI) analyses were accomplished from the same datasets that were used to generate the cortical ERP data reported in a previous publication (Ehlers et al., 1999). Data from each trial generated by the stimuli were processed by a time-frequency analysis algorithm, which utilizes the S-transform (Stockwell et al., 1996), a generalization of the Gabor transform (Gabor, 1946), defined as:
The equation for calculation of the S-transform of discrete time series h(kT) at time jT and frequency n/NT is where T is the sample period of the discrete time series, j is the sample index, N is the number of samples in the time series, n is the frequency index, and H[ ] is the Fourier spectrum of the discrete time series. The computer code used is based closely on a C language S-transform subroutine available from the NIMH MEG Core Facility web site (http://kurage.nimh.nih.gov/meglab/). The defining equation of the S-transform is a convolution integral in continuous time. The method used is equivalent to the finite discrete time version of this, but for computational efficiency, multiplications in frequency domain are used rather than convolution in time domain. The inputs to the S-transform are real, but the outputs are complex. We use the magnitudes squared of the time-frequency output values, discarding the corresponding phase angles.
The PLI was calculated to measure phase variability in relation to stimulus onset, as previously described (Schack and Klimesch, 2002). The PLI, which ranges between zero and one. An increased PLI indicates less phase variability and stronger phase locking to the onset, whereas a reduced PLI indicates higher phase variability and weaker phase locking (Schack and Klimesch, 2002).
To reduce consequences of resulting discontinuities, we use a cosine window over the initial and final 100 msec of the input time series of each trial. The output of the transform for each stimuli and electrode site was calculated by averaging the individual trials containing the time-frequency energy distributions. To quantify S transform magnitudes, a region of interest (ROI) was identified by specifying the band of frequencies and the time interval contained in the rectangular ROI and the energy in that region was estimated. PLI was estimated at the peak amplitude within the ROI. These analyses are similar to what has been previously described (Jones et al., 2004; Schack and Klimesch, 2002). Baseline corrected post-stimulus activity (900 ms) was calculated by subtracting averaged pre-stimulus ERO energy values (100 ms) from the post-stimulus ROI values, as previously described (Padmanabhapillai et al., 2006). Normalized pre-stimulus ERO energy values for each ROI was determined by calculating a normalized baseline, defined as: V1 − (Hb × A1), where V1 is the volume energy of ROI1, Hb is the average height of the baseline for ROI1, and A1 is area of ROI1. The area of ROI1 (A1) is calculated as the frequency range of ROI1 × the total duration of ROI1. Hb is defined as: Vb ÷ Ab, where Vb is the volume of the baseline for ROI1, and Ab is the area of the baseline for ROI1. The ROI frequencies were: delta (1–4 Hz), theta (4–8 Hz), alpha/beta (8–32 Hz). The ROI time intervals correspond to the P3 component (250–325 ms) and are consistent with our previous ERP studies (Ehlers et al., 1999).
Statistical Analysis
Statistical analyses were performed by using SPSS for the Macintosh (SPSS, Inc., Chicago, IL). Values are mean ± standard error of the mean (SEM). Data analyses were performed on parietal ERO energy and PLI for the ROIs in response to standard, rare and noise tones. Group (P vs. NP) was assessed as a between subject variable. Tone (standard, rare and noise) was assessed as within subject repeated measures. For all repeated measures analyses, Greenhouse-Geisser corrected P-values were reported to account for violations of sphericity. Post hoc analysis of two-way repeated measures ANOVA (Tone) utilized pairwise comparisons. One-way ANOVA was used to assess strain differences. For these analyses, P-value was set at P < 0.05 to determine the levels of statistical significance.
Results
ERO mean energy and phase locking in P and NP rats
Effect of tone type on ERO energy and PLI
Two-way repeated measures ANOVA with ERO energy as dependent variable revealed a significant main effect of Tone in the theta and alpha/beta frequency bands in the parietal cortex (Table 1). Post hoc pairwise comparisons showed lower ERO energy in the parietal theta and alpha/beta bands occurred in response to rare and noise tones, compared to standard tones (Table 1).
Table 1.
Tone main effects of event-related oscillations (ERO) energy in P and NP rats in the parietal cortex
| Standard Tone | Rare Tone | Noise Tone | Tone Effects | |
|---|---|---|---|---|
| Delta Band | 61.3 ± 8.7 | 49.2 ± 12.4 | 48.7 ± 14.8 | F(1.4,27.2)=1.0, P>0.05 |
| Theta Band | 161.8 ± 16.1* | 96.9 ± 20.2 | 40.9 ± 30.3 | F(1.8,33.9)=11.5, P<0.001 |
| Alpha/Beta Band | 513.8 ± 31.6* | 335.5 ± 49.6 | 266.3 ± 48.4 | F(1.9,36.9)=14.4, P<0.001 |
Represent statistically significant difference;
standard vs. rare and noise tones
Two-way repeated measures ANOVA with PLI as dependent variable revealed a significant main effect of Tone in the delta, theta and alpha/beta frequency bands in the parietal cortices, when collapsing across P and NP groups (Table 2). Post hoc pairwise comparisons showed lower PLI in response to standard tones (vs. rare and noise tones) in the parietal delta and theta bands. Lower PLI in response to rare tones (vs. noise tones) was also found in the parietal alpha/beta band (Table 2).
Table 2.
Tone main effects of phase-locking index (PLI) in P and NP rats in the parietal cortex
| Standard Tone | Rare Tone | Noise Tone | Tone Effects | |
|---|---|---|---|---|
| Delta Band | 0.135 ± 0.013* | 0.399 ± 0.033 | 0.514 ± 0.026 | F(1.6,30.4)=56.9, P<0.001 |
| Theta Band | 0.134 ± 0.010* | 0.368 ± 0.028 | 0.459 ± 0.033 | F(1.8,33.5)=47.7, P<0.001 |
| Alpha/Beta Band | 0.128 ± 0.004* | 0.381 ± 0.016** | 0.487 ± 0.019 | F(1.9,36.3)=202.0, P<0.001 |
Represent statistically significant difference,
standard vs. rare and noise tones;
rare vs. noise tones.
ERO differences between P and NP rats: Baseline activity
Baseline ERO energy in the parietal delta band was not different between P and NP rats (Data not shown). Baseline ERO energy in the parietal theta band was lower in NP than in P rats in response to standard tones [P rats: 337.1 ± 48.2 vs. NP rats: 188.5 ± 32.7; F(1,20)=4.9, P<0.05]. Lower baseline ERO energy in the parietal alpha/beta band was also observed in NP rats in response to standard [P rats: 880.1 ± 62.4 vs. NP rats: 516.3 ± 42.2; F(1,20)=17.6, P<0.001], rare [P rats: 1095.0 ± 97.8 vs. NP rats: 625.2 ± 51.8; F(1,20)=12.6, P<0.005] and noise [P rats: 1209.1 ± 87.7 vs. NP rats: 715.4 ± 94.8; F(1,20)=13.5, P<0.005] tones.
ERO differences between P and NP rats: ERO energy and PLI in the 250–325 ms window
ERO Energy
ERO energy in the parietal delta band was not different between NP than in P rats (Table 3). ERO energy in the parietal theta and alpha/beta bands was lower in NP than in P rats in response to standard tones (Table 3).
Table 3.
ERO energy in P and NP rats during the time interval corresponding to the P3 component (250–325 ms) in the parietal cortex.
| Standard Tone | Rare Tone | Noise Tone | |
|---|---|---|---|
| Delta Band | F(1,20)=2.4, NS | F(1,20)=1.3, NS | F(1,20)=0.4, NS |
| P | 71.8 ± 11.2 | 63.2 ± 16.7 | 58.5 ± 21.3 |
| NP | 50.7 ± 12.9 | 35.2 ± 16.1 | 38.9 ± 14.4 |
| Theta Band | F(1,20)=4.6, P<0.05 | F(1,20)=0.1, NS | F(1,20)=0.3, NS |
| P | 196.4 ± 22.8 | 102.6 ± 30.4 | 58.5 ± 45.2 |
| NP | 127.2 ± 17.1 | 91.2 ± 13.2 | 23.2 ± 21.6 |
| Alpha/Beta Band | F(1,20)=9.2, P<0.01 | F(1,20)=0.2, NS | F(1,20)=2.5, NS |
| P | 609.6 ± 45.0 | 355.7 ± 73.5 | 343.3 ± 68.8 |
| NP | 418.0 ± 32.3 | 315.2 ± 38.5 | 189.3 ± 50.9 |
PLI
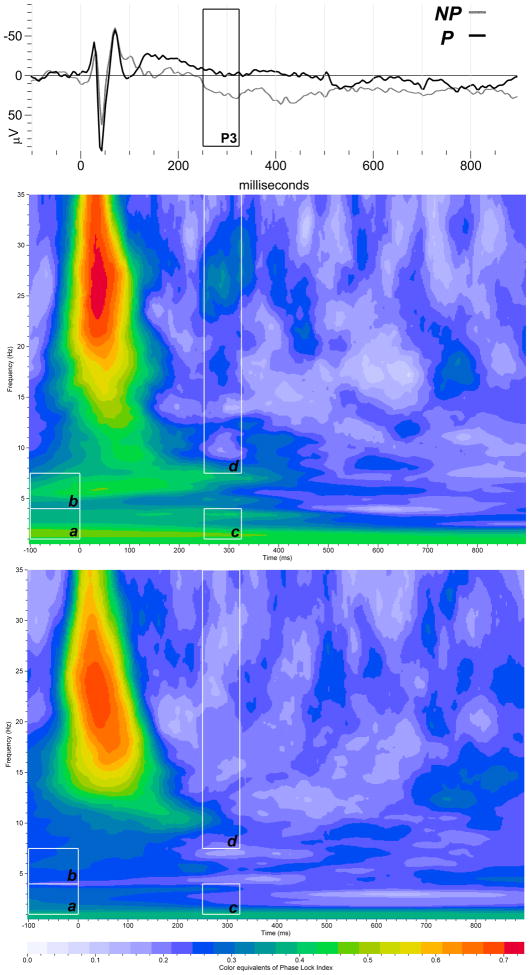
PLI in the parietal delta and alpha/beta bands was lower in P than in NP rats in response to noise tones (Table 4). PLI in the parietal theta band was not different between NP and P rats in response to standard, rare and noise tones (Table 4). Figure 1A–B illustrates grand-averaged PLI time-frequency representations of the noise tones for the delta (a, c), theta (b) and alpha/beta (d) frequency bands.
Table 4.
PLI in P and NP rats during the time interval corresponding to the P3 component (250–325 ms) in the parietal cortex.
| Standard Tone | Rare Tone | Noise Tone | |
|---|---|---|---|
| Delta Band | F(1,20)=1.8, NS | F(1,20)=0.1, NS | F(1,20)=5.1, P<0.05 |
| P | 0.152 ± 0.019 | 0.392 ± 0.041 | 0.455 ± 0.027 |
| NP | 0.117 ± 0.010 | 0.406 ± 0.050 | 0.572 ± 0.049 |
| Theta Band | F(1,20)=0.3, NS | F(1,20)=0.2, NS | F(1,20)=3.8, NS |
| P | 0.139 ± 0.015 | 0.380 ± 0.037 | 0.396 ± 0.036 |
| NP | 0.128 ± 0.012 | 0.355 ± 0.035 | 0.522 ± 0.060 |
| Alpha/Beta Band | F(1,20)=1.0, NS | F(1,20)=0.3, NS | F(1,20)=4.4, P=0.05 |
| P | 0.125 ± 0.004 | 0.373 ± 0.019 | 0.448 ± 0.020 |
| NP | 0.132 ± 0.008 | 0.389 ± 0.024 | 0.526 ± 0.034 |
Figure 1.
Time-frequency responses of evoked delta (a, c), theta (b) and alpha/beta (d) band PLI distribution to noise stimuli in P (A) and NP (B) rats in the parietal cortex. Time-frequency ROI windows used were −100 to 0 ms (baseline) and 250–325 ms (white squares). The inset shows representative ERP grand averages from NP (black line) and P (gray line) groups from the parietal cortex in response to the noise tones.
Discussion
Brain oscillations have been proposed to represent neurophysiological correlates of human information processing and cognitive function (Basar et al., 1999, Karakas et al., 2000). They have also been considered endophenotypes for complex genetic disorders, including drug addiction and psychiatric disorders (for reviews, see Begleiter and Porjesz, 2006; Porjesz et al., 2005). There is evidence to suggest that individuals with a positive family history of alcoholism have decreased P3 amplitude prior to significant ethanol drinking (Bauer et al., 1994; Begleiter et al., 1984; Pfefferbaum et al., 1991). Findings from the Collaborative Study on the Genetics of Alcoholism (COGA) have achieved significant progress identifying EROs associated with the human P3 component and several genes potentially involved in their regulation (for reviews, see Begleiter and Porjesz, 2006; Porjesz et al., 2005; Rangaswamy and Porjesz, 2008). Those studies have shown that ethanol dependent individuals manifest significantly less evoked delta and theta ERO power than age-matched controls (Jones et al., 2006a). Rangaswamy et al. (2007) have shown that adolescent offspring of ethanol dependent individuals have reduced delta and theta ERO power. These results suggest that a decrease in delta and theta EROs may antecede the development of ethanol dependence. Evidence from the COGA project has also shown a significant linkage and association between parietal delta ERO power and the cholinergic muscarinic receptor gene (CHRM2) on chromosome 7 (Jones et al., 2004, 2006b). These findings have provided a better understanding of the neurophysiological mechanisms and genes contributing to the human P3 component (for reviews, see Begleiter and Porjesz, 2006; Porjesz et al., 2005; Rangaswamy and Porjesz, 2008).
In contrast to the considerable progress understanding the relationship between human brain oscillations and an increased risk for ethanol dependence, brain oscillations have not been well characterized in animal models of high ethanol preference. The present study is part of our ongoing investigation characterizing cortical oscillatory activity in rodent models of high and low ethanol preference (see Criado and Ehlers, 2009; Ehlers and Criado 2009). Results from our earlier studies suggested that P rats have significantly lower P3 amplitudes than NP rats (Ehlers et al., 1999). The P3 component in rats is a broad positive going potential that peaks between 250 and 400 ms (e.g., Ehlers et al., 1994; Shinba, 1997). The present findings show that the decrease in parietal P3 amplitudes found in P rats in response to noise tones (Ehlers et al., 1999) is related to decreases in evoked delta and alpha/beta phase locking. Our findings also showed that ERO energy in the parietal theta and alpha/beta bands was lower in NP than in P rats. However, these differences were only observed in response to the standard tones, whereas reductions in P3 amplitudes in P rats were found in response to the noise tones. The results suggesting that baseline ERO energy in the parietal alpha/beta bands was higher in P than in NP rats are consistent with our previous studies characterizing EEG power in P and NP rats (Ehlers et al., 1999). These differences in baseline ERO energy in the alpha/beta bands were observed in response to standard, rare and noise tones and are likely independent to the reductions in P3 amplitude in P rats in response to noise tones (Ehlers et al., 1999).
Consistent with the present results, we recently showed that reductions in evoked delta phase locking in the parietal cortex are related to the decrease in P3 amplitudes in high ethanol preference C57BL/6 (B6) mice, compared to low ethanol preference DBA/2J (D2) mice (Criado and Ehlers, 2009). This previous study also demonstrated that reductions in evoked delta ERO energy and theta phase locking in the parietal cortex are associated with a decrease in P3 amplitudes in B6 mice (Criado and Ehlers, 2009). In contrast, the present study found that differences in ERO energy between P and NP rats do not play an important role in the decrease in P3 amplitudes found in P rats. These findings indicate that reduced P3 amplitudes in rat models of high ethanol preference may have different neurophysiological profiles than humans. These data also suggest different neurophysiological mechanisms associated with a reduction of P3 amplitudes in rat and mouse models of high ethanol preference (Ehlers et al., 1999; Ehlers and Somes, 2002; Slawecki et al., 2003).
Like most ERP studies using rodents as subjects a “passive” auditory oddball paradigm has been used to generate ERPs in rats. The advantages of a passive paradigm are that it can be administered to human and other animal subjects without extensive prior training, and it does not require the subject to respond to the stimuli. While ERPs have been successfully recorded in a number of animal species the use of ERO technology to study brain function in animal models has been less applied. The present studies extend and confirm our previous findings demonstrating that EROs can be recorded in rodents (Ehlers and Criado, 2009; Criado and Ehlers, 2009). Collapsing across lines, our findings showed that parietal theta and alpha/beta ERO energy was significantly reduced in response to the rare and noise tones, compared to the standard tones. Results from the present study also found that PLI in the parietal delta and theta bands was significantly increased in response to rare and noise tones, compared to the standard tones. These findings suggest that presentation of the standard tone produces an evoked response characterized by higher theta and alpha/beta ERO energy, but also shows an increase in delta and theta phase variability, compared to the noise and rare tones. The relationship between brain oscillations and P3 amplitudes has not been well characterized in animal models of high and low ethanol preference. Future studies are needed in these models to determine the relationship between their genetic and phenotypic profile with the expression of their different neurophysiological endophenotypes associated with reduced P3 amplitudes.
The phase-locking index, or PLI, reflects the degree of phase variation over trials and may have a significant effect on the amplitudes and latencies of ERP components (Gruber et al., 2005; Sauseng et al., 2007; Schack and Klimesch, 2002). PLI is similar to other measures of phase variation including ‘inter-trial coherence’ (Makeig et al., 2002), ‘phase-locking factor’ (Tallon-Baudry et al., 1996), and ‘phase-locking value’ (Rodriguez et al., 1999). Findings from the present study suggest that the decrease in P3 amplitudes in P rats is associated with a decrease in delta and alpha/beta band PLI. The mechanisms mediating the lower evoked delta and alpha/beta phase locking in adult P rats and their potential role increasing the risk for ethanol dependence are not clearly understood. Studies in humans have demonstrated that delta and theta EROs are the primary contributors to the human P3 ERP component (Basar et al., 1999; Basar-Eroglu et al., 1992; Demiralp et al., 2001; Karakas et al., 2000; Schurmann et al., 2001; Stampfer and Basar, 1985; Yordanova and Kolev, 1996). However, the primary contributors to the P3 ERP component in P and NP rats have not been determined. Further research needs to be done to understand the relationship between PLI, ERO energy and ERP amplitude.
Studies have demonstrated that, relative to NP rats, the P rat exhibit several phenotypes associated with alcohol abuse and alcoholism and meets the criteria of a valid animal model to assess alcohol-preference and excessive drinking (for review, see McBride et al., 2005; Bell et al., 2006). Consistent with studies in human subjects at risk for ethanol dependence (for review see Porjesz et al., 2005), our previous study showed that P rats have significantly lower P3 amplitude, when compared to NP rats (Ehlers et al., 1999). However, the relationship between an ethanol preference phenotype and changes in EROs are not well understood in genetic mouse models of high and low alcohol preference. There is evidence to suggest that multiple neurotransmitter systems contribute to differences in ethanol preference between P and NP rats (for review, see McBride et al., 2005; Bell et al., 2006). For instance, early-onset of ethanol drinking in P rats has been associated with higher density of cortical and hippocampal seronin-1A (5-HT1A) receptors and lower density in the expression of dopamine (DA) D2 receptors in the ventral tegmental area (VTA) (for review, see McBride et al., 2005). Previous studies in our laboratory in P and NP rats have also shown that differences in glutamate and GABA function may play an important role in their ethanol preference. We found that the acute effects of MK-801, an NMDA receptor antagonist, and diazepam, a GABA/benzodiazepine receptor agonist, on cortical and hippocampal EEG are significantly attenuated in P rats, in comparison to NP rats (Robledo et al., 1994). Whether deficits in these neurotransmitter systems play a role in the attenuation in evoked delta and alpha/beta phase locking in P rats remains unclear. While findings from the present studies contribute to our understanding of the neurophysiological mechanisms regulating the reduction in P3 amplitudes in P rats, further studies are needed to determine the relationship between the expression of these neurophysiological endophenotypes and the genetic profile of the P and NP rats. Understanding the relationship between evoked oscillatory activity, phase resetting and ERP responses in P and NP rats may provide insight into the brain processes underlying susceptibility to alcohol dependence.
Acknowledgments
Supported in part by National Institute on Alcoholism and Alcohol Abuse grant AA006059 and AA014339 and by the Stein Endowment fund. The computer programs were written by Dr. James Havstad. The authors thank Derek Wills, Evelyn Phillips, Phil Lau and Jennifer Roth for assistance in analyses, and Shirley Sanchez for assistance in editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basar-Eroglu C, Basar E, Demiralp T, Schurmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels. A review. Int J Psychophysiol. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-g. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci. Lett. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM, O’Connor S, Roberts L. P300 differences between nonalcoholic young men at average and above average risk for alcoholism: effects of distraction and task modality. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:263–277. doi: 10.1016/0278-5846(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Genetics of human brain oscillations. Int J Psychophysiol. 2006;60:162–171. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Breen TE, Morzorati SL. Innate differences in medial septal area burst firing neurons and the hippocampal theta rhythm during ambulation in selectively bred rat lines. Brain Res. 1996;714:156–164. doi: 10.1016/0006-8993(95)01520-5. [DOI] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Event-related oscillations as risk markers in genetic mouse models of high alcohol preference. Neuroscience. 2009;163:506–523. doi: 10.1016/j.neuroscience.2009.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Comerchero M, Polich J. Wavelet analysis of P3a and P3b. Brain Topogr. 2001;13:251–267. doi: 10.1023/a:1011102628306. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O’Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI, Lumeng L, Li TK. Electrophysiological response to ethanol in P and NP rats. Alcohol Clin Exp Res. 1991;15:739–744. doi: 10.1111/j.1530-0277.1991.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI, Wall TL, Lumeng L, Li TK, Owens MJ, Nemeroff CB. Corticotropin releasing factor (CRF): studies in alcohol preferring and non-preferring rats. Psychopharmacology (Berl) 1992;106:359–364. doi: 10.1007/BF02245418. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI. Long latency event related potentials in rats: The effects of changes in stimulus parameters and neurochemical lesions. J Neural Trans. 1992;88:61–75. doi: 10.1007/BF01245037. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Kaneko WM, Robledo P, Lopez AL. Long-latency event-related potentials in rats: effects of task and stimulus parameters. Neuroscience. 1994;62:759–769. doi: 10.1016/0306-4522(94)90474-x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Li TK, Lumeng L, Kinkead B, Owens MJ, Nemeroff CB. Neurontensin studies in alcohol naive, preferring and non- preferring rats. Neuroscience. 1999;93:227–236. doi: 10.1016/s0306-4522(99)00113-x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Somes C. Long latency event-related potentials in mice: effects of stimulus characteristics and strain. Brain Res. 2002;957:117–128. doi: 10.1016/s0006-8993(02)03612-0. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR. Event-related oscillations in mice: effects of stimulus characteristics. J Neurosci Methods. 2009;181:52–57. doi: 10.1016/j.jneumeth.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor D. Theory of Communication. J Inst Elec Eng. 1946;93:429–457. [Google Scholar]
- Gevins A, Smith ME, Leong H, McEvoy L, Whitfield S, Du R, Rush G. Monitoring working memory load during computer-based tasks with EEG pattern recognition methods. Hum Factors. 1998;40:79–91. doi: 10.1518/001872098779480578. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber WR, Klimesch W, Sauseng P, Doppelmayr M. Alpha phase synchronization predicts P1 and N1 latency and amplitude size. Cereb Cortex. 2005;15:371–377. doi: 10.1093/cercor/bhh139. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Goate A, Wang JC, Dick DM, Hinrichs A, Kwon J, Rice JP, Rohrbaugh J, Stock H, Wu W, Bauer LO, Chorlian DB, Crowe RR, Edenberg HJ, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O’Connor SJ, Schuckit MA, Stimus AT, Tischfield JA, Reich T, Begleiter H. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int J Psychophysiol. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Chorlian D, Rangaswamy M, Kamarajan C, Padmanabhapillai A, Stimus A, Begleiter H. S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clin Neurophysiol. 2006a;117:2128–2143. doi: 10.1016/j.clinph.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Dick D, Goate A, Hinrichs A, Rice JP, Wang JC, Bauer LO, Crowe R, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O’Connor SJ, Rohrbaugh J, Schuckit MA, Tischfield J, Edenberg HJ, Begleiter H. A cholinergic receptor gene (CHRM2) affects event-related oscillations. Behav Genet. 2006b;36:627–639. doi: 10.1007/s10519-006-9075-6. [DOI] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin Neuropathol. 2000;111:1719–1732. doi: 10.1016/s1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schimke H, Ripper B. Theta synchronization and alpha desynchronization in a memory task. Psychophysiology. 1997;34:169–176. doi: 10.1111/j.1469-8986.1997.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Schabus M, Doppelmayr M, Gruber W, Sauseng P. Phase-locked alpha and theta oscillations generate the P1-N1 complex and are related to memory performance. Brain Res Cogn Brain Res. 2004;19:302–316. doi: 10.1016/j.cogbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, Doolittle DP. Selective breeding for alcohol preference and associated responses. Behav Genet. 1993;23:163–170. doi: 10.1007/BF01067421. [DOI] [PubMed] [Google Scholar]
- Lumeng L, Hawkins TD, Li TK. New strains of rats with alcohol preference and non-preference. In: Thurman RG, Williamson JR, Drott H, Chance B, editors. Alcohol and aldehyde metabolizing systems III. Intermediary Metabolism and Neurochemistry. New York: Academic Press; 1977. pp. 537–544. [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences. Studies with animal models. Recent Dev Alcohol. 2005;17:123–142. doi: 10.1007/0-306-48626-1_6. [DOI] [PubMed] [Google Scholar]
- Morzorati S, Breen TE, Lumeng L, Li TK. Comparison of innate EEG parameters in rat lines selected for ethanol preference. Alcohol. 1994;11:253–258. doi: 10.1016/0741-8329(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Padmanabhapillai A, Tang Y, Ranganathan M, Rangaswamy M, Jones KA, Chorlian DB, Kamarajan C, Stimus A, Kuperman S, Rohrbaugh J, O’Connor SJ, Bauer LO, Schuckit MA, Begleiter H, Porjesz B. Evoked gamma band response in male adolescent subjects at high risk for alcoholism during a visual oddball task. Int J Psychophysiol. 2006;62:262–271. doi: 10.1016/j.ijpsycho.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. 2. New York: Plenum Press; 1979. [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Mathalon D. Event-related potentials in alcoholic men: P3 amplitude reflects family history but not alcohol consumption. Alcohol Clin Exp Res. 1991;15:839–850. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neuropathol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, Kuperman S, Rohrbaugh J, O’Connor SJ, Bauer LO, Schuckit MA, Begleiter H. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int J Psychophysiol. 2007;63:3–15. doi: 10.1016/j.ijpsycho.2006.10.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. Uncovering genes for cognitive (dys)function and predisposition for alcoholism spectrum disorders: a review of human brain oscillations as effective endophenotypes. Brain Res. 2008;1235:153–171. doi: 10.1016/j.brainres.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Lumeng L, Li TK, Ehlers CL. Effects of oral ethanol self-administration on the EEG of alcohol preferring and -nonpreferring rats. Psychopharmacology (Berl) 1993;113:60–66. doi: 10.1007/BF02244335. [DOI] [PubMed] [Google Scholar]
- Robledo P, Lumeng L, Li TK, Ehlers CL. Effects of MK 801 and diazepam on the EEG of P and NP rats. Alcohol Clin Exp Res. 1994;18:363–368. doi: 10.1111/j.1530-0277.1994.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception’s shadow: long-distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146:1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Schack B, Klimesch W. Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neurosci Lett. 2002;331:107–110. doi: 10.1016/s0304-3940(02)00846-7. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar-Eroglu C, Kolev V, Basar E. Delta responses and cognitive processing: single-trial evaluations of human visual P300. Int J Psychophysiol. 2001;39:229–239. doi: 10.1016/s0167-8760(00)00144-6. [DOI] [PubMed] [Google Scholar]
- Shinba T. Event-related potentials of the rat during active and passive auditory oddball paradigms. Electroencephalogr Clin Neurophysiol. 1997;104:447–452. doi: 10.1016/s0168-5597(97)00047-6. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Grahame NJ, Roth J, Katner SN, Ehlers CL. EEG and ERP profiles in the high alcohol preferring (HAP) and low alcohol preferring (LAP) mice: relationship to ethanol preference. Brain Res. 2003;961:243–254. doi: 10.1016/s0006-8993(02)03959-8. [DOI] [PubMed] [Google Scholar]
- Stampfer HG, Basar E. Does frequency analysis lead to better understanding of human event related potentials. Int J Neurosci. 1985;26:181–196. doi: 10.3109/00207458508985616. [DOI] [PubMed] [Google Scholar]
- Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: The S Transform. IEEE Trans on Signal Processing. 1996;44:998–1001. [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J, Kolev V. Brain theta response predicts P300 latency in children. Neuroreport. 1996;8:277–280. doi: 10.1097/00001756-199612200-00055. [DOI] [PubMed] [Google Scholar]