Abstract
Using two in vivo methods, microdialysis and rapid in situ electrochemistry, this study examined the modulation of extracellular glutamate levels by endogenously produced kynurenic acid (KYNA) in the prefrontal cortex (PFC) of awake rats. Measured by microdialysis, intraperitoneal (i.p.) administration of KYNA's bioprecursor L-kynurenine dose-dependently elevated extracellular KYNA and reduced extracellular glutamate (nadir after 50 mg/kg kynurenine: 60% decrease from baseline values). This dose-dependent decrease in glutamate levels was also seen using a glutamate-sensitive microelectrode array (MEA) (31% decrease following 50 mg/kg kynurenine). The kynurenine-induced reduction in glutamate was blocked (microdialysis) or attenuated (MEA) by co-administration of galantamine (3 mg/kg, i.p.), a drug that competes with KYNA at an allosteric potentiating site of the α7 nicotinic acetylcholine receptor. In separate experiments, extracellular glutamate levels were measured by MEA following the local perfusion (45 min) of the PFC with kynurenine (2.5 μM) or the selective KYNA biosynthesis inhibitor S-ethylsulfonylbenzoylalanine (S-ESBA; 5 mM). In agreement with previous microdialysis studies, systemic kynurenine application produced a reversible reduction in glutamate (nadir: −29%), whereas perfusion with S-ESBA increased glutamate levels reversibly (maximum: +38%). Collectively, these results demonstrate that fluctuations in the biosynthesis of KYNA in the PFC bi-directionally modulate extracellular glutamate levels, and that qualitatively very similar data are obtained by microdialysis and MEA. Since KYNA levels are elevated in the PFC of individuals with schizophrenia, and since prefrontal glutamatergic and nicotinic transmission mediate cognitive flexibility, normalization of KYNA levels in the PFC may constitute an effective treatment strategy for alleviating cognitive deficits in schizophrenia.
Keywords: α7 Nicotinic receptor, Astrocytes, Microelectrode array, Schizophrenia
Abnormal neurotransmitter interactions within the prefrontal cortex (PFC) play an important role in the deficits in executive function seen in patients with schizophrenia (SZ; Volk and Lewis, 2002; Barch, 2005; Tan et al., 2007). Pharmacological and genetic studies suggest that these cognitive impairments, which include abnormal attention and working memory, are critically shaped by dysregulations in cortical glutamatergic neurotransmission (Krystal, 2003; Lewis and Moghaddam, 2006; Robbins and Murphy, 2006). There is also good evidence for cholinergic dysfunction in SZ (for reviews, see Hyde and Crook, 2001; Sarter et al., 2005), including reductions in the expression of nicotinic (Guan et al., 1999; Mathew et al., 2007) and muscarinic (Dean et al., 2002; Scarr et al., 2009) receptors in the PFC, and an association between polymorphisms in the gene regulating nicotinic acetylcholine receptors (nAChR) and disease transmission (Mexal et al., 2010). Together, these impairments may constitute key components of the neurochemical disarray underlying prefrontal cognitive deficits in individuals with SZ (Sarter et al., 2005).
Elevated levels of kynurenic acid (KYNA) in the PFC may contribute to the abnormal glutamatergic and nicotinic function in SZ (Schwarcz et al., 2001; Erhardt et al., 2009). This concept is in part based on the finding that endogenous KYNA, an astrocyte-derived metabolite of the kynurenine pathway of tryptophan degradation (Kiss et al., 2003), acts preferentially as a non-competitive antagonist of the α7 nAChR (Hilmas et al., 2001). In the PFC, as elsewhere in the brain, these receptors are present in multiple locations, including glutamatergic and cholinergic nerve terminals (Lambe et al., 2003; Dickinson et al., 2008; Duffy et al., 2009), interneurons (Alkondon et al., 2000; Krenz et al., 2001) and astrocytes (Patti et al., 2007). It is likely, in particular, that the close association of cholinergic and glutamatergic terminals in the PFC accounts for the fact that α7 nAChR activity regulates prefrontal glutamate release (Marchi et al., 2002; Rousseau et al., 2005; Wang et al., 2006; Konradsson-Geuken et al., 2009). By inhibiting α7 nAChRs at a site that is very similar to that occupied by the pro-cognitive, allosteric potentiator galantamine (Samochocki et al., 2003; Lopes et al., 2007), elevated KYNA levels may therefore cause and/or exacerbate prefrontal hyponicotinergic and – secondarily – hypoglutamatergic transmission in SZ.
In the course of our studies investigating possible behavioral consequences of abnormal KYNA levels in the PFC, we recently began to examine the effects of acute KYNA manipulations on prefrontal glutamate release in intact, awake rats. In these experiments, we stimulated KYNA formation in the PFC locally by administering kynurenine, the immediate metabolic precursor of KYNA (Speciale et al., 1990; Swartz et al., 1990). In complementary studies, cortical KYNA levels were decreased pharmacologically by locally applied S-ethylsulfonylbenzoylalanine (S-ESBA), a selective but not brain-penetrable inhibitor of kynurenine aminotransferase II (KAT II), the major biosynthetic enzyme of KYNA in the mammalian brain (Pellicciari et al., 2006; Guidetti et al., 2007a,b). Using in vivo microdialysis, we observed that elevations and reductions in cortical KYNA levels resulted in reciprocal changes in extracellular glutamate levels in the PFC (Wu et al., 2010). These results were reminiscent of the bi-directional effects of fluctuating KYNA concentrations on extracellular dopamine and acetylcholine (Wu et al., 2006; Zmarowski et al., 2009).
The present study was designed to explore the relationship between KYNA and glutamate in the PFC in greater depth, using additional experimental approaches. Thus, we investigated the effects of systemically administered kynurenine, which raises KYNA levels throughout the brain and therefore better duplicates the situation in SZ (Rassoulpour et al., 2006). Moreover, we compared in vivo monitoring by microdialysis with a glutamate-sensitive microelectrode array (MEA), which allows the detection of extracellular glutamate levels with a temporal and spatial resolution not otherwise achievable (Burmeister and Gerhardt, 2001; Rutherford et al., 2007).
EXPERIMENTAL PROCEDURES
Animals
Male, adult Sprague-Dawley (Charles River Laboratories, Kingston, NY, USA) or Wistar rats (Charles River, Wilmington MA, USA) weighing 250–450 g were used in our experiments. Animals were maintained in a temperature- and humidity-controlled room on a 12:12 hour light:dark cycle (lights on at 0600 hr) and had access to food and water ad libitum. All procedures involving animals were approved by the University of Maryland or The Ohio State University Institutional Animal Care and Use Committees in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Materials
L-Kynurenine (sulfate form; `kynurenine'), KYNA, L-ascorbic acid (AA), dopamine (DA), L-glutamate (monosodium salt), glutaraldehyde [25% (w/v) in water], bovine serum albumin (BSA), and H2O2 were obtained from Sigma Aldrich Corp. (St. Louis, MO, USA). Galantamine was a research gift from Janssen Pharmaceutica (Beerse, Belgium). L-Glutamate oxidase (GluOx; EC 1.4.3.11) was purchased from Seikaghaku America, Inc. (East Falmouth, MA, USA). S-ESBA was synthesized as described previously (Pellicciari et al., 2006). Meta-phenylenediamine (m-PD) was purchased from Acros Organics (Fairlawn, NJ, USA). All solutions were prepared using distilled, deionized water.
Microdialysis
Rats used in Experiment 1 were anesthetized (chloral hydrate, 360 mg/kg, i.p.) and placed in a stereotaxic frame. A guide cannula (SciPro, Inc., Sanborn, NY, USA) was positioned unilaterally over the medial PFC (AP: 3.2 mm anterior to bregma, L: ± 0.6 mm from midline, DV: 2.0 mm below dura; hemispheres counterbalanced) and secured to the skull with acrylic dental cement. Coordinates were determined from the atlas of Paxinos and Watson (1995). On the next day, a microdialysis probe (CMA/10, membrane length: 3.0 mm, Carnegie Medicin, Stockholm, Sweden) was inserted and connected to a microperfusion pump set to a speed of 1 μL/min. The freely moving animals were perfused with Ringer solution containing (in mM) NaCl, 144; KCl, 4.8; MgSO4, 1.2; CaCl2, 1.7; pH 6.7. After the establishment of a stable baseline, test compounds were administered systemically. Microdialysis fractions were collected every 30 min, and KYNA and glutamate were determined in the same samples. Data were not corrected for recovery from the microdialysis probe.
HPLC-based measurements of KYNA and glutamate from dialysates
The content of KYNA and glutamate in microdialysate samples was determined according to established high performance liquid chromatography (HPLC) procedures (Swartz et al., 1990; Quarta et al. 2004). KYNA was measured following isocratic elution with a mobile phase containing 200 mM zinc acetate and 5% acetonitrile (pH 6.2) and detected fluorimetrically (excitation wavelength: 344 nm; emission wavelength: 398 nm). Glutamate (o-phthalaldehyde/β-mercaptoethanol derivatization; excitation wavelength: 390 nm; emission wavelength: 460 nm) was determined fluorimetrically after gradient elution.
The glutamate-sensitive microelectrode and the detection of glutamate-generated signals
The MEA for freely-moving animals consists of a ceramic paddle containing, at its tip, four platinum (Pt) recording sites, interfaced with a pre-amp headstage (see Konradsson-Geuken et al., 2009, for details on this assemblage). The four 15 × 333 μm Pt recording sites are arranged in two pairs beginning approximately 100 μm from the electrode tip. One pair of recording sites was designated to be sensitive to glutamate plus other endogenous electroactive species and was coated with GluOx (2%, 1 unit/1 μL, 100 nL), BSA (1%) and glutaraldehyde (0.125%). The remaining pair served as a background or sentinel site, sensitive to the oxidation of the same electroactive molecules except for glutamate. This coating arrangement and the design of the MEA allows for a self-referencing procedure in which the current derived exclusively from the oxidation of glutamate can be isolated (Day et al., 2006; Rutherford et al., 2007; Konradsson-Geuken et al., 2009). Coated MEAs were allowed to dry for ≥ 2 days at room temperature (25°C) and low humidity prior to in vitro calibration. m-PD (5.0 mM) was then electropolymerized onto all sites in order to reduce access of potential electroactive interferents, including AA and catecholamines, to the Pt recording sites (Mitchell, 2004). Electroplating was conducted in bubbled nitrogen using cyclic voltammetry (peak-to-peak amplitude of 0.25V every 0.05 sec for 25 min).
The enzyme detection scheme for generating the current derived by the selective oxidation of glutamate is more fully described elsewhere (Day et al., 2006; Rutherford et al., 2007; Konradsson-Geuken et al., 2009). Briefly, at the glutamate-sensitive sites, glutamate is oxidized by GluOx, generating α-ketoglutarate and H2O2. Because the MEA is maintained at a constant potential (+0.7V vs. Ag/AgCl reference), the H2O2 reporting molecule is oxidized, yielding two electrons. The resulting current is then amplified and recorded by a FAST-16 mkI recording system (Quanteon, LLC, Nicholasville, KY). On the sentinel channels, extracellular glutamate reaches the Pt surface but in the absence of GluOx no oxidation current is generated. Any current detected at these sites is due to endogenous electroactive molecules other than glutamate.
In vitro calibration of microelectrodes
MEAs were calibrated prior to implantation. Calibrations were performed in a stirred solution of phosphate-buffered saline (PBS; 0.05 M, 40 mL, pH 7.4, 37°C). After stabilization, AA (250 μM), glutamate (3 × 20 μM), DA (2 μM), and H2O2 (8.8 μM) were sequentially added to the calibration beaker. Amperometric signals were acquired at a rate of 2.0 Hz. The slope (sensitivity, nA/μM glutamate), limit of detection (μM glutamate), selectivity (ratio of glutamate over AA), and linearity (R2) were calculated. In order to be used for subsequent in vivo recordings, the MEAs had to conform to the following calibration criteria (single electrode mode): i) similar background current (i.e. no greater than a 20 pA difference between the glutamate-sensitive and sentinel channels), ii) linear response to increasing concentrations of glutamate (R2 > 0.998), iii) a minimum slope of −3.0 pA/μM glutamate, iv) a limit of detection of ≤ 0.5 μM, and v) a high selectivity for glutamate over either AA or DA (i.e. > 50:1).
Implantation of MEAs and dialysis probes
In Experiment 2, rats received, under isofluorane anesthesia, unilateral implants of MEAs in the PFC (AP: 2.7 mm anterior to bregma, L: ± 0.6 mm from midline, DV: 3.9 mm below dura; hemispheres counterbalanced). In some animals (Experiment 2B), a guide cannula for microdialysis probes (see above) was implanted 200 μm anterior to the MEA such that the membrane tip of the probe (3.0 mm) could be used for the intra-cortical delivery of test compounds in close proximity to the MEA. A dummy cannula was positioned in the guide cannula and extended 1.0 mm beyond the tip of the guide. An Ag/AgCl reference electrode was implanted in a contralateral site distant from the recording area.
In vivo recordings and intra-cortical perfusions
All electrochemical recordings were conducted in freely moving rats in a large wooden box (57.2 cm H × 41.9 cm W × 17.0 cm L) 2–6 days after implantation of the MEA (Experiment 2A) or MEA/dialysis probes (Experiment 2B). Animals were placed in the recording box and connected to the head stage. Stable baseline signals were recorded following a 3–4 hr habituation period. In Experiment 2A, the effects of systemic administration of test compounds on cortical glutamate were determined. In the first study, rats were treated with kynurenine (25 or 50 mg/kg, i.p.; n = 5/dose). In a second study, we tested the hypothesis that the effect of kynurenine was mediated by the antagonism of α7 nAChRs. Thus, we injected galantamine (3.0 mg/kg, i.p.), a positive allosteric modulator that binds at a site of the α7 nAChR that is very similar to that targeted by KYNA (Lopes et al., 2007), 5 min prior to kynurenine (50 mg/kg, n = 5). Recordings continued for 6 hrs post-injection. Another group of rats (n = 5) was injected with both kynurenine and galantamine except that, in this case, the administration of galantamine was delayed until 3 hrs post-kynurenine.
In Experiment 2B, the effects of local changes in KYNA on cortical glutamate levels were determined using counterbalanced perfusions of kynurenine (2.5 μM; to increase KYNA) or S-ESBA (5.0 mM; to decrease KYNA) on consecutive recording days (n = 5). A stable glutamate baseline was achieved during which artificial cerebrospinal fluid (aCSF, pH = 7.2; Zmarowski et al., 2009) was locally perfused (1.25 μL/min) for 45 min. Kynurenine or S-ESBA (solutions adjusted to pH 7.1–7.4) was then perfused at the same rate for 45 min, followed by a return to control aCSF perfusion for 45 min. Finally, in a separate group of rats, we determined the effects of S-ESBA (5.0 mM) on glutamate with the MEA potential reduced to +0.25 V (from +0.7 V). As glutamate-derived H2O2 does not oxidize at this lower potential, the elimination of the self-referenced signal at 0.25 V supports the interpretation that differences in current at the GluOx vs. sentinel sites reflected current derived from extracellular glutamate.
Histology
At the conclusion of each experiment, animals were anesthetized with isofluorane and then given an overdose of pentobarbital. Brains were removed and stored in formalin (10%) for at least 24 hrs, and then transferred to a sucrose solution (30%) for at least three days. Brains were sectioned using a cryostat; and coronal and sagittal sections (50 μm) were mounted on gelatin-coated slides, stained using cresyl violet and examined under a light microscope for verification of MEA and dialysis probe placements.
Data analysis
Microdialysis data were expressed as a percent change from baseline as baseline values did not differ significantly among treatment groups. The time-dependent effects of drug administration were determined using two-way analysis of variance (ANOVA) with DOSE and TIME as factors. A Huynh-Feldt correction was utilized in order to reduce Type I errors associated with repeated measures ANOVAs (Vasey and Thayer, 1987). Individual post-hoc comparisons were conducted using t-tests between dependent means and a Bonferroni adjustment of α level. For the microelectrode experiments, group comparisons of absolute glutamate levels or various temporal dimensions of the glutamate signal were analyzed using one- and two-way ANOVAs. In some instances, when only percentage data were being compared, t-tests between independent means were utilized. Significance was defined as P < 0.05. All statistical tests were performed using SPSS for Windows (Version 17.0; Chicago, IL).
RESULTS
Experiment 1: Effects of systemic kynurenine on cortical KYNA and glutamate levels as measured by microdialysis
The dose- and time-dependent effects of systemically administered kynurenine on extracellular levels of KYNA and glutamate in the PFC are illustrated in Fig. 1. The higher dose (50 mg/kg, i.p.) produced greater and longer-lasting elevations of KYNA than the lower dose (25 mg/kg, i.p.) (Panel A). These differences were evident as main effects of DOSE (F1,9 = 142.34, P < 0.001), TIME (F15,135 = 107.86, P < 0.001), and DOSE × TIME interaction (F15,135 = 29.54, P < 0.001). Panel B demonstrates that kynurenine also caused a dose-dependent reduction in prefrontal glutamate levels that was a mirror image of the elevations seen in KYNA. Again, the effect of the two doses on glutamate differed in terms of the magnitude of the decrease (F1,9 = 13.05, P = 0.006), its duration (F15,135 = 17.06, P < 0.001), and their interaction (F15,135 = 4.01, P < 0.001).
Figure 1.
Effects of systemically administered kynurenine (25 and 50 mg/kg, i.p.; arrows) on the extracellular levels of KYNA and glutamate in the rat PFC, assessed by in vivo microdialysis. KYNA and glutamate were determined in the same samples, as described in the text. Kynurenine produces a dose- and time-dependent increase in KYNA levels (A), which is closely mirrored by dose-dependent reductions in glutamate levels (B). Hatched symbols indicate baseline values (average of 4 samples: KYNA: 2.7 ± 0.2 nM; glutamate: 1.9 ± 0.1 μM), which were obtained from all animals. Data are the mean ± SEM of 5 (25 mg/kg) and 6 (50mg/kg) rats, respectively.
* = significantly different from last baseline value.
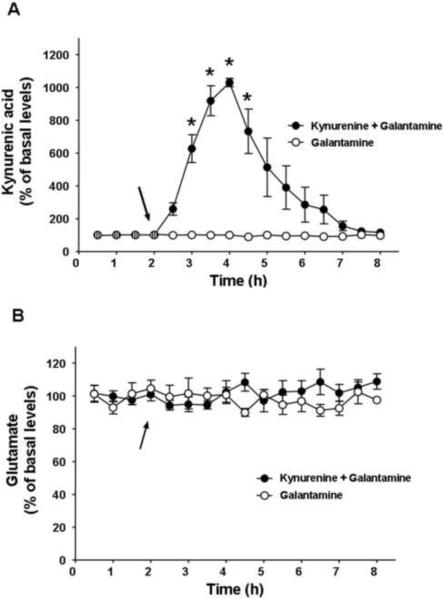
The effects of kynurenine on cortical glutamate levels are hypothesized to reflect increased α7 nAChR inhibition by elevated levels of KYNA (Wu et al., 2010). This was tested by applying galantamine, an α7 nAChR agonist that targets a very similar site of the α7 nAChR as KYNA (Lopes et al., 2007), in conjunction with kynurenine (Fig. 2). Co-administration of galantamine (3.0 mg/kg, i.p.) with kynurenine (50 mg/kg) had no effect on the ability of kynurenine to elevate KYNA levels (cf. Figs. 2A and 1A; F1,10 = 0.94, P = 0.36). Despite this persistent elevation of KYNA levels, galantamine completely blocked the ability of kynurenine to reduce cortical glutamate levels (cf. Figs 2B and 1B, F1,10 = 23.22, P = 0.001). Injection of galantamine (3.0 mg/kg, i.p.) alone had no effect on cortical levels of KYNA (Fig. 2A) or glutamate (Fig. 2B).
Figure 2.
Effects of systemically administered kynurenine (50 mg/kg, i.p.) + galantamine (3 mg/kg, i.p.) or galantamine alone (arrows) on the extracellular levels of KYNA and glutamate in the rat PFC, assessed by in vivo microdialysis. KYNA and glutamate were determined in the same samples, as described in the text. Co-administration of galantamine has no effect on kynurenine's ability to elevate KYNA (values and time course comparable to Fig. 1A) (A) but abolishes the ability of kynurenine to reduce glutamate (cf. Fig. 1B) (B). Galantamine alone has no effect on either KYNA or glutamate levels. Hatched symbols indicate baseline values (average of 4 samples: KYNA: 2.5 ± 0.2 nM; glutamate: 1.7 ± 0.2 μM), which were obtained from all animals. Data are the mean ± SEM of 6 (kynurenine + galantamine) and 4 (galantamine alone) rats, respectively.
* = significantly different from last baseline value.
Experiment 2A: Effects of systemic kynurenine on cortical glutamate levels as measured by the microelectrode array
The following experiments utilized the glutamate-sensitive MEA to examine the effects of fluctuating KYNA levels on prefrontal glutamate. Prior to implantation, MEAs were calibrated in a beaker with known concentration of potential analytes. Fig. 3 illustrates the results of a representative in vitro calibration. Current tracings following the administration of AA, glutamate, DA and H2O2 (indicated by arrows) are shown from each of the 4 channels. The top two tracings (GluOx) depict current at the glutamate-sensitive channels, whereas the bottom two tracings (sentinel) illustrate current from the sentinel or background channels. The addition of AA to the beaker produced only minor increases in oxidation current, which were, importantly, comparable on all 4 channels. Serial additions of glutamate (20 μM aliquots) produced large, reproducible and linear increases in current at the GluOx sites as the concentration of glutamate in the beaker was progressively elevated. In contrast, the signal on the sentinel channels did not change as a result of the addition of glutamate. Addition of DA produced only a slight change in current, indicating the effectiveness of m-PD, and this modest increase was comparable across the 4 channels. Finally, all 4 recording sites exhibited similar sensitivity to the reporter molecule, H2O2, which is a necessary condition for the self-referencing procedure used to isolate the current change caused by the oxidation of glutamate (Burmeister and Gerhardt, 2001).
Figure 3.
Representative in vitro calibration of the glutamate-sensitive MEA immediately prior to implantation into the PFC. Top two tracings: glutamate-sensitive (GluOx) recording channels. Bottom two tracings: sentinel background channels. Arrows indicate addition of various substances to the calibration beaker. Current (pAmp) is depicted along the vertical axis, and time (sec) along the horizontal axis. Successive additions of glutamate (raising concentration by 20 μM/aliquot) produce a linear increase in the glutamate signal. No glutamate-related changes in current are detected on the two sentinel channels. Note equivalent sensitivities to the reporter molecule H2O2 on all four channels. meta-Phenylenediamine (m-PD) (see Methods) coating blocked access of negatively charged in vivo interferents such as ascorbic acid (AA) or dopamine (DA).
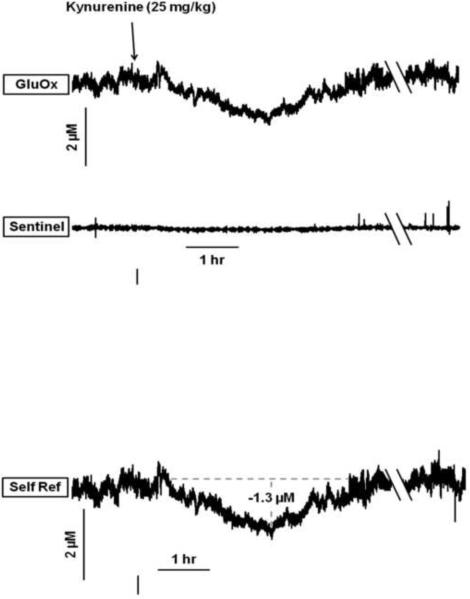
Fig. 4 shows a representative MEA tracing following the systemic administration of kynurenine (50 mg/kg, i.p.). After the establishment of a stable baseline, kynurenine produced a gradual and protracted reduction in signal at the glutamate-sensitive site (top tracing). There was only a minor negative drift in the current output at the background sentinel site (middle tracing). Self-referencing the sentinel from the GluOx sites yielded a signal that isolated current changes due only to the oxidation of glutamate (bottom tracing). The kynurenine-induced decrease in extracellular glutamate reached a maximum of −2.1 μM (−23%) from baseline by the end of the 6 hr recording session. Importantly, a control procedure conducted at the end of the session verified that the MEA had maintained its ability to record changes in extracellular glutamate levels. Thus, an infusion of exogenous glutamate (0.25 mM) at the nadir of the current output elicited a rapid and pronounced increase in signal. Control recordings conducted at +0.25 V eliminated any differences between the GluOx and sentinel sites, consistent with the interpretation that the self-referenced signal is generated by the oxidation of glutamate-derived H2O2 (data not shown). The bottom tracing in Fig. 4 illustrates a representative self-referenced record from an animal receiving a control injection of saline (0.9%, i.p.). This provides evidence that the injection per se did not lead to significant changes in current and that the signal was relatively stable over the entire recording session.
Figure 4.
Representative MEA tracings from a rat receiving kynurenine (KYN; 50 mg/kg, i.p.). Top two tracings: MEA signal from the glutamate-sensitive site (GluOx) and its adjacent background sentinel. The third trace from the top reflects the self-referenced signal (Self Ref) of the GluOx channel against the sentinel background. Deflections along the vertical axis reflect changes in concentration (μM), and the horizontal axis depicts time (hr). The total recording session extended for 6 hrs after kynurenine administration (note 3 hr break in time axis). Kynurenine produces a steady decline in the glutamate signal, reaching a maximum decrease of 2.1 μM by the end of the recording session (see Table 1 for group data). A control local infusion of glutamate (0.25 mM) rapidly elicits the characteristic phasic glutamate signal (8.5 μM increase), demonstrating that the MEA remained sensitive to changes in glutamate levels at the nadir of the kynurenine effect. Note that the abrupt drop in baseline, prior to the glutamate infusion, is an artifact reflecting disruption of background current due to the removal of the dummy cannula and the insertion of the infusion cannula. Bottom tracing: self-referenced signal from an animal that received a control saline injection (0.9%, i.p.), demonstrating stability of the basal glutamate signal.
Table 1 summarizes group data on the magnitude and timing of the changes in the glutamate signal. Overall, kynurenine (50 mg/kg, n = 5) caused a significant decrease (−31%) compared to basal glutamate levels (t4 = 4.67, P = 0.009). This reduction was evident within 6 min of the injection and reached a maximum 5 hrs later. This effect of kynurenine persisted throughout the 6 hr recording session.
TABLE 1.
GLUTAMATE LEVELS FOLLOWING SYSTEMIC INJECTIONS: GROUP MICROELECTRODE DATA
| MEASURE | KYNURENINE (25 mg/kg) | KYNURENINE (50 mg/kg) | GALANTAMINE (3 mg/kg) + KYNURENINE (50 mg/kg) |
|---|---|---|---|
| BASAL GLUTAMATE (μM) | 6.0 ± 1.3 | 6.2 ± 1.4 | 6.5 ± 1.6 |
| POST-INJECTION GLUTAMATE (% CHANGE FROM BASAL) | 5.4 ± 1.2 ( −10.6 ±1.1a) | 4.4 ± 1.1 (−30.8 ± 5.0) | 5.7 ± 1.4 (−10.8 ± 2.3a) |
| TIME TO EFFECT (min) | 16.2 ± 4.9 | 5.4 ± 3.6 | 16.3 ± 8.9 |
| TIME TO MAX EFFECT (min) | 111.2 ± 23.3a | 314.0 ± 20.0 | 77.8 ± 20.2a |
| DURATION OF EFFECT (min) | 210.3 ± 21.6a | > 6 hr | 146.6 ± 13.4a,b |
All pharmacological treatments were administered via intraperitoneal (i.p.) injections. Values are means ± S.E.M. with n = 5 rats/treatment group.
= significantly different from kynurenine (50 mg/kg)
= significantly different from kynurenine (25 mg/kg)
As in the microdialysis study (Fig. 1B), the ability of systemic kynurenine to decrease prefrontal glutamate levels was dose-dependent. Fig. 5 illustrates the tracings from a representative animal treated with 25 mg/kg kynurenine (i.p.) (n = 5). While the baseline glutamate signal was similar to that seen when studying the effects of the higher dose, administration of the lower dose produced an effect that was smaller in magnitude (−1.3 μM, −12% from baseline) and of shorter duration (return to baseline within 4 hrs) than the reduction caused by the higher dose (cf. Fig. 4). As summarized in the group data (Table 1), 25 mg/kg kynurenine caused a significant decrease (−11%) from basal glutamate levels (t4 = 3.83, P = 0.019). Glutamate levels were reduced within 16 min after kynurenine and returned to basal values within 3 ½ hrs of the injection. 50 mg/kg kynurenine produced greater reductions in glutamate levels than 25 mg/kg (F1,8 = 7.71, P = 0.02). While there was no significant dose-dependent difference in the onset of the effect, the time to reach maximal effect (F1,9 = 44.70, P < 0.001) and effect duration (F1,9 = 47.99, P < 0.001) were significantly longer for the higher dose than for the smaller dose.
Figure 5.
Representative MEA tracings from a rat receiving an injection of kynurenine (25 mg/kg, i.p.). The self-referenced glutamate signal (bottom tracing; Self Ref; note 3 hr break in time axis) reveals that 25 mg/kg kynurenine produces a smaller maximal decrease in glutamate (1.3 μM) than 50 mg/kg of kynurenine (cf. Fig. 4). Glutamate levels return to basal values within 3 ½ hrs (see Table 1 for group data).
The kynurenine-induced reduction of prefrontal glutamate levels reflected the ability of enhanced KYNA to antagonize the α7 nAChR. Thus, as in the microdialysis experiment (Fig. 2B), pre-treatment with galantamine (3.0 mg/kg, i.p.) markedly attenuated the effects of 50 mg/kg kynurenine. Fig. 6 illustrates a representative current tracing from the galantamine/kynurenine combination. The third trace from the top depicts the self-referenced glutamate signal following the combined treatment. Compared to the profile seen following 50 mg/kg kynurenine alone (Fig. 4), the combination of the two compounds produced a smaller maximal effect on glutamate (−1.3 μM), which reverted more quickly to basal levels (2 ½ hrs). The group data listed in Table 1 show that the combination of galantamine and kynurenine (n = 5) led to smaller decreases in glutamate (F1,9 = 13.34, P = 0.006) and also shorter durations of the inhibition (F1,9 = 255.36, P < 0.001) relative to kynurenine alone. In fact, pre-treatment with galantamine rendered the effects of 50 mg/kg kynurenine similar to the profile seen following the application of 25 mg/kg kynurenine alone. However, treatment with galantamine reduced the time to maximum effect relative to either dose of kynurenine (F1,9 = 6.28, P = 0.04).
Figure 6.
Representative MEA tracings from a rat receiving an injection of galantamine (3 mg/kg, i.p.), followed, 5 min later, by kynurenine (50 mg/kg, i.p.). Top two tracings: MEA signal from the glutamate-sensitive site (GluOx) and its adjacent background sentinel. The third tracing, illustrating the self-referenced (Self Ref) glutamate signal, reveals that galantamine attenuates the amplitude of the decline and the time course to values similar to those seen following 25 mg/kg kynurenine (cf. Fig. 5 and Table 1). Bottom tracing: self-referenced record from an animal that first received kynurenine (50 mg/kg, arrow) and then, 3 hrs later at the nadir of glutamate levels, galantamine (3 mg/kg, arrow). The glutamate signal gradually returns to basal values following delayed galantamine administration, suggesting that the kynurenine-induced attenuation of glutamate release is consistently mediated by α7 nAChRs.
The bottom trace in Fig. 6 (“Kyn/Gal”) illustrates an experiment in which the administration of galantamine was delayed until 3 hrs after the injection of kynurenine (50 mg/kg), i.e. a time when the inhibitory effect of kynurenine on glutamate was pronounced. Galantamine caused a clear reversal of the glutamate signal, which returned to baseline values within 90 min. These results demonstrated that the kynurenine-induced decrease in glutamate is consistently mediated by the α7 nAChR.
Experiment 2B: Effects of local KYNA changes on cortical glutamate levels as measured by the microelectrode array
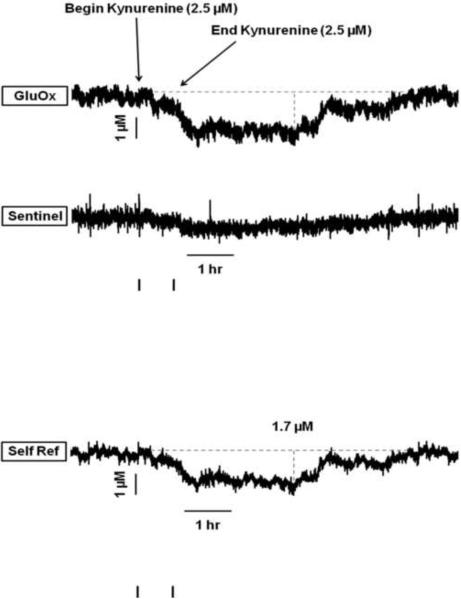
The next series of experiments determined the effects of local changes in KYNA on the prefrontal glutamate signal. In these studies, 2.5 μM kynurenine was perfused directly into the PFC to elevate KYNA levels. We had shown previously by microdialysis that this treatment causes a 125% increase in KYNA levels in the PFC (Wu et al., 2010). In the present experiment, perfusion of kynurenine (45 min in duration) produced a clear decrease in glutamate levels (Fig. 7). The isolated glutamate signal (bottom trace) from this representative animal revealed that glutamate was reduced by 1.7 μM (−25% from baseline) following kynurenine application. The decrease was apparent as soon as 6 min following the onset of the perfusion with the KYNA precursor and persisted for 5 ½ hrs. Analysis of the group data (n = 5) revealed that basal glutamate levels (7.73 ± 2.20 μM) lwere comparable to those reported in Table 1. Local administration of kynurenine decreased basal glutamate levels by 28.8 ± 8.5% (mean ± S.E.M.; t4 = 3.41, p = 0.04). As a group, the decreases occurred within 13 ± 7 min, reached a nadir by 112 ± 30 min and returned to baseline by 320 ± 38 min after the start of the kynurenine perfusion.
Figure 7.
Representative MEA tracings from a rat receiving an intracortical perfusion of kynurenine (2.5 μM, 45 min duration). The self-referenced glutamate signal (Self Ref) shows a kynurenine-induced decline in glutamate that begins within 6 min of the start of the perfusion, reaches a maximal decrease of 1.7 μM (25% decrease from baseline), and persists for 5 ½ hrs.
In contrast, local perfusion of S-ESBA (5.0 mM), an inhibitor of KYNA's biosynthetic enzyme KAT II, produced the opposite effect on basal glutamate levels in the PFC. Previous microdialysis experiments had demonstrated that local perfusion of the PFC with 3.0 mM S-ESBA causes a 35% reduction in extracellular KYNA levels and a concomitant significant increase in extracellular glutamate (Wu et al., 2010). In line with these results, the synthesis inhibitor produced a rapid and marked elevation in prefrontal glutamate levels measured by the MEA. Fig. 8 depicts a representative tracing from an S-ESBA recording session. Following a stable baseline, the 45 min perfusion of S-ESBA led to a clear increase in signal on the GluOx site (top trace) with very little change on the sentinel site (middle trace). The relative stability of the sentinel site confirmed the effectiveness of the m-PD exclusion layer. This was especially important since S-ESBA also stimulates extracellular DA levels in the PFC (Wu et al., 2006). The resultant, self-referenced glutamate signal (bottom trace) revealed a rapid increase to a maximum of 2.1 μM (37% from baseline). The increase was apparent as early as 5 min from the start of the infusion and persisted for approximately 1 hr. As a group (n = 5), basal glutamate levels (7.96 ± 2.82 μM) were similar to those reported in Experiment 2A (cf. Table 1). Local perfusion of S-ESBA increased basal glutamate levels by 38.4 ± 13.7% (mean ± S.E.M.; t4 = −3.61, p = 0.02). On average, the increase occurred within 4 ± 1 min, reached a maximum by 26 ± 4 min and returned to baseline by 65 ± 4 min after the start of perfusion with the enzyme inhibitor.
Figure 8.
Representative MEA tracings from a rat receiving an intracortical perfusion of the KAT II inhibitor S-ESBA (5 mM, 45 min duration). The bottom trace illustrates the self-referenced glutamate signal (Self Ref) and reveals a S-ESBA-induced increase in glutamate levels, which is apparent 5 min after the start of the perfusion. The increase reaches a maximum of 2.1 μM (37% above baseline) and persists for approximately 1 hr.
DISCUSSION
The present study revealed several novel facets of the recently described modulation of extracellular glutamate levels by endogenous KYNA (Wu et al., 2010). First, we showed that significant reductions in prefrontal glutamate are seen following the systemic administration of KYNA's precursor kynurenine. Second, the reduction of extracellular glutamate following the peripheral application of kynurenine was prevented by the co-application of galantamine, indicating that α7 nAChRs constitute a crucial link between excessive KYNA and glutamate. Third, qualitatively very similar results were obtained when the effect of systemic kynurenine administration was monitored by microdialysis or with a selective glutamate MEA. Finally, as measured by the MEA, local perfusion of the specific KYNA synthesis inhibitor S-ESBA produced an unexpectedly rapid elevation in prefrontal glutamate levels. Taken together, these results, which have functional implications for the cognitive deficits seen in schizophrenia patients, shed new light on the mechanisms that underlie the regulation of cortical glutamate release by endogenous KYNA.
KYNA is an astrocyte-derived neuromodulator
KYNA, initially described as a broad spectrum antagonist of ionotropic glutamate receptors with low potency (Perkins and Stone, 1982), inhibits the glycine co-agonist site of the N-methyl-D-aspartate (NMDA) receptor (NMDAR) competitively with an IC50 of ~8–15 μM in the absence of glycine. In the presence of glycine, the IC50 of KYNA increases to 239 μM (Kessler et al., 1989; Hilmas et al., 2001). More recently, electrophysiological and biochemical studies revealed that KYNA antagonizes α7 nAChRs, albeit non-competitively, at physiological (i.e. nanomolar) concentrations (Hilmas et al., 2001). α7 nAChRs are therefore assumed to constitute an important, and possibly preferential, target of endogenous KYNA in vivo (Stone, 2007; see below).
In the rat brain, KAT II is the major enzyme catalyzing the irreversible transamination of kynurenine to KYNA (Guidetti et al., 2007a). Brain KAT II is almost exclusively localized in astrocytes (Guidetti et al., 2007b), and in vitro studies have demonstrated that newly produced, astrocyte-derived KYNA is readily released into the extracellular milieu (Kiss et al., 2003) where it can modulate neuronal α7 nAChRs and NMDARs. Notably, extracellular KYNA levels are elevated when synthesis is driven by kynurenine and reduced when KAT II activity is compromised by pharmacological or genetic means (Turski et al., 1989; Swartz et al., 1990; Yu et al., 2004; Pellicciari et al., 2006). In the present study, manipulations with either kynurenine or the specific KAT II inhibitor S-ESBA (which does not enter the brain after peripheral administration) were used experimentally to explore the link between KYNA and glutamate in the PFC.
In contrast to KYNA, which penetrates the blood-brain barrier only poorly due to its polar nature, kynurenine enters the brain promptly after systemic application (Gál and Sherman, 1978) using the large neutral amino acid carrier that also transports more abundant, competing amino acids such as tryptophan and phenylalanine (Fukui et al., 1991). In agreement with a previous study in the rat striatum (Swartz et al., 1990), we demonstrated here that peripheral administration of kynurenine dose-dependently raised extracellular KYNA levels in the PFC. In quantitative terms, the increase in cortical KYNA levels following the systemic application of 25 mg/kg kynurenine was comparable to the effect caused by a local perfusion of 2.5 μM kynurenine, which we described recently (Wu et al., 2010). Of note, this dose of systemic kynurenine also duplicated the reduction in extracellular glutamate seen after a local perfusion of kynurenine in the PFC. Increases in KYNA in other brain areas therefore do not appear to interfere with the control of prefrontal glutamate by KYNA.
Regulation of prefrontal glutamate by KYNA: the role of α7 nAChRs
Although there is consensus that local application of nicotine enhances cortical glutamatergic transmission, the relative roles of specific cholinergic receptor subtypes appear to be intricate. Thus, initial electrophysiological and microdialysis studies implied both muscarinic and nicotinic (Toth et al., 1993; Vidal and Changeux, 1993) receptors, whereas subsequent work with genetic models and specific pharmacological agents provided convincing evidence that α4β2 nAChRs play a crucial role in nicotine-induced glutamate release in the PFC (Gioanni et al., 1999; Lambe et al., 2003; Parikh et al., 2008).
Work from several laboratories has shown that selective activation of α7 nAChRs, too, stimulates prefrontal glutamate release (Rousseau et al., 2005; Wang et al., 2006; Konradsson-Geuken et al., 2009). We posit that this effect is responsible for the bi-directional modulation of extracellular glutamate by endogenous KYNA described here. Our conclusion is based on the ability of galantamine, which functions as a selective allosteric potentiator of the α7 nAChR at the dose used (3 mg/kg, i.p.; Samochocki et al., 2003; Geerts et al., 2005), to attenuate the kynurenine-induced reduction in extracellular glutamate without affecting KYNA neosynthesis (cf. Figs. 1A and 2A). This effect of galantamine, which was observed here with either of the two monitoring procedures used, is most readily explained by the fact that the drug acts as an agonist at a site of the α7 nAChR that is very similar to the one that is inhibited by KYNA (Lopes et al., 2007). Notably, our experiments using the MEA revealed that α7 nAChRs are continuously involved in the KYNA-induced reduction in prefrontal glutamate levels, since administration of galantamine at the point of maximal kynurenine-induced decrease resulted in a reversal of glutamate levels toward basal values (Fig. 6).
In vivo experiments in other areas of the rat brain, using choline, methyllycaconitine and α-bungarotoxin as specific pharmacological tools (Rassoulpour et al., 2005; Lopes et al., 2007; Konradsson-Geuken et al., 2009), had previously also indicated that α7 nAChRs rather than NMDA receptors are the primary target of KYNA. The events linking fluctuations in extracellular KYNA levels, α7 nAChR activity, and glutamatergic transmission are complex, however. Thus, a recent ultrastructural immunogold study demonstrated that the majority (65%) of α7 nAChRs in the rat PFC are localized within dendrites and dendritic spines. These structures are receptive to axon terminals that lack α7 nAChRs but form asymmetric excitatory-type (i.e. probably glutamatergic) synapses. In contrast, a significant proportion (22%) of α7 nAChRs was found to be associated with presynaptic cholinergic nerve terminals, suggesting that acetylcholine release in the PFC is autoregulated through these receptors (Duffy et al., 2009). These anatomical data, together with earlier studies (Csillik et al., 1998; Alkondon et al., 2000; Krenz et al., 2001), do not support the proposition that ACh regulates glutamate release in the PFC solely by activating α7 nAChRs situated on glutamatergic nerve terminals (Marchi et al., 2002; Dickinson et al., 2008; Livingstone et al., 2010). Thus, the effect of KYNA on extracellular glutamate described here likely involves inhibition of heterogeneously distributed α7 nAChRs receptors - either within the PFC or in brain areas with reciprocal links to the PFC (Del Arco and Mora, 2005, 2008; Biton et al., 2007; Couey et al., 2007).
Convergence between microdialysis and microelectrode methods
The present study utilized two methods to assess extracellular glutamate levels, i.e. conventional microdialysis and rapid electrochemistry using a glutamate-sensitive MEA. Overall, both methods revealed qualitatively similar, α7 nAChR-mediated (galantamine-sensitive) reductions in prefrontal glutamate following systemically administered kynurenine. Moreover, the increase in cortical glutamate levels seen using the microelectrode following local perfusion of S-ESBA paralleled our recent microdialysis study (Wu et al., 2010). This convergence of results obtained with microelectrode and microdialysis/HPLC methodologies not only substantiates our experimental results but also provides additional indirect validation of the specificity of the self-referenced glutamate signal (Day et al., 2006; Rutherford et al., 2007).
The use of the microelectrode, which provides second-by-second resolution, allowed for a precise quantification of the onset and offset of our experimental interventions – in contrast to the temporal constraint imposed by the 30 min collection intervals in the microdialysis studies. These experiments demonstrated that systemic administration of kynurenine (50 mg/kg) causes a significant reduction in prefrontal glutamate within 5 min, and local perfusion of the KYNA synthesis inhibitor S-ESBA produces a similarly timed elevation (within 4 min) of glutamate levels. Although our methodological limitations did not permit the measurement of extracellular KYNA in 5-min intervals, these findings show that astrocytes can be efficiently manipulated to adjust KYNA production and, in functional terms, that KYNA can be mobilized to influence glutamatergic tone.
While the two methods yielded qualitatively identical patterns of glutamate modulation by KYNA, there were quantitative differences. For example, compared to microdialysis, MEA analyses showed a smaller response to kynurenine (50 mg/kg), yet the effect was longer in duration. Also, the increase in prefrontal glutamate levels following perfusion of S-ESBA was more pronounced when assessed by microdialysis (Wu et al., 2010) than by MEA. The nature and significance of the differences between the two in vivo monitoring techniques, which have also been noted by others (Rutherford et al., 2007; van der Zeyder, 2008), are currently under investigation in our laboratories.
Functional implications
Although the bi-directional modulation of prefrontal glutamate levels by KYNA is of obvious relevance to brain physiology, our findings may be especially pertinent for the etiology and treatment of several of the cognitive deficits seen in SZ. Individuals with SZ have abnormally high KYNA levels in the PFC (Schwarcz et al., 2001) and exhibit deficits in several prefrontally-mediated executive functions such as working memory (Barch and Smith, 2008), attention (Nuechterlein et al., 2009) and cognitive flexibility (Leeson et al., 2009). Dysregulations in cortical glutamatergic and cholinergic transmission have been proposed to underlie these cognitive deficits and may, in fact, be causally related to increased KYNA function (see Introduction). Consistent with this hypothesis, experimentally-induced elevations in brain KYNA levels in animals are associated with performance deficits in several behavioral tasks that require cognitive operations similar to those that are impaired in SZ (Shepard et al., 2003; Erhardt et al., 2004; Chess and Bucci, 2006; Chess et al., 2007).
Collectively, these data suggest that strategies designed to reduce the production of KYNA in brain may constitute efficacious adjunctive therapies for the treatment of cognitive deficits in SZ (Wonodi and Schwarcz, 2010). This approach would be in line with recent clinical studies, which showed modest pro-cognitive effects in SZ patients treated with partial α7 nAChR agonists, positive α7 nAChR modulators such as galantamine (Schubert et al., 2006; Freedman et al., 2008; Thomsen et al., 2010) or various glutamatergic agonists (Coyle, 2006). In light of the present results and the recent demonstration that KAT II-deficient mice show enhanced cognitive behaviors (Potter et al., 2010), we propose that selective, systemically active KAT II inhibitors might be especially efficacious as cognition enhancing agents in patients.
Acknowledgements
This work was supported by USPHS grant MH083729 to J.P.B. and R.S. R.S and R.P. are listed as inventors on a patent claiming cognitive enhancement by S-ESBA and its congeners. Å.K.-G. was partially supported by The Swedish Research Council (grant M2008-7218), Signe och Olof Wallenius Foundation, Fredrik och Ingrid Thurings Foundation, and PKF stiftelsen för Psykosomatisk Forskning. We are grateful for the contributions of Yasemin Sozeri and Andrew Campbell for their assistance in collecting the MEA data.
ABBREVIATIONS
- ACh
acetylcholine
- aCSF
artificial cerebrospinal fluid
- AA
ascorbic acid
- ANOVA
analysis of variance
- BSA
bovine serum albumin
- DA
dopamine
- GluOx
glutamate oxidase
- HPLC
high-performance liquid chromatography
- KAT
kynurenine aminotransferase
- KYNA
kynurenic acid
- m-PD
meta-phenylenediamine
- MEA
microelectrode array
- nAChR
nicotinic acetylcholine receptor
- NMDA
N-methyl-D-aspartate
- PFC
prefrontal cortex
- PBS
phosphate-buffered saline
- Pt
platinum
- S-ESBA
S-ethylsulfonylbenzoylalanine
- SZ
schizophrenia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alkondon M, Pereira EF, Eisenberg HJ, Albuquerque EX. Nicotinic receptor activation in human cerebral cortical interneurons: a mechanism for the inhibition and disinhibition of neuronal networks. J Neurosci. 2000;20:66–75. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Barch DM, Smith E. The cognitive neuroscience of working memory: relevance to CNTRICS and schizophrenia. Biol Psychiatry. 2008;64:11–17. doi: 10.1016/j.biopsych.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biton B, Bergis OE, Galli F, Nedelec A, Lochead AW, Jegham S, Godet D, Lanneau C, Santamaria R, Chesney F, Léonardon J, Granger P, Debono MW, Bohme GA, Sgard F, Besnard F, Graham D, Coste A, Oblin A, Curet O, Vigé X, Voltz C, Rouquier L, Souilhac J, Santucci V, Gueudet C, Françon D, Steinberg R, Griebel G, Oury-Donat F, George P, Avenet P, Scatton B. SSR180711, a novel selective alpha7 nicotinic receptor partial agonist:, (1) binding and functional profile. Neuropsychopharmacology. 2007;32:1–16. doi: 10.1038/sj.npp.1301189. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Gerhardt GA. Self referencing ceramic based multisite microelectrodes for the detection and elimination of interferences from the measurement of L-glutamate and other analytes. Anal Chem. 2001;73:1037–1042. doi: 10.1021/ac0010429. [DOI] [PubMed] [Google Scholar]
- Chess AC, Bucci DJ. Increased concentration of cerebral kynurenic acid alters stimulus processing and conditioned responding. Behav Brain Res. 2006;170:326–332. doi: 10.1016/j.bbr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33:797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, Mansvelder HD. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- Csillik B, Nemcsók J, Boncz I, Knyihár-Csillik E. Nitric oxide synthase and the acetylcholine receptor in the prefrontal cortex: metasynaptic organization of the brain. Neurobiology (Bp) 1998;6:383–404. [PubMed] [Google Scholar]
- Day BK, Pomerleau F, Burmesiter J, Huettl P, Gerhardt GA. Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain. J Neurochem. 2006;96:1626–1635. doi: 10.1111/j.1471-4159.2006.03673.x. [DOI] [PubMed] [Google Scholar]
- Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E. Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2002;7:1083–1091. doi: 10.1038/sj.mp.4001199. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Glutamate-dopamine in vivo interaction in the prefrontal cortex modulates the release of dopamine and acetylcholine in the nucleus accumbens of the awake rat. J Neural Transm. 2005;112:97–109. doi: 10.1007/s00702-004-0172-5. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav. 2008;90:226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Dickinson JA, Kew JN, Wonnacott S. Presynaptic α7- and β2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol Pharmacol. 2008;74:348–359. doi: 10.1124/mol.108.046623. [DOI] [PubMed] [Google Scholar]
- Duffy A, Zhou P, Milner TA, Pickel VM. Spatial and intracellular relationships between the alpha7 nicotinic acetylcholine receptor and the vesicular acetylcholine transporter in the prefrontal cortex of rat and mouse. Neuroscience. 2009;161:1091–1103. doi: 10.1016/j.neuroscience.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Olsson SK, Engberg G. Pharmacological manipulation of kynurenic acid. CNS Drugs. 2009;23:91–101. doi: 10.2165/00023210-200923020-00001. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry. 2004;56:255–260. doi: 10.1016/j.biopsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Johnson L, Allensworth D, Guzman-Bonila A, Clement B, Ball M, Kutnick J, Pender V, Martin L, Stevens K, Wagner B, Zerbe G, Soti F, Kem W. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Gál EM, Sherman AD. Synthesis and metabolism of L-kynurenine in rat brain. J Neurochem. 1978;30:607–613. doi: 10.1111/j.1471-4159.1978.tb07815.x. [DOI] [PubMed] [Google Scholar]
- Geerts H, Guillaumat PO, Grantham C, Bode W, Anciaux K, Sachak S. Brain levels and acetylcholinesterase inhibition with galantamine and donepezil in rats, mice, and rabbits. Brain Res. 2005;1033:186–193. doi: 10.1016/j.brainres.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Gioanni Y, Rougeot C, Clarke PBS, Lepousé C, Thierry M, Vidal C. Nicotinic receptors in the rat prefrontal cortex: increase in glutamate release and facilitation of mediodorsal thalmo-cortical transmission. Eur J Neurosci. 1999;11:18–30. doi: 10.1046/j.1460-9568.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10:1779–1782. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J Neurochem. 2007a;102:103–111. doi: 10.1111/j.1471-4159.2007.04556.x. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007b;55:78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EFR, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde TM, Crook JM. Cholinergic systems and schizophrenia: primary pathology or epiphenomena? J Chem Neuroanat. 2001;22:53–63. doi: 10.1016/s0891-0618(01)00101-6. [DOI] [PubMed] [Google Scholar]
- Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Kiss C, Ceresoli-Borroni G, Guidetti P, Zielke CL, Zielke HR, Schwarcz R. Kynurenate production by cultured human astrocytes. J Neural Transm. 2003;110:1–14. doi: 10.1007/s00702-002-0770-z. [DOI] [PubMed] [Google Scholar]
- Konradsson-Geuken A, Gash CR, Alexander K, Pomerleau F, Huettl P, Gerhardt GA, Bruno JP. Second-by-second analysis of alpha7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse. 2009;63:1069–1082. doi: 10.1002/syn.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz I, Kalkan D, Wevers A, de Vos RA, Steur EN, Lindstrom J, Pilz K, Nowacki S, Schütz U, Moser N, Witter B, Schröder H. Parvalbumin-containing interneurons of the human cerebral cortex express nicotinic acetylcholine receptor proteins. J Chem Neuroanat. 2001;21:239–246. doi: 10.1016/s0891-0618(01)00112-0. [DOI] [PubMed] [Google Scholar]
- Krystal JH. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology. 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, Joyce EM. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Livingstone PD, Dickinson JA, Srinivasan J, Kew JN, Wonnacott S. Glutamate-dopamine crosstalk in the rat prefrontal cortex is modulated by alpha7 nicotinic receptors and potentiated by PNU-120596. J Mol Neurosci. 2010;40:172–176. doi: 10.1007/s12031-009-9232-5. [DOI] [PubMed] [Google Scholar]
- Lopes C, Pereira EFR, Wu H-Q, Purushottamachar P, Njar V, Schwarcz R, Albuquerque EX. Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at α7* nicotinic receptors. J Pharmacol Exp Therap. 2007;322:48–58. doi: 10.1124/jpet.107.123109. [DOI] [PubMed] [Google Scholar]
- Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. Direct evidence that release-stimulating alpha7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J Neurochem. 2002;80:1071–1078. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- Mathew SV, Law AJ, Lipska BK, Dávila-García MI, Zamora ED, Mitkus SN, Vakkalanka R, Straub RE, Weinberger DR, Kleinman JE, Hyde TM. Alpha7 nicotinic acetylcholine receptor mRNA expression and binding in postmortem human brain are associated with genetic variation in neuregulin 1. Hum Mol Genet. 2007;16:2921–2932. doi: 10.1093/hmg/ddm253. [DOI] [PubMed] [Google Scholar]
- Mexal S, Berger R, Logel J, Ross RG, Freedman R, Leonard S. Differential regulation of alpha7 nicotinic receptor gene (CHRNA7) expression in schizophrenic smokers. J Mol Neurosci. 2010;40:185–195. doi: 10.1007/s12031-009-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KM. Acetylcholine and choline amperometric enzyme sensors characterized in vitro and in vivo. Anal Chem. 2004;76:1098–1106. doi: 10.1021/ac034757v. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Luck SJ, Lustig C, Sarter M. CNTRICS final task selection: control of attention. Schizophr Bull. 2009;35:182–196. doi: 10.1093/schbul/sbn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Man K, Decker MW, Sarter M. Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. J Neurosci. 2008;28:3769–3780. doi: 10.1523/JNEUROSCI.5251-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti L, Raiteri L, Grilli M, Zappettini S, Bonanno G, Marchi M. Evidence that α7 nicotinic receptor modulates glutamate release from mouse neocortical gliosomes. Neurochem Internat. 2007;51:1–7. doi: 10.1016/j.neuint.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1985. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Rizzo RC, Costantino G, Marinozzi M, Amori L, Guidetti P, Wu H-Q, Schwarcz R. Modulators of the kynurenine pathway of tryptophan metabolism: synthesis and preliminary biological evaluation of S-4-(Ethylsulfonyl)benzoylalanine, a potent and selective kynurenine aminotransferase II (KAT II)) inhibitor. Chem Med Chem. 2006;1:528–531. doi: 10.1002/cmdc.200500095. [DOI] [PubMed] [Google Scholar]
- Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Potter MC, Elmer GI, Bergeron R, Albuquerque EX, Guidetti P, Wu H-Q, Schwarcz R. Reduction of endogenous kynurenate formation enhances extracellular glutamate, hippocampal plasticity and cognitive behavior. Neuropsychopharmacology. 2010 March 24; doi: 10.1038/npp.2010.39. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D, Ferré S, Solinas M, You ZB, Hockemeyer J, Popoli P, Goldberg SR. Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. J Neurochem. 2004;88:1151–1158. doi: 10.1046/j.1471-4159.2003.02245.x. [DOI] [PubMed] [Google Scholar]
- Rassoulpour A, Wu H-Q, Ferré S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem. 2005;93:762–765. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- Rassoulpour A, Guidetti P, Conley RR, Roberts RC, Schwarcz R. Kynurenine pathway metabolism in the neostriatum of individuals with schizophrenia. Soc Neurosci Abstr. 2006;32:588.11. [Google Scholar]
- Robbins TW, Murphy ER. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci. 2006;27:141–148. doi: 10.1016/j.tips.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau SJ, Jones WW, Pullar IA, Wonnacott S. Presynaptic α7 and non-α7 nicotinic acetylcholine receptors modulate [3H]D-aspartate release from rat frontal cortex in vitro. Neuropharmacology. 2005;49:59–72. doi: 10.1016/j.neuropharm.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Rutherford EC, Pomerleau F, Huettl P, Strömberg I, Gerhardt GA. Chronic second-by-second measures of L-glutamate in the central nervous system of freely moving rats. J Neurochem. 2007;102:712–722. doi: 10.1111/j.1471-4159.2007.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samochocki M, Höffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, Radina M, Zerlin M, Ullmer C, Pereira EF, Lübbert H, Albuquerque EX, Maelicke A. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;305:1024–1036. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- Sarter M, Nelson CL, Bruno JP. Cortical cholinergic transmission and cortical information processing in schizophrenia. Schizophr Bull. 2005;31:117–138. doi: 10.1093/schbul/sbi006. [DOI] [PubMed] [Google Scholar]
- Scarr E, Cowie TF, Kanellakis S, Sundram S, Pantelis C, Dean B. Decreased cortical muscarinic receptors define a subgroup of subjects with schizophrenia. Mol Psychiatry. 2009;14:1017–1023. doi: 10.1038/mp.2008.28. [DOI] [PubMed] [Google Scholar]
- Schubert MH, Young KA, Hicks PB. Galantamine improves cognition in schizophrenic patients stabilized on risperidone. Biol Psychiatry. 2006;60:530–533. doi: 10.1016/j.biopsych.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu H-Q, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Joy B, Clerkin L, Schwarcz R. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology. 2003;28:1454–1462. doi: 10.1038/sj.npp.1300188. [DOI] [PubMed] [Google Scholar]
- Speciale C, Wu HQ, Gramsbergen JB, Turski WA, Ungerstedt U, Schwarcz R. Determination of extracellular kynurenic acid in the striatum of unanesthetized rats: effect of aminooxyacetic acid. Neurosci Lett. 1990;116:198–203. doi: 10.1016/0304-3940(90)90410-b. [DOI] [PubMed] [Google Scholar]
- Stone TW. Kynurenic acid blocks nicotinic synaptic transmission to hippocampal interneurons in young rats. Eur J Neurosci. 2007;25:2656–2665. doi: 10.1111/j.1460-9568.2007.05540.x. [DOI] [PubMed] [Google Scholar]
- Swartz KJ, During MJ, Freese A, Beal MF. Cerebral synthesis and release of kynurenic acid: an endogenous antagonist of excitatory amino acid receptors. J Neurosci. 1990;10:2965–2973. doi: 10.1523/JNEUROSCI.10-09-02965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes, and the pathogenesis of schizophrenia. Cerebral Cortex. 2007;17(Suppl 1):171–181. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD. Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr Pharm Des. 2010;16:323–343. doi: 10.2174/138161210790170094. [DOI] [PubMed] [Google Scholar]
- Toth E, Vizi ES, Lajtha A. Effect of nicotine on levels of extracellular amino acids in regions of the rat brain in vivo. Neuropharmacology. 1993;32:827–832. doi: 10.1016/0028-3908(93)90192-6. [DOI] [PubMed] [Google Scholar]
- Turski WA, Gramsbergen JBP, Traitler H, Schwarcz R. Rat brain slices produce and liberate kynurenic acid upon exposure to L-kynurenine. J Neurochem. 1989;52:1629–1636. doi: 10.1111/j.1471-4159.1989.tb09218.x. [DOI] [PubMed] [Google Scholar]
- Van der Zeyder M, Oldenziel WH, Rea K, Cremers TI, Westerink BH. Microdialysis of GABA and glutamate: analysis, interpretation and comparison with microsensors. Pharmacol Biochem Behav. 2008;90:135–147. doi: 10.1016/j.pbb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: a multivariate solution. Psychophysiology. 1987;24:479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Vidal C, Changeux JP. Nicotinic and muscarinic modulations of excitatory synaptic transmission in the rat prefrontal cortex in vitro. Neuroscience. 1993;56:23–32. doi: 10.1016/0306-4522(93)90558-w. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Impaired prefrontal inhibition in schizophrenia: relevance for cognitive dysfunction. Physiol Behav. 2002;77:501–505. doi: 10.1016/s0031-9384(02)00936-8. [DOI] [PubMed] [Google Scholar]
- Wang HW, Liao WN, Chang CT, Wang SJ. Facilitation of glutamate release by nicotine involves the activation of a Ca2+/calmodulin signaling pathway in rat prefrontal cortex nerve terminals. Synapse. 2006;59:491–501. doi: 10.1002/syn.20267. [DOI] [PubMed] [Google Scholar]
- Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in schizophrenia. Schizophr Bull. 2010;36:211–218. doi: 10.1093/schbul/sbq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HQ, Pellicciari R, Schwarcz R. Bidirectional regulation of extracellular dopamine by endogenous kynurenic acid in the rat medial prefrontal cortex. Soc Neurosci Abstr. 2006;32:624.3. [Google Scholar]
- Wu H-Q, Pereira EFR, Bruno JP, Pellicciari R, Albuquerque EX, Schwarcz R. The astrocyte-derived α7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in prefrontal cortex. J Mol Neurosci. 2010;40:204–210. doi: 10.1007/s12031-009-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Di Prospero NA, Sapko MT, Cai T, Chen A, Melendez-Ferro M, Du F, Whetsell WO, Jr, Guidetti P, Schwarcz R, Tagle DA. Biochemical and phenotypic abnormalities in kynurenine aminotransferase II-deficient mice. Mol Cell Biol. 2004;24:6919–6930. doi: 10.1128/MCB.24.16.6919-6930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmarowski A, Wu H-Q, Brooks JM, Potter MC, Pellicciari R, Schwarcz R, Bruno JP. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur J Neurosci. 2009;29:529–538. doi: 10.1111/j.1460-9568.2008.06594.x. [DOI] [PubMed] [Google Scholar]