Summary
When viewing a different stimulus with each eye we experience the remarkable phenomenon of binocular rivalry: alternations in consciousness between the stimuli [1, 2]. According to a popular theory first proposed in 1901, neurons encoding the two stimuli engage in reciprocal inhibition [3-8] so that those processing one stimulus inhibit those processing the other, yielding consciousness of one, dominant, stimulus at any moment, the other being suppressed. Also according to the theory, neurons encoding the dominant stimulus adapt, weakening their activity and the inhibition they can exert, while neurons encoding the suppressed stimulus recover from adaptation, until the balance of activity reverses, triggering an alternation in consciousness. Despite its popularity, this theory has one glaring inconsistency with data: during an episode of suppression, visual sensitivity to brief probe stimuli in the dominant eye should decrease over time, and should increase in the suppressed eye, yet sensitivity appears constant [9, 10]. Using more appropriate probe stimuli (Experiment 1) in conjunction with a new method (Experiment 2) we found that sensitivities in dominance and suppression do show the predicted complementary changes.
Highlights
We devised a new method to probe contrast sensitivity during rivalry episodes
Sensitivity of the dominant eye declines; sensitivity of the suppressed eye improves
Sensitivities are similar just prior to a switch of perceptual dominance
This confirms predictions from reciprocal-inhibition theory of binocular rivalry
Results and Discussion
The previous studies that failed to find changes in sensitivity during an episode of suppression suffered two limitations. First, the probe stimuli used to test sensitivity were very different from the stimuli engaged in rivalry. Fox and Check [9] used gratings as rival stimuli and briefly flashed monocular letters as probes, measuring letter-identification performance. Norman et al. [10] also used gratings as rival stimuli and very small monocular spots of light as probes, measuring luminance detection thresholds. In both cases, the probes had very different spatial frequency and orientation content from the rival stimuli. Because there is good evidence that adaptation is specific to the spatial properties of the adapting stimulus [11], such probes are unsuitable to quantify the supposed changing state of adaptation of the units responding to the rival stimuli. We addressed this limitation in Experiment 1.
Second, the previous studies examined only the first half of a suppression episode. It is possible that the effects of adaptation reveal themselves only late in an episode of suppression. In Experiment 2 we introduce a new method allowing us to study all of a suppression episode.
Experiment 1
We measured thresholds for detecting a brief increment in the contrast of either the upper or lower half of the rival target itself [12] (Figure 1). This increment was presented either early (200 ms) or late (the median of each observer's dominance durations) after onset of an episode of dominance or suppression. By pressing one of two keys, observers judged whether the probe increment was in the upper or lower half; over trials, the size of the contrast increment was adjusted using a staircase procedure to find the threshold.
Figure 1.
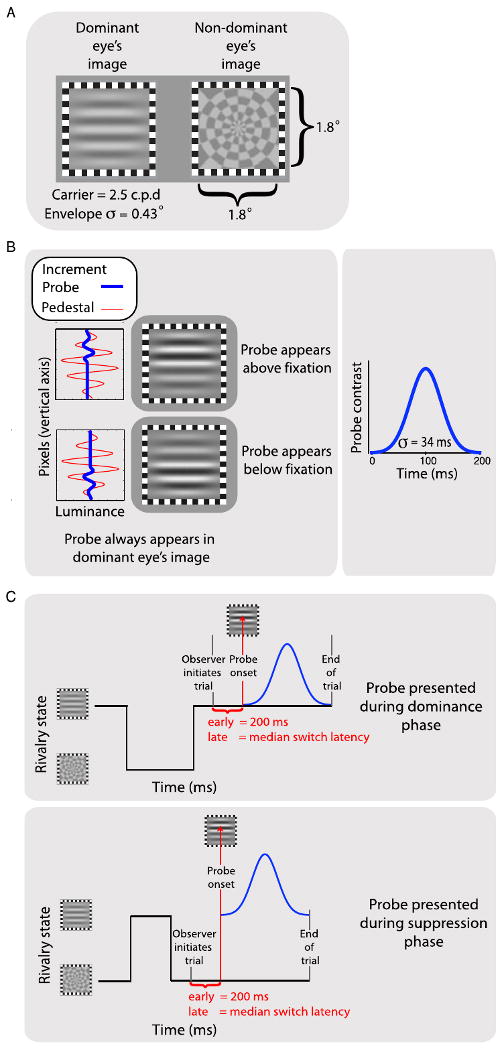
(A) Illustrations of the rival targets used in Experiments 1 and 2. The observer's rivalry-dominant eye always received the grating. (B) The two panels in the left-hand column plot the luminance profiles of the grating (red curve; pedestal) and the probe (blue curve). The probes were contrast increments added to the upper or lower half of the grating (illustrated in the right-hand column), with the magnitude of the contrast increment varied adaptively to find the contrast increment threshold. The probe contrast increment was added smoothly over time with a Gaussian profile (right-hand plot). (C) In Experiment 1, a series of short trials was used. Observers waited for rivalry to stabilise, then pressed a key to show the probe once the desired state was achieved (dominance or suppression, depending on condition). A brief period elapsed, either 200 ms for early probes or the median dominance duration for late probes (approximately 3 seconds), after which the probe contrast increment was presented. The trial terminated after the probe and the screen went blank. When the rivalry state changed prior to the onset of the probe, observers released the key, leading to the trial's being aborted.
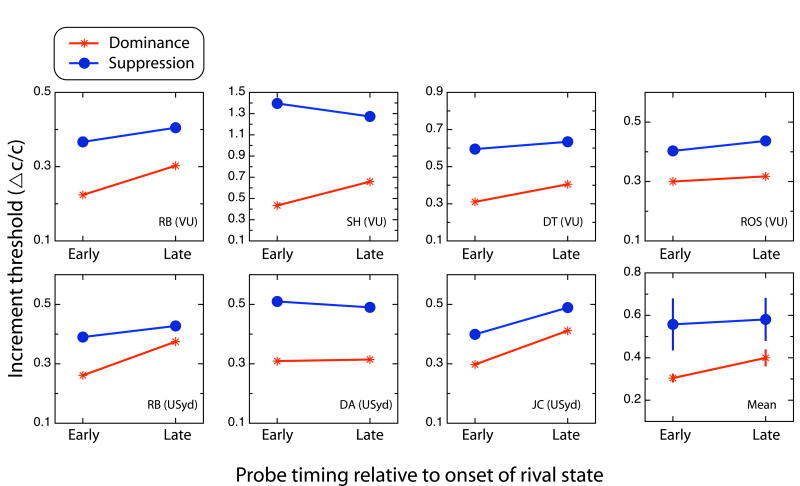
Figure 2 summarizes mean probe thresholds for each observer and condition. Note that thresholds are low when sensitivity is high and vice versa. Plotted in each panel is the average increment threshold for each of the four probe conditions (early and late probes delivered during dominance and during suppression). There is considerable variability among observers in the values of these thresholds, but this could be because different observers were purposefully tested using different background grating contrasts (to slow their rivalry state durations). Observer RB was tested both at Vanderbilt University and at University of Sydney, and his results measured at those two sites are comparable. RB's two data sets, although shown separately in Figure 2, were averaged and treated as one observer for statistical analyses.
Figure 2.
Results from Experiment 1. Contrast increment thresholds (ΔC/C: the contrast value necessary to detect an increment divided by the contrast of the background grating against which the increment appeared) for probe detection as a function of probe latency, for dominance and suppression states. Each point is the mean of at least four staircases. Data from all six observers are shown, including RB's data from two sites, together with the overall mean (bottom right panel) and standard errors of those means. On average, dominance thresholds are higher for late probes (i.e., sensitivity is lower), although suppression thresholds tend to remain stable.
All observers had lower thresholds under dominance than under suppression, replicating the well-established loss in visual sensitivity that accompanies suppression phases of rivalry [13]. The magnitude of this suppression effect ranges from 0.11 to 0.52 (mean = 0.20; SE = 0.03)—within the range reported in many previous reports.
The plots of individual data in Figure 2 show that every observer's thresholds rose during dominance from early to late. This is significant overall, one-tailed t(5) = 2.85, p < .05. Although some observers' thresholds fell during suppression from early to late (RB, SWH, DT), others' did not. Overall there was no significant change over time in suppression thresholds, t(5) = 0.32, p > .05. When the dominance and suppression thresholds are combined into suppression depth (the ratio of dominance to suppression thresholds), as is typical [14, 15], there is a significant reduction of suppression depth between early and late probes, one-tailed t(5) = 2.32, p < .05. This result differs from those of earlier studies that found no change in suppression depth over time [9, 10] supporting our contention that those earlier studies used inappropriate probe stimuli. Nevertheless, the change in suppression we found is rather small.
At least three factors may have worked against finding clearer evidence of weakening suppression over time in this and in previous studies. First, on any particular trial in the late-probe condition, observers had to abort about 50% of trials because the rivalry state spontaneously changed before the probe could be presented (Figure S3A). This made the experiment very long and tiring, preventing us from measuring potentially stronger adaptation-related changes occurring at later times.
Second, some observers may unwittingly have tried to use attention to keep rivalry in a desired state until the probe was presented. This would be undesirable because attention can alter rivalry dynamics [16, 17].
Third, the option to abort trials when the rivalry state appeared to change prior to probe presentation led to an unexpected criterion problem in that observers were more likely to abort suppression trials than dominance trials (Figure S3B). This probably resulted from observers' applying a strict criterion to ensure no trace of the suppressed grating was visible during suppression trials (as instructed), with the consequence that some legitimate trials in which the probe was visible were interpreted as a break in suppression just prior to the probe and were therefore aborted. This would tend to raise thresholds, counteracting the expected lowering of suppression thresholds due to recovery from adaptation.
What is needed is a new approach to avoid these problems; we developed one for Experiment 2.
Experiment 2
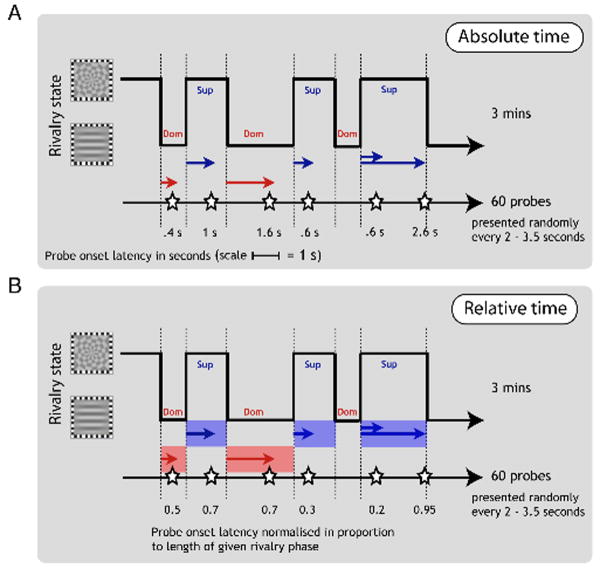
With our new method, observers continuously tracked their experience of rivalry over 3-minute trials by pressing one of two keys for exclusive visibility of one or the other rival stimuli, and also responded to fixed-contrast probes presented intermittently every few seconds (Figure 3A), marked by a brief tone, by pressing one of two other keys for upper or lower increments. Probe increment contrast was optimized for each observer as the mean of thresholds for dominance and suppression.
Figure 3.
Methods for Experiment 2. Observers continuously tracked their rivalry alternations while 60 contrast-increment probes were delivered at irregular intervals to the grating viewed by one eye. We then used the tracking sequence to divide the probes into dominance and suppression phases and to determine the timing of a given probe from the onset of the current rivalry phase. Probe timing could be coded as absolute time (A), or as relative time (B).
Because of response latency, the reported state changes during rivalry tracking necessarily lagged the perceived rivalry changes by between 400 and 500 ms. To correct for this, we advanced the key tracking records of all observers by 450 ms.
Figure 4A shows probe performance, as points and lines, plotted against the left-hand y-axis as a function of absolute time (time elapsed since onset of rivalry state). As expected, detection performance for dominance begins well above 75% correct, and performance for suppression begins below 75%. In the last time bin, performance reverses, but it is clear from the size of the associated error bars and the small number of observations per bin (plotted as grey bars against the right-hand y-axis) that these performance estimates are not reliable.
Figure 4.
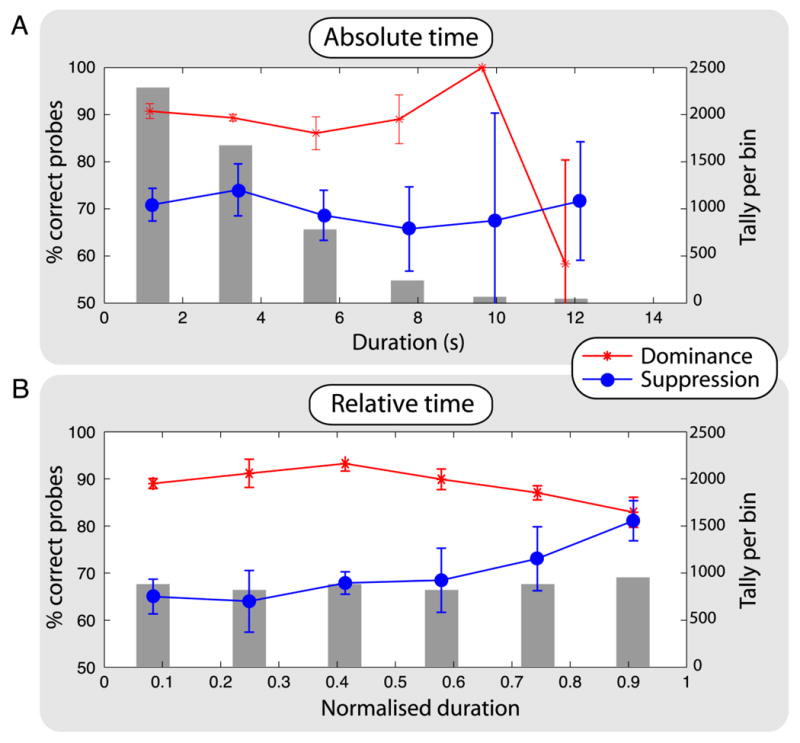
Results from Experiment 2. (A) The data points and lines show group means with standard error of those means for probe-detection performance (left-hand y-axis) for each rivalry state, as a function of time after onset of that state (advanced by 450 ms to correct for observers' latency in responding to perceptual changes, although any value from 400 to 650 ms produces similar results) plotted on the x axis. We sorted times into six equal-width bins for dominance and for suppression (the range of times for dominance was slightly smaller than that for suppression). The grey bars show the number of observations in each time bin (right-hand y-axis), summed over dominance and suppression with all observers pooled. Performance is better for dominance (red trace) than for suppression (blue trace), and there is no clear change in relative performance as a function of time. The grey bars show the typical gamma-shaped distribution of dominance times, explaining why probe performance data become so noisy in the last two time bins: there are very few observations in them. (B) The data from panel A recoded as relative time by normalising to the maximum time of each episode of rivalry. Normalising the state durations equalises the number of probes in each bin and reveals a clear change in relative performance over time. In the later time bins, dominance performance drops and suppression performance increases, consistent with the effects of adaptation on reciprocal inhibition.
Each episode of suppression had a random duration [18, 19]; it is possible this is caused by random variations in the time constants of any underlying neural adaptation, fast adaptation yielding a short episode of suppression and slow adaptation yielding a long episode. Another way to present the data is to plot probe performance as a function of the relative timing of a probe in a given rivalry state as a proportion of that state's duration (Figure 4B). Standardising has the added benefit of equalising the number of observations per bin, as the gray bars show, equating statistical reliability across all bins. In the later time bins it is clear that dominance performance declines and suppression performance improves. The performance levels are stable until after the median, illustrating the problem with the methodology of Experiment 1 and of previous studies using absolute time [9, 10]. Critically, the performance levels converge just prior to a perceptual reversal.
The complementary relationship between dominance and suppression performance is the expected signature of adaptation on reciprocal inhibition: as neurons signalling the dominant stimulus adapt over time, their ability to inhibit neurons signalling the suppressed stimulus wanes. This then is consistent with adaptation's playing a key role in binocular rivalry, as has long been proposed [3].
General Discussion
Although other conceptualizations of rivalry have been proposed (See Supplemental Information), theories based on reciprocal-inhibition have enjoyed enduring popularity. Adaptation is a cornerstone in most of those theories [6-8, 20]. The results obtained using our new method show for the first time the predicted reciprocal changes in suppression and dominance sensitivity during phases of rivalry which are expected on the basis of neural adaptation.
Why does this reciprocal pattern of sensitivity change emerge only when timing is expressed as a proportion of the time in a particular state of rivalry? Although the exponential decay used to describe neural adaptation is described by a time constant (implying a fixed rate), state durations during binocular rivalry alternations are close to random [21]. One possibility is that there are various sources of noise that disguise the correlation one might expect between the duration of one episode of suppression and the previous ones. Eye-blinks [22] and eye movements [23] represent possible sources of noise, and internal neural noise is another possibility [24-26]. Perhaps, too, adaptation time constants themselves are variable [27] so that adaptation rate varies over episodes of rivalry. Indeed, we demonstrate using a simple model how noisy adaptation could account for the typical positively-skewed durations of rivalry states (Figure S2). In such a model, normalising timing to the state duration would standardise all decay functions and better reveal patterns of data related to that decay. A fully developed model of bistable alternation dynamics based on the principle of noisy adaptation can be found elsewhere [28].
If adaptation is the cause of the changing sensitivity patterns over time, there is one query we must consider. Specifically, the dominance curve in Figure 4B does not conform to an exponential decline in effective contrast, as adaptation functions generally do [22]. Nor, for that matter, does the suppression curve resemble an exponential recovery function. Note, however, that growth and decay functions index adaptation's effects on contrast thresholds whereas Figure 4B shows changes in percent-correct performance over time measured using a fixed-contrast probe target. But our results are consistent with an exponential adaptation function if we assume that performance is governed by a compressive contrast response function, as we show in Figure S1.
Although these reciprocal changes in sensitivities are consistent with adaptation and point to a causal role for adaptation in rivalry alternations, the evidence is correlational rather than definitive. It remains to be seen whether experiments in which adaption is directly manipulated will show the same relation between adaptation and sensitivity as we do in Figure 4B, although we predict they will. We know, for example, that adapting one of the rival stimuli for a second or so makes it invisible when the second rival stimulus is shown [29].
As a final point, we have shown that probe sensitivity changes in the way predicted by adaption and reciprocal-inhibition theory, yet this does not explain the conscious experience of rivalry: we see a dominant stimulus as dominant for all of the duration of one episode of suppression until it is replaced by the previously suppressed stimulus. One might expect to see fading of the currently dominant stimulus or some visible hints of the suppressed stimulus towards the end of an episode of dominance. Of course, at transitions between dominance of rivalry stimuli, we do briefly see combinations of the two stimuli, so-called composites [2], but we excluded these experiences from our analyses. Perhaps perceptual experience exhibits hysteresis: seeing one rival stimulus at one moment promotes seeing that same rival stimulus at the next moment despite its neurons now weakly inhibiting the neurons encoding the other stimulus. Indeed, there are examples of hysteresis effects in rivalry [30-32]. Alternatively, there may be a separate, winner-take-all neural process determining experience. Such a process could arise at higher stages in the visual hierarchy, where competition is based on stimulus representations and not on eyes [33, 34].
In conclusion, we have used a new method that reveals clear reciprocal changes in contrast sensitivity during the course of a rivalry phase. For the first time, this is consistent with expected role of adaptation in the reciprocal-inhibition model of rivalry. This adds binocular rivalry to the phenomena reciprocal inhibition theory can explain, including linked reflexes in movement [4, 35], control of eye movements [36], and interactions between vision and balance [37], suggesting that reciprocal inhibition is a general mechanism in the nervous system that can be exploited in diverse contexts.
Experimental procedures
Observers
Six males, with ages ranging from 32 to 63, participated in these experiments after giving informed consent. Two observers were naïve about the hypothesis under test, and the other four were the authors of this paper. All have normal or corrected-to-normal vision and excellent stereopsis. Four of the six were tested at Vanderbilt University, and the other two were tested at the University of Sydney along with one of the observers previously tested at Vanderbilt (RB). Experiments were approved by the ethics boards of the respective institutions.
Apparatus and stimuli
Stimuli were created using Matlab with the Psychophysics Toolbox extensions [38, 39]running on a Macintosh computer. For test sessions administered at Vanderbilt University, stimuli were presented on a calibrated, Mitsubishi, colour, cathode-ray-tube (CRT) monitor (Diamond Pro 2020u; 1024 × 768 pixels, 100Hz) the mean luminance of which was 31 cd/m2. For test sessions administered at Sydney University, stimuli were presented on a LaCie, colour, CRT monitor (Electron Blue 22” series 3; 1024 × 768 pixels, 100 Hz) the mean luminance of which was 27 cd/m2.
At both testing locations, the two rival stimuli (Figure 1A) were a horizontal Gabor patch, presented to the observer's dominant eye, and a radial checkerboard pattern presented to the non-dominant eye (where eye dominance was estimated based on relative predominance derived in pilot tracking sessions that employed equal-contrast rival targets). The Gabor patch had a circular Gaussian envelope with a standard deviation of 0.43°, and contained within this 1.8° circular patch was a horizontal sine-wave grating whose spatial frequency was 2.5 cpd. The radial checkerboard was centered within a square region 1.8° on a side and had 16 wedges and six bands radially, with sharp transitions between light and dark regions. These rival stimuli were presented simultaneously on left and right halves of the monitor, with a centre-to-centre separation of 13.3°. Surrounding each rival stimulus was a square frame (0.4° in width) comprising black and white checks (see Figure 1A). These checks were binocularly matched and therefore provided dioptic stimuli for maintaining stable binocular alignment. These stimuli were viewed, one to each eye, through a four, front-surfaced, mirror stereoscope, giving an optical distance from the monitor to the eye of 82 cm. The entire apparatus was housed in a darkened test chamber. Observers gave their responses by pressing keys on the computer's keyboard.
For both testing locations, a bit-stealing technique was used to achieve an effective resolution of 10-bits on our gamma corrected CRT monitors. The contrast of the Gabor (governed by the amplitude of the Gaussian window over the sinusoid) and the radial checkerboard were determined by pilot experiments measuring the alternation dynamics between the two stimuli. These experiments involved varying the relative contrasts of the stimuli to find low contrasts that would slow the rivalry process to a median duration of at least 3 s (allowing easier probing of a given rivalry phase) and which would produce equal predominance of each stimulus. In practice, this meant a mean stimulus contrast on the order of 25%.
Brief test probes were delivered to the eye viewing the Gabor patch, to permit measurement of visual sensitivity during dominance and suppression phases. A test probe consisted of a contrast increment added to either the top or bottom half of the horizontal grating (Figure 1B). The sinusoid within the Gabor had a zero crossing that horizontally bisected the stimulus into equal upper and lower regions, allowing the contrast increment to be applied uniformly over each region. A temporal Gaussian profile was used to ramp the probe's contrast smoothly up and down to help reduce transients that can break rivalry suppression [40]. The standard deviation of the temporal Gaussian was 34 ms and was truncated at ±3 standard deviations (±100 ms).
Procedure
Experiment 1 comprised two phases: 1) measurement of each observer's dominance duration distributions for the Gabor and radial checkerboard so that the median duration could be determined, and 2) measurement of contrast increment thresholds for the Gabor test probes.
For the measurement of dominance durations, we required observers to track rivalry between the Gabor and the radial checkerboard for at least five consecutive 60-s periods, using one computer key to indicate when the Gabor was completely dominant, another key when it was completely suppressed, and neither key when a piecemeal mixture of both was visible. From the resulting tracking records we computed the median durations for dominance and suppression for the Gabor patch and used those values as the late probe delays for the probe detection portion of the experiment.
For the measurement of contrast increment thresholds for the Gabor test probes, we used two delays for presenting probes: an early condition, 200 ms after an observer's key-press indicated a stable dominance or suppression phase had been achieved (Figure 1C), and a late condition, when an observer's median suppression duration had elapsed after the key-press. We varied the magnitude of the brief contrast increment applied to the top or bottom half of the Gabor using a staircase procedure driven by performance on a two-alternative forced-choice (2AFC) task. Over trials, the region of the Gabor patch receiving the increment was varied randomly, and the observer's task was to report using key presses whether the contrast increment appeared in the top or the bottom half of the grating (with error feedback provided). Each trial began with the appearance of the rival targets, and the observer waited for at least one complete cycle of rivalry before pressing and holding the space bar to signal onset of the rivalry state being tested, either dominance (grating completely visible) or suppression (radial checkerboard completely visible).
Observers were instructed never to press the space bar during the transitory phases between dominance and suppression. Observers were instructed to release the space bar if the designated rival state changed before the probe appeared, aborting the trial. This latter instruction meant, therefore, that approximately half of trials were aborted during the late probe conditions, because approximately 50% of rivalry phase durations were shorter than the median state. Those trials had no influence on the staircase procedure used to vary the probe contrast increment; we kept a tally of the number of aborted trials.
For testing performed at Vanderbilt University, the contrast increment was initially set to 50% of the contrast of the Gabor. Three consecutive correct responses on the 2AFC task reduced the contrast increment for the next trial by 30% of the current trial. A single incorrect response set the contrast increment for the next trial 30% higher. After 4 reversals, the contrast adjustment was reduced to 15%. When the number of reversals reached 12, the staircase was terminated, and the contrast increment threshold for that staircase was defined as the average contrast increment value associated with the last six staircase reversals. For testing at University of Sydney, contrast was varied over trials using the adaptive QUEST procedure, with six QUEST staircases each for early and late probe conditions administered during dominance and during suppression; their means were averaged into a final threshold estimate.
Experiment 2 also comprised two phases: 1) measurement of each observer's thresholds under dominance and suppression so as to choose a suitable contrast increment for the second phase, and 2) measurement of forced-choice detection of probe location while observers were continually reporting their experiences of rivalry.
Supplementary Material
Acknowledgments
We are grateful for Urte Roeber's careful reading of a previous version of the manuscript. This research was supported by ARC grants DP0770299 to DA, DP0774697 to JC, NIH grant EY13358 to RB and by the WCU program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R32-10142).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wheatstone C. Contributions to the physiology of vision.—Part the First. On some remarkable, and hitherto unobserved, phænomena of binocular vision. Philosophical Transactions of the Royal Society of London. 1838;128:371–394. [Google Scholar]
- 2.Breese BB. On inhibition. Psychological Monographs. 1899;3:1–65. [Google Scholar]
- 3.McDougall W. IV.—Some new observations in support of Thomas Young's theory of light- and colour-vision (I.) Mind. 1901;10:52–97. [Google Scholar]
- 4.Sherrington CS. The integrative action of the nervous system. London: Constable; 1906. [Google Scholar]
- 5.Sugie N. Neural models of brightness perception and retinal rivalry in binocular vision. Biological Cybernetics. 1982;43:13–21. doi: 10.1007/BF00337283. [DOI] [PubMed] [Google Scholar]
- 6.Lehky SR. An astable multivibrator model of binocular rivalry. Perception. 1988;17:215–228. doi: 10.1068/p170215. [DOI] [PubMed] [Google Scholar]
- 7.Noest AJ, van Ee R, Nijs MM, van Wezel RJ. Percept-choice sequences driven by interrupted ambiguous stimuli: A low-level neural model. Journal of Vision. 2007;7:10. doi: 10.1167/7.8.10. [DOI] [PubMed] [Google Scholar]
- 8.Klink PC, van Ee R, Nijs MM, Brouwer GJ, Noest AJ, van Wezel RJA. Early interactions between neuronal adaptation and voluntary control determine perceptual choices in bistable vision. Journal of Vision. 2008;8:1–18. doi: 10.1167/8.5.16. [DOI] [PubMed] [Google Scholar]
- 9.Fox R, Check R. Independence between binocular rivalry suppression duration and magnitude of suppression. Journal of Experimental Psychology. 1972;93:283–289. doi: 10.1037/h0032455. [DOI] [PubMed] [Google Scholar]
- 10.Norman HF, Norman JF, Bilotta J. The temporal course of suppression during binocular rivalry. Perception. 2000;29:831–841. doi: 10.1068/p3085. [DOI] [PubMed] [Google Scholar]
- 11.Blakemore C, Nachmias J. The orientation specificity of two visual after-effects. Journal of Physiology. 1971;213:157–174. doi: 10.1113/jphysiol.1971.sp009374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe K, Paik Y, Blake R. Preserved gain control for luminance contrast during binocular rivalry suppression. Vision Research. 2004;44:3065–3071. doi: 10.1016/j.visres.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Wales R, Fox R. Increment detection thresholds during binocular rivalry suppression. Perception & Psychophysics. 1970;8:90–94. [Google Scholar]
- 14.Blake R, Fox R. Binocular rivalry suppression: Insensitive to spatial frequency and orientation change. Vision Research. 1974;14:687–692. doi: 10.1016/0042-6989(74)90065-0. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen VA, Freeman AW, Alais D. Increasing depth of binocular rivalry suppression along two visual pathways. Vision Research. 2003;43:2003–2008. doi: 10.1016/s0042-6989(03)00314-6. [DOI] [PubMed] [Google Scholar]
- 16.Chong SC, Blake R. Exogenous attention and endogenous attention influence initial dominance in binocular rivalry. Vision Research. 2006;46:1794–1803. doi: 10.1016/j.visres.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Paffen CLE, Alais D, Verstraten FAJ. Attention speeds binocular rivalry. Psychological Science. 2006;17:752–756. doi: 10.1111/j.1467-9280.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- 18.Levelt WJM. Note on the distribution of dominance times in binocular rivalry. British Journal of Psychology. 1967;58:143–145. doi: 10.1111/j.2044-8295.1967.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 19.van Ee R, Noest AJ, Brascamp JW, van den Berg AV. Attentional control over either of the two competing percepts of ambiguous stimuli revealed by a two-parameter analysis: Means do not make the difference. Vision Research. 2006;46:3129–3141. doi: 10.1016/j.visres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Wilson HR. Computational evidence for a rivalry hierarchy in vision. Proceedings of the National Academy of Science, USA. 2003;100:14499–14503. doi: 10.1073/pnas.2333622100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Ee R. Dynamics of perceptual bi-stability for stereoscopic slant rivalry and a comparison with grating, house-face, and Necker cube rivalry. Vision Research. 2005;45:29–40. doi: 10.1016/j.visres.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 22.Ridder WH, III, Tomlinson A. Spectral characteristics of blink suppression in normal observers. Vision Research. 1995;35:2569–2578. doi: 10.1016/0042-6989(95)00011-n. [DOI] [PubMed] [Google Scholar]
- 23.Sabrin HW, Kertesz AE. Microsaccadic eye movements and binocular rivalry. Perception & Psychophysics. 1980;28:150–154. doi: 10.3758/bf03204341. [DOI] [PubMed] [Google Scholar]
- 24.Brascamp JW, van Ee R, Noest AJ, Jacobs RH, van den Berg AV. The time course of binocular rivalry reveals a fundamental role of noise. Journal of vision. 2006;6:1244–1256. doi: 10.1167/6.11.8. [DOI] [PubMed] [Google Scholar]
- 25.Kim YJ, Grabowecky M, Suzuki S. Stochastic resonance in binocular rivalry. Vision Research. 2006;46:392–406. doi: 10.1016/j.visres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Shpiro A, Moreno-Bote R, Rubin N, Rinzel J. Balance between noise and adaptation in competition models of perceptual bistability. Journal of Computational Neuroscience. 2009;27:37–54. doi: 10.1007/s10827-008-0125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vautin RG, Berkley MA. Responses of single cells in cat visual cortex to prolonged stimulus movement: Neural correlates of visual aftereffects. Journal of Neurophysiology. 1977;40:1051–1065. doi: 10.1152/jn.1977.40.5.1051. [DOI] [PubMed] [Google Scholar]
- 28.van Ee R. Stochastic variations in sensory awareness are driven by noisy neuronal adaptation: Evidence from serial correlations in perceptual bistability. Journal of the Optical Society of America A. 2009;26:2612–2622. doi: 10.1364/JOSAA.26.002612. [DOI] [PubMed] [Google Scholar]
- 29.Tsuchiya N. Flash suppression. Scholarpedia. 2008;3:5640. [Google Scholar]
- 30.Brascamp JW, Pearson J, Blake R, van den Berg AV. Intermittent ambiguous stimuli: Implicit memory causes periodic perceptual alternations. Journal of Vision. 2009;9:1–23. doi: 10.1167/9.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Julesz B, Tyler CW. Neurontropy, an entropy-like measure of neural correlation, in binocular fusion and rivalry. Biological Cybernetics. 1976;23:25–32. doi: 10.1007/BF00344148. [DOI] [PubMed] [Google Scholar]
- 32.O'Shea RP, Crassini B. Binocular rivalry occurs without simultaneous presentation of rival stimuli. Perception & Psychophysics. 1984;36:266–276. doi: 10.3758/bf03206368. [DOI] [PubMed] [Google Scholar]
- 33.Logothetis NK, Leopold DA, Sheinberg DL. What is rivalling during binocular rivalry? Nature. 1996;380:621–624. doi: 10.1038/380621a0. [DOI] [PubMed] [Google Scholar]
- 34.Alais D, Melcher D. Strength and coherence of binocular rivalry depends on shared stimulus complexity. Vision Research. 2007;47:269–279. doi: 10.1016/j.visres.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Li L, Goulding M, Frank E. Early postnatal development of reciprocal Ia inhibition in the murine spinal cord. Journal of Neurophysiology. 2008;100:185–196. doi: 10.1152/jn.90354.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi M, Sugiuchi Y, Shinoda Y. Commissural mirror-symmetric excitation and reciprocal inhibition between the two superior colliculi and their roles in vertical and horizontal eye movements. Journal of Neurophysiology. 2007;98:2664–2682. doi: 10.1152/jn.00696.2007. [DOI] [PubMed] [Google Scholar]
- 37.Hospedales TM, van Rossum MC, Graham BP, Dutia MB. Implications of noise and neural heterogeneity for vestibulo-ocular reflex fidelity. Neural Computation. 2008;20:756–778. doi: 10.1162/neco.2007.09-06-339. [DOI] [PubMed] [Google Scholar]
- 38.Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- 39.Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- 40.Walker P, Powell DJ. The sensitivity of binocular rivalry to changes in the nondominant stimulus. Vision Research. 1979;19:247–249. doi: 10.1016/0042-6989(79)90169-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.