Abstract
Neural information is processed based on integrated activities of relevant neurons. Concerted population activity is one of the important ways for retinal ganglion cells to efficiently organize and process visual information. In the present study, the spike activities of bullfrog retinal ganglion cells in response to three different visual patterns (checker-board, vertical gratings and horizontal gratings) were recorded using multi-electrode arrays. A measurement of subsequence distribution discrepancy (MSDD) was applied to identify the spatio-temporal patterns of retinal ganglion cells’ activities in response to different stimulation patterns. The results show that the population activity patterns were different in response to different stimulation patterns, such difference in activity pattern was consistently detectable even when visual adaptation occurred during repeated experimental trials. Therefore, the stimulus pattern can be reliably discriminated according to the spatio-temporal pattern of the neuronal activities calculated using the MSDD algorithm.
Keywords: Multi-unit recording, Population activity, Multiple spike train analysis, Pattern recognition
Introduction
The visual information processing first occurs in the retinal circuitry before the signals are further transmitted to the central visual system via optic nerve fibers (Carcieri et al. 2003; Dacey 1996; Masland 2001). Retinal ganglion cells are the first stage where visual information is encoded in a form of spikes (Dacey 1996; Meister and Berry 1999). However, the number of retinal ganglion cells is the fewest among all kinds of neurons in the visual pathway (Barlow 1981; Usrey and Reid 1999). This anatomy limitation makes it necessary that visual information is efficiently organized in the retinal ganglion cell layer before it is transferred to the central visual part.
The classical view of neural coding emphasizes the importance of information carried by changes in the neurons’ spike discharge rates. However, the firing rate of individual cell may be similar in response to different stimulation patterns because of the similarity of the local stimulus falling in the cell’s receptive field; on the other hand, single neuronal firing activity in response to repeated stimulation of the same visual pattern is changeable due to adaptation (Chen et al. 2005; Jin et al. 2005; Van Steveninck et al. 1997). In this situation, the visual information can hardly be encoded by single neuronal activities; so retinal ganglion cells might need to encode different visual stimuli via different population activity patterns (DeVries 1999; Feldman 2010; Frechette et al. 2005; Meister et al. 1995; Schnitzer and Meister 2003; Singer 2009). To date, there is short of relevant multi-dimensional data analysis methods for analyzing concerted population activity. Common methods were basically designed for pair-wise neuron analysis and did not afford the necessity for dealing with population activity including more than two neurons. Although techniques were also explored to deal with multiple spike trains at the same time (Gerstein and Aertsen 1985; Schnitzer and Meister 2003), but these proposed methods were seriously subject to the selection of parameters.
The purpose of this study try to develop an effective method to identify the different spatio-temporal patterns of the retinal ganglion cells activities which are related to different stimulation patterns. In the present study, the concerted property of population activity of the retinal ganglion cells in response to specific stimulation patterns were investigated by using a measurement of subsequence distribution discrepancy (MSDD) (Fang 1994; Fang et al. 2001; Wang et al. 2006a). The results obtained in the present study reveal that the population activity patterns were different in response to different stimulation patterns although the firing rates could be similar. The difference in activity patterns was consistently detectable during repeated experimental trials even when visual adaptation occurred. Therefore, the result of MSDD algorithm can be used as the index representing the population activity in response to different stimulation pattern; and different stimulation patterns can be discriminated according to the different population activity pattern represented by the result of MSDD algorithm.
Methods
Electrophysiology recordings and visual stimulation
Extracellular recordings were made in isolated bullfrog retinas using coplanar multi-electrode arrays (MEA, MMEP-4, CNNS UNT, USA) consisted of 64 electrodes (8 μm in diameter) arranged in an 8 × 8 matrix (covering an area of 1.05 × 1.05 mm2) with 150 μm tip-to-tip distances between nearest horizontal and vertical electrodes. All procedures strictly conformed to the humane treatment and use of animals as prescribed by the Association for Research in Vision and Ophthalmology. Bullfrogs were dark adapted for at least 30 min before experiment. Under dim red light, the bullfrog was double pithed and eyes were enucleated. The eyeball was hemisected and the eyecup was cut into several pieces, then the retina was isolated carefully. A small piece (4 × 4 mm2) of isolated retina was placed on the MEA with the ganglion cell side contacting the electrodes and superfused with oxygenated (95% O2 and 5% CO2) standard solution, which contained (in mM): NaCl 100.0, KCl 2.5, MgCl2 1.6, CaCl2 2.0, NaHCO3 25.0, glucose 10.0.
Retinal ganglion cells’ responses to visual stimulation were simultaneously recorded by the MEA and the signals were amplified through a 64-channel amplifier (MEA workstation, Plexon Inc., USA). Signals from the selected channels along with the stimulus were sampled at a rate of 40 kHz and stored in a Pentium-IV-based computer. Spikes from individual neurons were sorted using principal component analysis (PCA) method (Wang et al. 2006b; Zhang et al. 2004) as well as the spike-sorting unit in the commercial software OfflineSorter (Plexon Inc. Texas, USA). In order to get accurate data for firing activity comparison, these two spike-sorting methods were applied separately, only single-neuron events clarified by all these two spike-sorting methods were used for further analyzes in the present study.
Light stimulus was generated from a computer monitor (Iiyama, Vision Master Pro 456, Japan) and was focused to form a 1.1 × 1.1 mm2 image on the isolated retina via a lens system. The stimulation protocols were: (1) Pseudo-random checker-board which consisted of 16 × 16 sub-squares was displayed on the computer monitor at a frame refresh rate of 20 Hz. Each sub-square covered an area of 66 × 66 μm2 on the retinal piece and was assigned with a value either “1” (white light, 77.7 nW/cm2) or “0” (dark) following a pseudo-random binary sequence. (2) Three different stationary stimuli with 1-s duration and 2-s intervals (gray screen, 38.9 nW/cm2) were given (in random order) for 20 repeats: (1) checker-board stimulus consisted of 8 × 8 sub-squares, with each sub-square covering an area of 132 × 132 μm2 on the retinal piece and was assigned with a value either “1” (white light, 77.7 nW/cm2) or “0” (dark, 0 nW/cm2); (2) vertical gratings consisted of 3 light bars (77.7 nW/cm2) and 3 dark bars (0 nW/cm2), with the width of each bar being 177 μm when projected on the retina; (3) horizontal gratings consisted of 3 light bars (77.7 nW/cm2) and 3 dark bars (0 nW/cm2), with the width of each bar being 177 μm when projected on the retina. The mean illumination of these three different stimuli was of the same intensity.
Measuring the retinal ganglion cells’ receptive field profiles
To map the receptive field profile of each individual retinal ganglion cells, spike triggered average (STA) algorithm was applied (Devries and Baylor 1997), with pseudo-random checker-board flickering as the stimuli. The profile of the spatial receptive field was fitted with a two-dimensional Gaussian distribution:
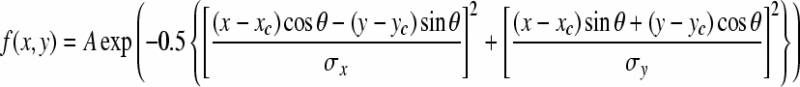 |
1 |
where (x, y) is the position of the stimulus, (xc, yc) is the position of the estimated mass center of the receptive field, A is the estimated maximum amplitude of the two-dimensional Gaussian distribution, θ is the angle between the major axis (long axis) of the receptive field and the x axis, σx and σy are the standard deviation of the major and minor axes of the two-dimensional Gaussian distribution, respectively.
Multiple spike train analysis
In the present study, MSDD (Fang 1994; Fang et al. 2001) analysis was applied to deal with a group of spike train sequences (n > 2) and analyze the spatio-temporal patterns of concerted activity among the neurons. Detailed method was previously reported (Liu et al. 2009a; Wang et al. 2006a).
Briefly, the spike trains were symbolized into “0–1” sequences (bin size = 2 ms), where “1” represents that there is one spike in the time bin of interest and “0” represents that there is no spike in the time bin. When MSDD method is applied to measure discrepancy among multiple sequences, the constructive information of a sequence is transformed into a set of subsequence distributions, which is defined as follows (Fang 1994, 2000; Fang et al. 2001):
Let 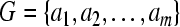 be a set of m symbols, and suppose
be a set of m symbols, and suppose 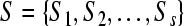 is a set of s sequences (firing sequences recorded from s neurons, in our case) formed from the symbol set G. Then the total number of all different subsequences formed from G with length l should be equal to ml. In the present study, the neuronal firing activities were represented by two symbols “0” and “1” (m = 2). All the sequences to be analyzed were separated into 6-letter overlapping subsequences (l = 6), with moving step being one time bin, so ml = 26 = 64.
is a set of s sequences (firing sequences recorded from s neurons, in our case) formed from the symbol set G. Then the total number of all different subsequences formed from G with length l should be equal to ml. In the present study, the neuronal firing activities were represented by two symbols “0” and “1” (m = 2). All the sequences to be analyzed were separated into 6-letter overlapping subsequences (l = 6), with moving step being one time bin, so ml = 26 = 64.
Suppose Sk is the k-th sequence of L bins, and given the subsequence length of l, the probability distribution of the subsequences in Sk of our data is:
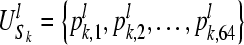 |
2 |
where pk,j denotes the probability of subsequence pattern j in sequence k, 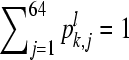 , l ≪ L, k = 1, 2, …, s.
, l ≪ L, k = 1, 2, …, s.
Given a set of s sequences, we have:
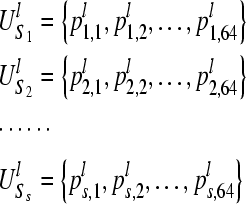 |
3 |
Then the Bk value for the k-th sequence can be calculated as:
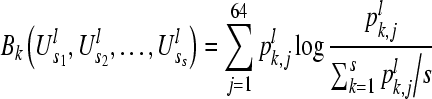 |
4 |
Therefore, if the two sequences Si and Sj(i ≠ j, i, j ≤ s) have the same subsequence distribution 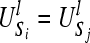 , then we should have the result
, then we should have the result 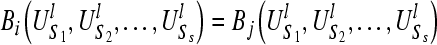 . It implies that if two spike trains are completely synchronized, they will have exactly the same values for Bk at the same time, a large Bk value is related to profound difference between the temporal structure of the k-th sequence and the rest of the group.
. It implies that if two spike trains are completely synchronized, they will have exactly the same values for Bk at the same time, a large Bk value is related to profound difference between the temporal structure of the k-th sequence and the rest of the group.
Results
The stimulation patterns and receptive field properties of retinal ganglion cells
In the present study, the activities of bullfrog retinal ganglion cells in response to three different stationary visual patterns (checker-board, vertical gratings and horizontal gratings) were recorded from two retinas. Data from an example retina are presented, while similar results were also obtained from another retina.
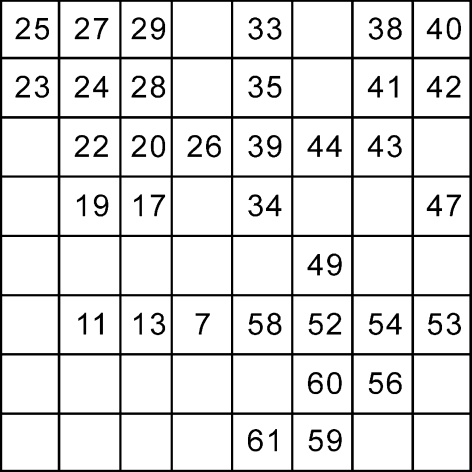
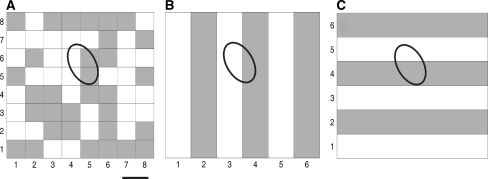
Figure 1 illustrates the geometric position of 34 electrodes on the multi-electrode arrays (MEA) by which the firing activities of 34 retinal ganglion cells were recorded from one example retina. Receptive field properties of ganglion cells were estimated by STA method (see methods Section “Measuring the retinal ganglion cells’ receptive field profiles”) using pseudo-random checker-board flickering stimulation. Figure 2a, b and c show three stimulation patterns and the receptive field profile of one example retinal ganglion cell, which demonstrate that these stimuli were similar to this example cell in a sense that the receptive field of this retinal ganglion cell was partially lit up by each of these stimuli, although the global stimulation patterns were different from one another. This was actually true for almost all the cells investigated in the present study that each cell’s receptive field center was always partially illuminated when different visual patterns were applied.
Fig. 1.
Geometric positions of 34 electrodes by which the activities of 34 neurons from one example retina were recorded
Fig. 2.
Three stimulation patterns applied in the present study and one example RGC’s receptive field profiles. a Checker-board; b Vertical gratings; c Horizontal gratings. The ellipse indicates the 1-s.d. boundary of the neuron’s receptive field fitted with a two-dimensional Gaussian distribution (scale bar, 200 μm)
Retinal ganglion cells’ firing activities in response to different stimulation patterns
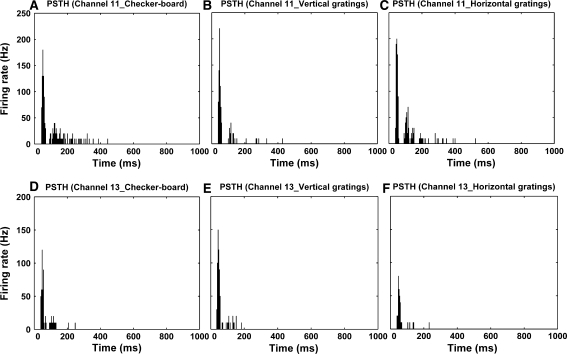
In order to investigate the retinal ganglion cells activities in response to different stimulation patterns, we firstly compared the firing rates of individual neurons in response to different stimulations. Figure 3 demonstrates the recordings obtained from two example retinal ganglion cells in response to different stimulations. The firing rates of neuron #11 were similar in exposure to three different stimulation patterns (Fig. 3a–c). The peak value in the PSTH of this cell’s activities (averaged over 20 trials) in response to checker-board was 180 Hz as given in Fig. 3a. During vertical gratings stimulation, the peak value in the PSTH was 220 Hz (Fig. 3b) and the peak value in response to horizontal gratings was 200 Hz (Fig. 3c). However, the firing rates of neuron #13 were significantly different in response to different stimulation patterns (Fig. 3d–f). The peak value in the PSTH of the cell’s activities elicited by checker-board was 120 Hz (Fig. 3d). During vertical gratings stimulation, the peak value in the PSTH was 150 Hz (Fig. 3e) and the peak value in response to horizontal gratings was 80 Hz (Fig. 3f). These results show that the single neuronal firing rates in response to different stimulation patterns could either be similar or different.
Fig. 3.
The averaged PSTHs of two example neurons (upper & lower rows, respectively) in response to (a & d) checker-board stimulation; (b & e) vertical gratings; (c & f) horizontal gratings. (20 repeats, bin width 5 ms)
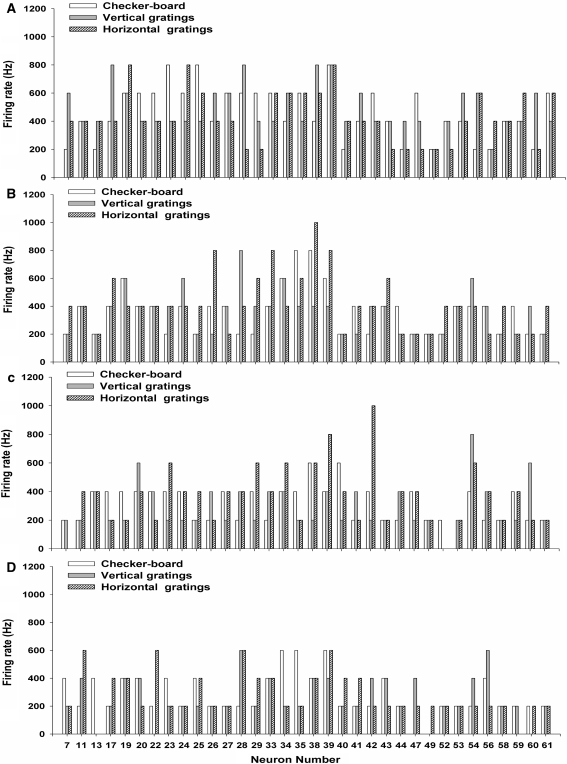
The peak values in the PSTHs of the 34 recorded retinal ganglion cells elicited by three different stimulation patterns during different experimental trials are plotted in Fig. 4a–d (the 1st, 5th, 10th and 15th trials, respectively). The results show that the single neuronal activity in response to the same stimulation pattern was not stable during the repeated experimental trails. In addition, for each individual neuron, although there could be some differences in the firing rates elicited by different stimulation patterns, the difference was not consistent across repeated trials. Therefore, different stimulation patterns can hardly be distinguished according to the changes in single neuronal firing rates.
Fig. 4.
The peak values in the PSTHs of 34 neurons in response to three different stimulation patterns during (a) the 1st trial; b the 5th trial; c the 10th trial; d the 15th trial
The retinal ganglion cells population activities elicited by different stimulation patterns
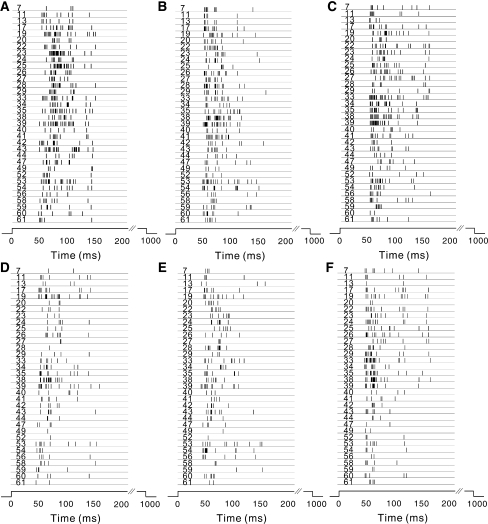
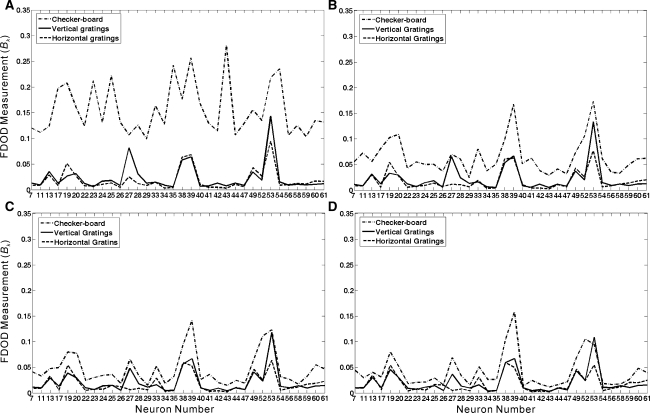
To describe the concerted pattern among multiple spike trains, we applied MSDD to perform multi-dimensional data analysis. The example spike trains of 34 neurons in response to three different stimulation patterns (the 1st & 5th trials) are plotted in Fig. 5, where each trace corresponds to a 200-ms recording of one cell’s response. The MSDD method was applied, and Bk values for each neuron’s activities in response to three different stimulation patterns were calculated and plotted in Fig. 6. It is shown in Fig. 6a that when checker-board stimulus was applied (the 1st trial), the neuronal activities were with larger Bk values as compared to the neuronal responses elicited by the vertical and horizontal gratings, and this is true for all the 34 neurons recorded from this retina. According to the principle of MSDD method, larger Bk values reflect that the firing patterns were less coincident among the group cells in response to checker-board stimulus than the gratings. MSDD analysis performed on repeated experimental trials show that the Bk values had a decreasing tendency when the pattern stimulations were given repeatedly, but it was persistently true that the population activity pattern for the checker-board-response was significantly different from that of others, which is reflected by larger Bk values (Fig. 6b–d).
Fig. 5.
Raster plot of 34 neurons’ firing activities (200 ms after the stimulus onset) in response to (a & d) checker-board; (b & e) vertical gratings; (c & f) horizontal gratings. (Upper and lower rows: the 1st and the 5th trials, respectively)
Fig. 6.
Bk values calculated for the neuronal activities in response to checker-board, vertical gratings and horizontal gratings during a the 1st trial; b the 5th trial; c the 10th trial; d the 15th trial
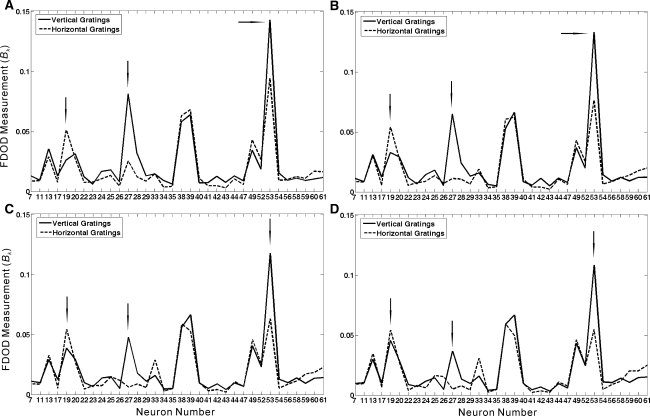
On the other hand, the population activity patterns in response to vertical gratings and horizontal gratings were somewhat similar in a sense that the Bk values obtained for these situations were quite similar for most cells as illustrated in Figs. 6 and 7. However, the results plotted in Fig. 7a also show that there were a small group of neurons (#19, #27 and #53) whose Bk values were remarkably different in response to two grating stimulation patterns. These neurons were therefore chosen for further analysis. Table 1 lists the summed Bk values of these 3 neurons’ firing sequences (B27 + B53 − B19) in response to checker-board, vertical gratings and horizontal gratings during each experimental trial. The results show that the summed Bk values of the neuronal responses to vertical gratings are persistently larger than those in response to horizontal gratings during repeated experimental trials. Thus, the vertical- and the horizontal-gratings can also be consistently detectable according to the difference of population activity patterns represented by the Bk values calculated for the selected neurons.
Fig. 7.
Bk values of the neuronal activities in response to vertical gratings and horizontal gratings during a the 1st trial; b the 5th trial; c the 10th trial; d the 15th trial. The arrows indicate the positions of 3 selected ganglion cells (#19, #27 and #53), respectively
Table 1.
Comparison the summed Bk values in response to vertical gratings and horizontal gratings of 3 selected RGCs (#19, #27 and #53)
| Summed Bk values (vertical gratings) | Summed Bk values (horizontal gratings) | |
|---|---|---|
| 1st trial | 0.3915 | 0.1852 |
| 2nd trial | 0.3720 | 0.1627 |
| 3rd trial | 0.3576 | 0.1501 |
| 4th trial | 0.3414 | 0.1307 |
| 5th trial | 0.3392 | 0.1169 |
| 6th trial | 0.3246 | 0.1080 |
| 7th trial | 0.3175 | 0.1004 |
| 8th trial | 0.2958 | 0.0928 |
| 9th trial | 0.2885 | 0.0852 |
| 10th trial | 0.2748 | 0.0814 |
| 11th trial | 0.2672 | 0.0773 |
| 12th trial | 0.2580 | 0.0727 |
| 13th trial | 0.2518 | 0.0699 |
| 14th trial | 0.2400 | 0.0669 |
| 15th trial | 0.2336 | 0.0652 |
| 16th trial | 0.2237 | 0.0610 |
| 17th trial | 0.2204 | 0.0583 |
| 18th trial | 0.2202 | 0.0563 |
| 19th trial | 0.2167 | 0.0535 |
| 20th trial | 0.2111 | 0.0515 |
Taken together, these results reveal that: (1) Different stimulation patterns can hardly be distinguished according to the changes in single neuronal firing rates. (2) The population activity patterns were different in response to different stimulus patterns, such differences are consistently detectable during repeated experimental trials.
Although all the detailed descriptions presented here are based on data collected from one piece of retina, similar results (comparing the neuronal activity patterns elicited by three different stimulation patterns) were obtained from 2 retinas. We also did some additional experiments to investigate the neuronal activity patterns elicited by two different stimulation patterns (checker-board and vertical gratings) from 4 retinas. The results lead to the same conclusion.
Discussion
Characterizing the relationship between stimulus and response has been difficult because neuronal responses are complex and variable. Traditional coding theory emphasizes the importance of information carried by the neurons’ firing rates. However, it has been reported that the neuronal firing activity tends to be adapted in exposure to sustained or repeated stimulus (Chen et al. 2005; Fairhall et al. 2001; Jin et al. 2005; Liu et al. 2009b). Such adaptation process was also observed in our present study such that the single neuron’s firing rates had a decreasing tendency when the same stimulation pattern was applied repeatedly. In this case, the stimulation pattern can hardly be represented by single neuronal activities. On the other hand, it is also observed in the present study that single cell’s responses elicited by different stimulation patterns could be similar because of the similarity of the local stimulus falling in the ganglion cell’s receptive field (Figs. 3, 4), which means that different stimulation patterns can hardly be distinguished based on the changes in single neuronal firing activities.
Typically, while many neurons respond to a given stimulus, the stimulus features can be encoded by the activities of the neural population. Considering the concerted population activity as one of the important ways for retinal ganglion cells to transmit visual information to the central visual part (Field and Chichilnisky 2007; Frechette et al. 2005), we performed MSDD algorithm to analyze population activity pattern among a group of retinal ganglion cells. Our results reveal that the population activity patterns were different in response to different stimulation patterns and such differences were consistently detectable during repeated experimental trials, even if visual adaptation occurred. The neuronal activities elicited by checker-board stimulus were with larger Bk values as compared to that in response to the vertical- and horizontal-gratings. Larger Bk values reflect that the firing patterns were with less coincidence among the group cells in response to checker-board stimulus than the gratings stimulation patterns (Liu et al. 2009a; Wang et al. 2006a). This is not surprising, because checker-board stimulus has lower spatial correlation as compared to vertical- and horizontal-gratings (Cai et al. 2008; Lesica and Stanley 2004). Given that single retinal ganglion cell’s firing activity is determined by the visual input in its receptive field as well as its lateral connection with the neighboring neurons (Masland 2001), the highly correlated spatial pattern in vertical and horizontal gratings will both result in concerted activities among neighboring cells, which is reflected by overall small Bk values of the neuronal firing sequences.
However, although some cells’ responses could be similar in response to vertical and horizontal grating, some might be different—this is reflected by the remarkable difference in the Bk values of several cells in the group (#19, #27 and #53 in the example retina). Further analysis show that the Bk values of neurons #27 and #53 in responses to vertical gratings are always larger than that in response to horizontal gratings during repeated experimental trials (see Fig. 7), which means that for these cells, their responses were less coincident with other cells in the group in response to the vertical gratings. On the other hand, there should be another part of ganglion cells within the retina area whose activities were less coincident with that of others in response to horizontal gratings—neuron #19 is one of them (see Fig. 7). However, since the electrodes are sparsely distributed in the array, therefore the neurons in the retinal area can not be fully or evenly sampled in our experiment, we can only obtain the response properties from a small randomly sampled group of cells. Even though, the different visual patterns can be consistently detectable according to the difference of population activity patterns represented by the Bk values.
The results obtained in the present study revealed that the population activity patterns calculated by the MSDD algorithm were different in response to different stimulation patterns. The differences in activity patterns were consistently detectable during repeated experimental trials. MSDD method can be employed to efficiently distinguish different population patterns in response to different stimulation patterns, which also confirms the notion that concerted population activity is an important way for retinal ganglion cells to transmit visual information to the central visual part.
Acknowledgments
This work was supported by grants from the State Key Basic Research and Development Plan (No. 2005CB724301) and National Foundation of Natural Science of China (No. 30670519).
References
- Barlow HB. The ferrier lecture, 1980. Critical limiting factors in the design of the eye and visual cortex. Proc R Soc Lond B Biol Sci. 1981;212:1–34. doi: 10.1098/rspb.1981.0022. [DOI] [PubMed] [Google Scholar]
- Cai CF, Zhang YY, Liu X, Liang PJ, Zhang PM. Detecting determinism in firing activities of retinal ganglion cells during response to complex stimuli. Chin Phys Lett. 2008;25(5):1595–1598. doi: 10.1088/0256-307X/25/5/020. [DOI] [Google Scholar]
- Carcieri SM, Jacobs AL, Nirenberg S. Classification of retinal ganglion cells: a statistical approach. J Neurophysiol. 2003;90:1704–1713. doi: 10.1152/jn.00127.2003. [DOI] [PubMed] [Google Scholar]
- Chen AH, Zhou Y, Gong HQ, Liang PJ. Luminance adaptation increased the contrast sensitivity of retinal ganglion cells. Neuroreport. 2005;16(4):371–375. doi: 10.1097/00001756-200503150-00013. [DOI] [PubMed] [Google Scholar]
- Dacey DM. Circuitry for color coding in the primate retina. Proc Natl Acad Sci USA. 1996;93:582–588. doi: 10.1073/pnas.93.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries SH. Correlated firing in rabbit retinal ganglion cells. J Neurophysiol. 1999;81(2):908–920. doi: 10.1152/jn.1999.81.2.908. [DOI] [PubMed] [Google Scholar]
- Devries SH, Baylor DA. Mosaic arrangement of ganglion cell receptive fields in rabbit retina. J Neurophysiol. 1997;78(4):2048–2060. doi: 10.1152/jn.1997.78.4.2048. [DOI] [PubMed] [Google Scholar]
- Fairhall AL, Lewen GD, Bialek W, de Ruyter Van Steveninck RR. Efficiency and ambiguity in an adaptive neural code. Nature. 2001;412(6849):787–792. doi: 10.1038/35090500. [DOI] [PubMed] [Google Scholar]
- Fang WW. Disagreement degree of multi-person judgements in an additive structure. Math Soc Sci. 1994;28:85–111. doi: 10.1016/0165-4896(94)00751-9. [DOI] [Google Scholar]
- Fang WW. The characterization of a measure of information discrepancy. Inform Sci. 2000;125:207–232. doi: 10.1016/S0020-0255(00)00008-6. [DOI] [Google Scholar]
- Fang WW, Roberts FS, Ma ZR. A measure of discrepancy of multiple sequences. Inform Sci. 2001;137:75–102. doi: 10.1016/S0020-0255(01)00108-6. [DOI] [Google Scholar]
- Feldman J. Ecological expected utility and the mythical neural code. Cogn Neurodyn. 2010;4:25–35. doi: 10.1007/s11571-009-9090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field GD, Chichilnisky EJ. Information processing in the primate retina: circuitry and coding. Annu Rev Neurosci. 2007;30:1–30. doi: 10.1146/annurev.neuro.30.051606.094252. [DOI] [PubMed] [Google Scholar]
- Frechette ES, Sher A, Grivich MI, Petrusca D, Litke AM, Chichilnisky EJ. Fidelity of the ensemble code for visual motion in primate retina. J Neurophysiol. 2005;94(1):119–135. doi: 10.1152/jn.01175.2004. [DOI] [PubMed] [Google Scholar]
- Gerstein GL, Aertsen AM. Representation of cooperative firing activity among simultaneously recorded neurons. J Neurophysiol. 1985;54(6):1513–1528. doi: 10.1152/jn.1985.54.6.1513. [DOI] [PubMed] [Google Scholar]
- Jin X, Chen AH, Gong HQ, Liang PJ. Information transmission rate changes of retinal ganglion cells during contrast adaptation. Brain Res. 2005;1055(1–2):156–164. doi: 10.1016/j.brainres.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Lesica N, Stanley G. Encoding of natural scene movies by tonic and burst spikes in the lateral geniculate nucleus. J Neurosci. 2004;24(47):10731. doi: 10.1523/JNEUROSCI.3059-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li H, Liang PJ (2009a) Estimation of concerted activities based on subsequence distribution discrepancy calculation. iCBBE
- Liu YQ, Zhao LY, Hong B. Rate and temporal characteristics of adaptation to repetitive stimulus of rat inferior colliculus neurons. Acta Biophysica Sinica. 2009;23(3):209–218. [Google Scholar]
- Masland R. The fundamental plan of the retina. Nat Neurosci. 2001;4(9):877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- Meister M, Berry MJ. The neural code of the retina. Neuron. 1999;22:435–450. doi: 10.1016/S0896-6273(00)80700-X. [DOI] [PubMed] [Google Scholar]
- Meister M, Lagnado L, Baylor DA. Concerted signaling by retinal ganglion cells. Science. 1995;270(5239):1207–1210. doi: 10.1126/science.270.5239.1207. [DOI] [PubMed] [Google Scholar]
- Schnitzer MJ, Meister M. Multineuronal firing patterns in the signal from eye to brain. Neuron. 2003;37(3):499–511. doi: 10.1016/S0896-6273(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Singer W. Distributed processing and temporal codes in neuronal networks. Cogn Neurodyn. 2009;3:189–196. doi: 10.1007/s11571-009-9087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Reid RC. Synchronous activity in the visual system. Annu Rev Physiol. 1999;61:435–456. doi: 10.1146/annurev.physiol.61.1.435. [DOI] [PubMed] [Google Scholar]
- Steveninck S, Lewen GD, Strong SP, Koberle R, Bialek W. Reproducibility and variability in neural spike trains. Science. 1997;275(5307):1805–1808. doi: 10.1126/science.275.5307.1805. [DOI] [PubMed] [Google Scholar]
- Wang GL, Liu X, Zhang P, Liang PJ. A new method for multiple spike train analysis based on information discrepancy. Lecture Notes Comp Sci. 2006;4232:30–38. doi: 10.1007/11893028_4. [DOI] [Google Scholar]
- Wang GL, Zhou Y, Chen AH, Zhang PM, Liang PJ. A robust method for spike sorting with automatic overlap decomposition. IEEE Trans Biomed Eng. 2006;53(6):1195–1198. doi: 10.1109/TBME.2006.873397. [DOI] [PubMed] [Google Scholar]
- Zhang PM, Wu JY, Zhou Y, Liang PJ, Yuan JQ. Spike sorting based on automatic template reconstruction with a partial solution to the overlapping problem. J Neurosci Methods. 2004;135(1–2):55–65. doi: 10.1016/j.jneumeth.2003.12.001. [DOI] [PubMed] [Google Scholar]