Abstract
Successful axonal repair following injury is critical for nerve regeneration and functional recovery. Nerve repair relies on three functionally distinct events involving membrane trafficking. First, axonally transported vesicles accumulate, while others are generated at the cut end to restore a selective barrier to the severed axon. Then, retrograde transport of vesicles along microtubules informs the cell body that damage has occurred in the distal axon. Finally, membrane addition to a newly formed growth cone, or to the axonal membrane is required to promote axonal re-growth and elongation. Yet, how these membrane trafficking events are regulated and what are the identities of the molecules and organelles involved remains largely unknown. Several potential factors have been recently identified. Members of the SNARE machinery appear to regulate fusion of vesicles in a calcium-dependent manner to promote axolemmal resealing. Retrograde transport of endosomes powered by the dynein-dynactin molecular motor complex represents a potential injury-signaling platform. Several classes of secretory and endocytic vesicles may coordinate axonal membrane extension and re-growth. Here we discuss recent advances in understanding the mechanisms of the membrane trafficking involved in nerve repair.
Key words: regeneration, repair, axon, membrane trafficking, vesicular transport, injury signaling, axon growth
Introduction
The extreme polarized morphology of neurons poses a challenging problem for intracellular membrane trafficking. The transport of numerous distinct classes of organelles and vesicles is required to establish and maintain the polarized morphology of neurons. This transport system also controls survival and stress signaling between the cell body and the distantly located dendritic and synaptic terminals. Similarly to non-polarized cells, membrane trafficking in neurons can be subdivided in two main categories, the biosynthetic and the endocytic pathway. In the biosynthetic pathway, proteins and lipids are synthesized in the endoplasmic reticulum and then trafficked through the Golgi apparatus where a series of post-translational modifications occur. Vesicles exiting the Golgi are targeted to the plasma membrane or join elements of the endocytic pathway. Endocytosis is the process by which cell surface molecules, membrane proteins, receptors and extracellular solutes are sequestered away from the plasma membrane and delivered to early endosomes. Then, some membrane proteins and receptors are recycled back to the cell surface through the recycling endosomes, while others are transported to late endosomes and lysosomes for degradation, or join the biosynthetic pathway.1 Given their morphological complexity, neurons have mastered the art of membrane specialization.2 Neurons appear to possess a more complex population of endosomal carriers than non-neuronal cells3,4 and unlike non-polarized cells, trafficking through the endoplasmic reticulum and the Golgi complex is not restricted to the cell body but also occurs in axons5 and dendrites.6
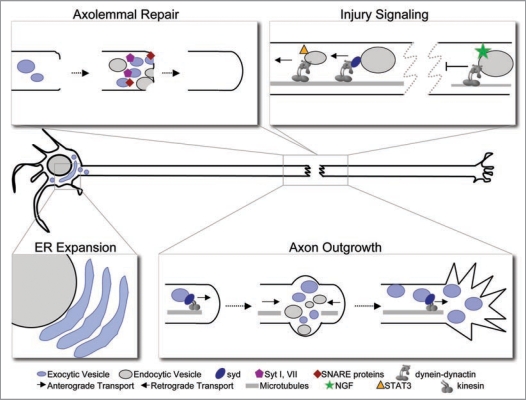
Recent studies have begun to reveal the identity of organelles and membrane compartments that contribute to neurite extension and axon guidance during development (reviewed in refs. 7 and 8). Early endosomes play a role in axon guidance, while recycling and late endosomes were shown to contribute to membrane addition during neurite outgrowth and extension.8 The mechanisms regulating neurite outgrowth during development may be recapitulated during nerve regeneration. However, given the significantly greater distances that axons typically need to regrow in adult animals, the mechanisms may differ. In addition to growth per se, membrane trafficking events are required for membrane resealing and intracellular signaling. A three-step process requiring distinct membrane trafficking events can be proposed to drive nerve regeneration (Fig. 1). First, following nerve transection, the axonal plasma membrane, also known as axolemma, must be locally repaired. Second, retrograde injury signals traveling from the injury site back to the cell body increase the intrinsic growth capacity of injured neurons to promote successful regeneration. Third, membrane trafficking and cargo delivery contribute to membrane expansion during axonal re-growth and elongation. Here we discuss recent findings on the distinct roles of membrane trafficking in nerve repair. Based on the current knowledge of membrane trafficking during neuronal development, we also discuss the mechanisms that might be involved in axonal extension during regeneration.
Figure 1.
Model of membrane trafficking events during axon repair and regeneration. Repair of the axolemma is mediated by the accumulation and fusion of exocytic vesicles derived from the Golgi complex or lysosomal/secretory organelles. Calcium-dependent fusion mechanisms involving synaptotagmin I and VII, SNARE proteins TI-VAMP/VAMP-7 and syntaxins 1 and 4 are important for successful membrane resealing. Similarly, calciumdependent endocytic mechanisms also play a role in membrane repair. Retrograde injury signaling informs the cell body that damage has occurred in the distal axon. Retrograde transport of Sunday Driver (syd)-associated endocytic vesicles powered by the dynein-dynactin molecular motor complex represents a potential injury-signaling platform. Lack of NGF delivery to the cell body represents other mechanisms to signal injury. Expansion of the endoplasmic reticulum allows for increased synthesis of proteins and lipids necessary for repairing the lesion and extending the axon over a large distance to successfully reconnect with its target. Establishment of a new growth cone is mediated by the accumulation and fusion of Golgi-derived and endocytic vesicles just proximal to the cut end. Continuous supply of anterogradely transported vesicles then contribute to the addition of membrane at the growth cone and potentially along the length of the axon.
Axolemmal Repair
One of the earliest responses of neurons to axotomy is the resealing of cut axons. Failure to reseal or a delay in resealing may be detrimental to a neuron’s ability to survive axotomy or to regenerate a new axon. Although restoring a selective barrier to severed axons is a prerequisite for restoring function, it has received relatively little attention during a century of research on axon regrowth after injury. In the early 1990s, confocal and electron microscope studies demonstrated the accumulation of membranous, injuryinduced vesicles that appeared to occlude the open, cut ends of isolated squid giant axons.9,10 Further studies in the pseudo-myelinated axons of the earthworm, Lumbricus terrestris, revealed a role for calcium in vesicle fusion with each other or with the plasma membrane for resealing.11 Membrane resealing in lesioned mammalian axons was shown to take up to several hours, and depend on axon diameter and on calcium in the extracellular environment. 12 The level of intra-axonal calcium is also important for membrane resealing. In Aplysia neurons, calcium influx after axotomy occurs by direct entrance through the disrupted plasma membrane at the lesion site and also through voltage-gated calcium channels.13,14 An increase in intra-axonal calcium induced by injury was also observed in rat sciatic nerve.15 This rapid shift in calcium concentrations induces at least two major events that are necessary for plasmalemmal repair: proteolysis and membrane fusion/fission events. Calcium-dependent activation of proteases such as calpain is a necessary event for membrane resealing in rat septal neurons16 and in myelinated dorsal root axons.12 Cleavage of submembrane spectrin skeleton by calpain is required for the formation of a competent growth cone after axotomy.17,18 This protease activity presumably releases membrane tension allowing for more efficient vesicle fusion with the plasma membrane.17
Since the observation 20 years ago that vesicles accumulate at the axon cut end9,10 (reviewed in ref. 19), progress has been made towards defining the nature of these vesicles and the molecules mediating their trafficking. It appears that both exo- and endocytosis are triggered upon elevated intra-axonal calcium to mediate membrane resealing. Calcium-dependent exocytosis is known to mediate membrane resealing in nonneuronal cell types like fibroblasts,20 and also in the squid and crayfish giant axons.21 Recently, Norma Andrews group has identified the lysosomes as exocytotic vesicles involved in membrane resealing and synaptotagmin VII, a member of the synaptotagmin family of Ca2+-binding proteins, as a regulator of this process.22 The machinery regulating vesicle fusion has been extensively studied in the case of synaptic vesicle exocytosis. It involves an interaction between synaptotagmin and the SNARE machinery, which includes vSNAREs on the vesicle and the t-SNAREs such as syntaxin on the target membrane. Synaptotagmin VII-dependent lysosomal fusion in fibroblasts requires the v-SNARE TI-VAMP/VAMP7 and the t-SNARE syntaxin4.23 Whether synaptotagmin VII is also involved in membrane resealing in mammalian neurons remains to be determined. However, the recent finding that in peripheral nerves, synaptotagmin VII is anterogradely transported in vesicles bearing the molecular motor binding protein Sunday Driver (syd), also known as JIP3 or JSAP1, suggests a mechanism for targeted delivery of synaptotagmin VII to distal nerve sites.24 Synaptotagmin VII thus emerges as a strong candidate regulating membrane repair in vertebrate axons. This does not exclude the possibility that other organelles and molecules participate in resealing. Synaptotagmin1 and syntaxin1, which are known to regulate fusion of synaptic vesicles for neurotransmitter release, are also needed for membrane repair in the squid and crayfish giant axons.21 In addition to exocytosis, calciumdependent endocytosis mediates plasma membrane repair in the squid giant axon.19,25 Endocytic vesicles bearing synaptotagmin on their surfaces are formed at the lesion site and promote membrane resealing.25 Calcium-dependent endocytosis is also necessary for wound removal induced by the pore-producing bacterial toxin streptolysinO in NRK cells or in mechanically wounded cells.26 Future work is needed to understand whether the organelles involved in membrane resealing are specialized for this task or whether several types of molecules and organelles present at the injury site randomly facilitate fusions events essential for the repair of damaged axolemmal membranes.
Transformation of a transected axonal tip into a growth cone is a critical step in the cascade leading to neuronal regeneration. Some of the vesicles that accumulate at axonal cut ends arise from continuous axonal transport from the cell body. In addition to assisting with the necessary immediate repair, the accumulation of vesicles at the cut end contributes to growth cone formation. Spira and co-workers have shown that microtubules form a trap in which anterogradely and retrogradely transported vesicles accumulate just proximal to the cut end and assist with growth cone formation after axotomy.27 Cleavage of the submembrane spectrin skeleton by calcium-activated proteases is crucial to allow membrane expansion and growth cone formation.17,18,28 Failure to remove the spectrin barrier results in end-bulb formation, a classical structure observed in central nervous system (CNS) neurons, which generally fail to regenerate.17 Interestingly, axonal resealing is slower in central than peripheral axons, which may contribute to their different ability to regenerate.29 In addition to intrinsic differences, chronic, non-disruptive axonal injury that often occurs in the central nervous system may not elicit the necessary changes, such as increased calcium and proteolytic activity required for repair and may instead lead to endbulb formation or axonal degeneration.30 A detailed analysis of the molecular processes that enable vesicles to restore a plasma membrane seal in central and peripheral neurons could help understand the poor regenerative capacity of CNS neurons.
Injury Signaling
Injury to peripheral nerves provokes a series of morphologic and biochemical changes in neuronal cell bodies that were initially described more than 40 years ago. The most prominent change was described as chromatolysis and refers to the disruption and dispersion of Nissl Bodies due to disintegration of stacked rough endoplasmic reticulum.31 In addition to chromatolysis, rearrangement of the cytoskeleton and increased protein synthesis was also reported.31,32 In 1970, Cragg proposed several injury signaling mechanisms that could account for initiating the cell body reaction.32 Later studies in Aplysia33 and in mice34–36 led to a model in which three types of injury signals functioning in a temporal sequence assist with nerve regeneration (reviewed in ref. 37): injury-induced discharge of axonal potentials, interruption of the normal supply of retrogradely transported targetderived factors, and retrograde injury signals traveling from the injury site back to the cell body, also called positive injury signals. While some components of the positive retrograde injury signals, including the importin complex, directly associate with the retrograde motor machinery (reviewed in ref. 38), others hitchhike on axonal vesicles. For a more complete discussion of the current knowledge of injury signals, see ref. 38–40.
Endosomes might represent the carrier of choice to convey information about distant event in the axon back to the cell body. Distinct endosomal populations exist in neurons.41 Each specialized endosome may facilitate the formation of signaling platforms by clustering a selected pool of signaling components, therefore providing a precise temporal and spatial regulation of signal transduction.4 Early studies on intra-axonal organelle transport have revealed an anterograde to retrograde conversion after injury,42 which could in part depend upon local proteolysis at the site of injury.43 Since these early studies on axonal transport, much research has been devoted to understanding how these organelles move by characterizing the molecular motors they are associated with, dynein-dynactin and kinesin. However, we still know little about how motors discriminate among their many potential cargoes and how transport directionality is achieved. The motorbinding protein syd represents a potential motor adaptor on axonal endosomes24 to convey information about axonal injury back to the cell body. In peripheral axons, the stress-activated protein kinase JNK is present on syd-associated vesicles and is transported in both anterograde and retrograde directions.44 Nerve injury induces a transport switch, such that syd and JNK preferentially move retrogradely, most likely due to a preferred interaction between syd and the retrograde motor complex dynein-dynactin.44 JNK signaling has been implicated in promoting nerve regeneration, as sciatic nerve transection induces a rapid and prolonged increase in activated JNK in the cell body, which returns to basal levels once regeneration is completed.45 These studies suggest that syd mediates the axonal transport of endosomes carrying JNK on their surface, and that the directional switch induced by axon injury provides a mechanism for propagation of retrograde injury signals back to the cell body. Whether syd-endosomes are derived from the endocytic events at the nerve terminal or emerge at the site of injury from the local endocytic events25 remains to be determined.
In addition to activation of mitogen-activated protein kinases such as JNK44 and Erk46 at the site of injury, axonal damage activates transcription factors, such as STAT3, which is important for regeneration. Indeed, STAT3 activation through the Jak2 signaling pathway occurs in DRG neurons cell body after peripheral, but not central lesion,47,48 strongly supporting a role for STAT3 in neuronal regeneration. The retrograde transport of locally activated STAT3 has been suggested49,50 and STAT3 associates with early endosomes in Hep3B liver cells.51 However, whether STAT3 itself is associated with vesicular structures in axons remains unknown. Nevertheless, STAT3 signaling following injury is likely to depend in part on vesicular transport since in vitro studies using compartmentalized cultures have suggested a signaling endosome model in which the gp130/JAK complex is endocytosed and then retrogradely transported to activate STAT3 in the cell body.52 Whether the gp130/JAK complex travels on the classical signaling endosome or another specialized organelle originating at the site of injury will require further studies.
Another type of endosome that plays an indirect role in injury signaling is the so called “signaling endosome”. Signaling endosomes contain neurotrophic factors secreted by the target tissue and are transported retrogradely back to the cell body. Loss of such signal has the potential to initiate a regenerative response and was first postulated by Cragg to explain chromatolysis.32 Later experiments have indeed shown that loss of trophic factors might act as an injury signal. Sciatic nerve axotomy leads to decreased levels of retrogradely transported nerve gowth factor (NGF)53 and artificial interruption of the NGF supply by injections of antiserum to NGF mimics the changes in gene expression induced by axotomy.54 The signaling endosome hypothesis has been put forward to explain retrograde neurotrophin signaling, amongst a number of other possible models.55 The precise identity of such signaling endosome remains controversial and includes both early and late endosomes, as well as lysosome and multivesicular bodies (reviewed in ref. 55). Yet the role of multivesicular bodies in neurotrophic signaling has been recently challenged56 and they may instead represent a population of organelles that arise upon injury in axons.57 This is an interesting concept since storage of signaling molecules within intralumenal vesicles of multivesicular bodies may prevent their deactivation during the long journey from the axon back to the cell body.58 As intraluminal vesicles are not always destined for lysosomal degradation, but can fuse back with the limiting membrane of late endosomes,59 multivesicular bodies may offer a protected environment during transport and release their cargo at destination in the cell body. The identity of the various endocytic vesicular carriers, and the regulatory mechanisms that determine their transport direction remain unclear. Future proteomic studies, such as those carried out for syd-endosome24 or dynein associated axonal vesicle60 are needed to shed light on the precise nature of organelles transported along axons.
Axon Outgrowth and Elongation
The balance between constitutive plasma membrane retrieval and insertion that determines the shape and dimension of a neuron has to be modified to allow a neuron to regrow its axon after injury.61 The importance of membrane trafficking, especially endocytosis, for neurite outgrowth and guidance during development is being unraveled (reviewed in ref. 62). Sadly, virtually nothing is known about how membrane trafficking assists with regeneration in vivo. Regenerative axonal outgrowth may recapitulate a developmental program.63 However, given the significantly greater distances that axon need to cover to reconnect with their target in an adult animal, it is conceivable that axonal regeneration depends on somehow distinct mechanisms. What are the mechanisms regulating membrane insertion, and how is the vast amount of membrane material required for axonal extension following injury supplied to the growing axon? The classical paradigm implicates that proteins and lipids are synthesized in the cell body through the endoplasmic reticulum and the Golgi complex and are then co-transported along microtubules in the form of vesicles to reach the membrane insertion site. This paradigm largely rests on the long-standing assumption that only the cell body contains the necessary biosynthetic activity to synthesize protein and lipids. During the last 10 years, it became clear that axons possess components of endoplasmic reticulum and Golgi, and are capable of local protein5 and lipid synthesis.64 Merianda et al. report that inhibition of Golgi function in isolated adult DRG axons attenuates translation-dependent axonal growth responses.5 Furthermore, they show that the capacity for secreting locally synthesized proteins in axons appears to be increased by injury. This is in contrast with earlier studies showing that protein synthesis in distal axons is not required for axon growth in the embryonic spinal cord,65 but may emphasize a difference in outgrowth mechanisms in peripheral versus central neurons.
How and where locally synthesized membrane proteins are inserted into the plasma membrane remains largely unknown. In cultured developing neurons, the site of membrane insertion for axonal outgrowth appears to be primarily at the growth cone.66 This is not the case for mature neurons, in which, at least in dendrites, membrane appears to be added to multiple sites along the dendritic surface.66 Dendritic Golgi outposts distributed along dendrites may play a role in membrane insertion given their requirement for dendritic growth.6 It is conceivable that equivalent Golgi outposts along axon may play a similar role in membrane addition during regenerative axonal growth. Indeed, in growing Xenopus neurites, membrane is inserted at the cell body and all along the neurite, creating an anterograde bulk membrane flow that correlates with neurite elongation.67
The massive demand for membrane biogenesis in regenerating neurons must be different to that of neurons not actively growing. The expansion of the endoplasmic reticulum in the cell body that occurs after injury reflects the increase in membrane synthesis. The mechanisms regulating this increase in membrane biogenesis are not completely understood. Recently, it has been proposed that transcription factors may play new, non-genomic roles in regulating regeneration. The transcription factor c-fos has, in addition to its transcription factor activity, the capacity to activate the biosynthesis of phospholipids and glycolipids necessary for membrane biogenesis.68,69 This activity is required for neurite elongation, at least in PC12 cells.68 Whether this non- transcriptional c-fos activity is required to stimulate membrane biogenesis in vivo has to be further examined, given the observation that c-fos expression is not induced in DRG neurons following nerve injury.70
Axolemmal expansion requires fusion of vesicles with the plasma membrane. Similarly to its role in new membrane addition for extension of developing neurites,71 synaptotagmin VII may function in regenerative outgrowth. A population of axonally transported vesicles carrying syd and synaptotagmin VII on their surface24 may mediate the delivery of synaptotagmin VII to distal axons. This would agree with the observed axonal growth defects observed in syd knockout animals.72,73 Another recently identified molecular pathway that may control membrane extension is the IGF1-exocyst pathway. In cultured hippocampal neurons, membrane expansion at the axonal growth cone is regulated by IGF-1 via a signaling cascade involving TC10 and the exocyst complex.74 Interestingly, IGF-1 is required for peripheral nerve regeneration,75 indicating that IGF-1 may regulate a signaling cascade for membrane insertion.
In addition to proteins, axon elongation requires insertion of lipids to the growing axons. Axons do not need to obtain all of their lipids from the cell body since they have the capacity to synthesize most phospholipids in the axon. Importantly, axonal synthesis of phospholipids is required for axon elongation.64 In contrast, cholesterol is not made in axons, but exclusively in the cell body. While endogenous supply of cholesterol may be sufficient for developmental growth, it may not be the case for regenerative growth. Several studies (reviewed in ref. 64) have suggested that lipoproteins such as apoE may function in lipid recycling following injury to repackage and reuse lipids to promote membrane assembly and regeneration. The transfer of lipids from axon to Schwann cells76 together with the transfer of ribosomes from Schwann cells to axons77 reflect the intimate relationship between the Schwann cell and its ensheathed axon. In case of very long axons in which the cell body may not be able to provide sufficient material to support regeneration at the axon tip, the possibility that the distal axon can rely on non-neuronal sources to support its autonomy from the cell body and regenerate effectively is intriguing.
The field of axonal repair and extension remains filled with open questions. Future work will need to define the mechanisms regulating membrane trafficking in axonal repair in peripheral nerves and their implications to the lack of regenerative capacity of CNS neurons.
Acknowledgements
This work was supported in part by NINDS National Institute of Health grant RO1NS060709 (to V. Cavalli).
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/11555
References
- 1.Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- 2.Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003;40:277–295. doi: 10.1016/s0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- 3.Parton RG, Simons K, Dotti CG. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992;119:123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salinas S, Bilsland LG, Schiavo G. Molecular landmarks along the axonal route: axonal transport in health and disease. Curr Opin Cell Biol. 2008;20:445–453. doi: 10.1016/j.ceb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Merianda TT, et al. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol Cell Neurosci. 2009;40:128–142. doi: 10.1016/j.mcn.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci. 2003;23:6188–6199. doi: 10.1523/JNEUROSCI.23-15-06188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfenninger KH. Plasma membrane expansion: a neuron’s Herculean task. Nat Rev Neurosci. 2009;10:251–261. doi: 10.1038/nrn2593. [DOI] [PubMed] [Google Scholar]
- 8.Sann S, Wang Z, Brown H, Jin Y. Roles of endosomal trafficking in neurite outgrowth and guidance. Trends Cell Biol. 2009;19:317–324. doi: 10.1016/j.tcb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Fishman HM, Tewari KP, Stein PG. Injury-induced vesiculation and membrane redistribution in squid giant axon. Biochim Biophys Acta. 1990;1023:421–435. doi: 10.1016/0005-2736(90)90135-b. [DOI] [PubMed] [Google Scholar]
- 10.Krause TL, Fishman HM, Ballinger ML, Bittner GD. Extent and mechanism of sealing in transected giant axons of squid and earthworms. J Neurosci. 1994;14:6638–6651. doi: 10.1523/JNEUROSCI.14-11-06638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballinger ML, et al. Delaminating myelin membranes help seal the cut ends of severed earthworm giant axons. J Neurobiol. 1997;33:945–960. doi: 10.1002/(sici)1097-4695(199712)33:7<945::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Howard MJ, David G, Barrett JN. Resealing of transected myelinated mammalian axons in vivo: evidence for involvement of calpain. Neuroscience. 1999;93:807–815. doi: 10.1016/s0306-4522(99)00195-5. [DOI] [PubMed] [Google Scholar]
- 13.Ziv NE, Spira ME. Spatiotemporal distribution of Ca2+ following axotomy and throughout the recovery process of cultured Aplysia neurons. Eur J Neurosci. 1993;5:657–668. doi: 10.1111/j.1460-9568.1993.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 14.Ziv NE, Spira ME. Axotomy induces a transient and localized elevation of the free intracellular calcium concentration to the millimolar range. J Neurophysiol. 1995;74:2625–2637. doi: 10.1152/jn.1995.74.6.2625. [DOI] [PubMed] [Google Scholar]
- 15.Mata M, Staple J, Fink DJ. Changes in intra-axonal calcium distribution following nerve crush. J Neurobiol. 1986;17:449–467. doi: 10.1002/neu.480170508. [DOI] [PubMed] [Google Scholar]
- 16.Xie XY, Barrett JN. Membrane resealing in cultured rat septal neurons after neurite transection: evidence for enhancement by Ca2+-triggered protease activity and cytoskeletal disassembly. J Neurosci. 1991;11:3257–3267. doi: 10.1523/JNEUROSCI.11-10-03257.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamber D, Erez H, Spira ME. Local calcium-dependent mechanisms determine whether a cut axonal end assembles a retarded endbulb or competent growth cone. Exp Neurol. 2009;219:112–125. doi: 10.1016/j.expneurol.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Gitler D, Spira ME. Real time imaging of calciuminduced localized proteolytic activity after axotomy and its relation to growth cone formation. Neuron. 1998;20:1123–1135. doi: 10.1016/s0896-6273(00)80494-8. [DOI] [PubMed] [Google Scholar]
- 19.Fishman H, Bittner G. Vesicle-mediated restoration of a plasmalemmal barrier in severed axons. News Physiol Sci. 2003;18:115–118. doi: 10.1152/nips.01429.2002. [DOI] [PubMed] [Google Scholar]
- 20.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 21.Detrait E, et al. Axolemmal repair requires proteins that mediate synaptic vesicle fusion. J Neurobiol. 2000;44:382–391. doi: 10.1002/1097-4695(20000915)44:4<382::aid-neu2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 22.Andrews NW. Membrane resealing: synaptotagmin VII keeps running the show. Sci STKE. 2005. p. 19. [DOI] [PubMed]
- 23.Rao S, Huynh C, Proux-Gillardeaux V, Galli T, Andrews N. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J Biol Chem. 2004;219:20471–20479. doi: 10.1074/jbc.M400798200. [DOI] [PubMed] [Google Scholar]
- 24.Abe N, et al. Sunday driver interacts with two distinct classes of axonal organelles. J Biol Chem. 2009;284:34628–34639. doi: 10.1074/jbc.M109.035022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edelman CS, Ballinger ML, Smyers ME, Fishman HM, Bittner GD. Endocytotic formation of vesicles and other membranous structures induced by Ca2+ and axolemmal injury. J Neurosci. 1998;18:4029–4041. doi: 10.1523/JNEUROSCI.18-11-04029.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Idone V, et al. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol. 2008;180:905–914. doi: 10.1083/jcb.200708010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erez H, et al. Formation of microtubule-based traps controls the sorting and concentration of vesicles to restricted sites of regenerating neurons after axotomy. J Cell Biol. 2007;176:497–507. doi: 10.1083/jcb.200607098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziv NE, Spira ME. Induction of growth cone formation by transient and localized increases of intracellular proteolytic activity. J Cell Biol. 1998;140:223–232. doi: 10.1083/jcb.140.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed FA, Ingoglia NA, Sharma SC. Axon resealing following transection takes longer in central axons than in peripheral axons: implications for axonal regeneration. Exp Neurol. 2001;167:451–455. doi: 10.1006/exnr.2000.7562. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma. 1997;14:419–440. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- 31.Lieberman AR. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- 32.Cragg BG. What is the signal for chromatolysis? Brain Res. 1970;23:1–21. doi: 10.1016/0006-8993(70)90345-8. [DOI] [PubMed] [Google Scholar]
- 33.Ambron RT, Dulin MF, Zhang XP, Schmied R, Walters ET. Axoplasm enriched in a protein mobilized by nerve injury induces memory-like alterations in Aplysia neurons. J Neurosci. 1995;15:3440–3446. doi: 10.1523/JNEUROSCI.15-05-03440.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309:791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- 35.Smith DS, Skene JH. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci. 1997;17:646–658. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 37.Ambron RT, Walters ET. Priming events and retrograde injury signals. A new perspective on the cellular and molecular biology of nerve regeneration. Mol Neurobiol. 1996;13:61–79. doi: 10.1007/BF02740752. [DOI] [PubMed] [Google Scholar]
- 38.Ben-Yaakov K, Fainzilber M. Retrograde Injury Signaling in Lesioned Axons. Results Probl Cell Differ. 2009;48:327–338. doi: 10.1007/400_2009_14. [DOI] [PubMed] [Google Scholar]
- 39.Abe N, Cavalli V. Nerve injury signaling. Curr Opin Neurobiol. 2008;18:276–283. doi: 10.1016/j.conb.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rishal I, Fainzilber M. Retrograde signaling in axonal regeneration. Exp Neurol. 2009. In press. [DOI] [PubMed]
- 41.Wilson JM, et al. EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells and fibroblasts. Mol Biol Cell. 2000;11:2657–2671. doi: 10.1091/mbc.11.8.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bisby MA, Bulger VT. Reversal of axonal transport at a nerve crush. J Neurochem. 1977;29:313–320. doi: 10.1111/j.1471-4159.1977.tb09624.x. [DOI] [PubMed] [Google Scholar]
- 43.Sahenk Z, Lasek RJ. Inhibition of proteolysis blocks anterograde-retrograde conversion of axonally transported vesicles. Brain Res. 1988;460:199–203. doi: 10.1016/0006-8993(88)91224-3. [DOI] [PubMed] [Google Scholar]
- 44.Cavalli V, Kujala P, Klumperman J, Goldstein LS. Sunday Driver links axonal transport to damage signaling. J Cell Biol. 2005;168:775–787. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kenney AM, Kocsis JD. Peripheral axotomy induces long-term c-Jun amino-terminal kinase-1 activation and activator protein-1 binding activity by c-Jun and junD in adult rat dorsal root ganglia in vivo. J Neurosci. 1998;18:1318–1328. doi: 10.1523/JNEUROSCI.18-04-01318.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perlson E, et al. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 47.Schwaiger FW, et al. Peripheral but not central axotomy induces changes in Janus kinases (JAK) and signal transducers and activators of transcription (STAT) Eur J Neurosci. 2000;12:1165–1176. doi: 10.1046/j.1460-9568.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- 48.Qiu J, Cafferty WB, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci. 2005;25:1645–1653. doi: 10.1523/JNEUROSCI.3269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee N, Neitzel KL, Devlin BK, MacLennan AJ. STAT3 phosphorylation in injured axons before sensory and motor neuron nuclei: potential role for STAT3 as a retrograde signaling transcription factor. J Comp Neurol. 2004;474:535–545. doi: 10.1002/cne.20140. [DOI] [PubMed] [Google Scholar]
- 50.Sheu JY, Kulhanek DJ, Eckenstein FP. Differential patterns of ERK and STAT3 phosphorylation after sciatic nerve transection in the rat. Exp Neurol. 2000;166:392–402. doi: 10.1006/exnr.2000.7508. [DOI] [PubMed] [Google Scholar]
- 51.Shah M, et al. Membrane-associated STAT3 and PY-STAT3 in the cytoplasm. J Biol Chem. 2006;281:7302–7308. doi: 10.1074/jbc.M508527200. [DOI] [PubMed] [Google Scholar]
- 52.O’Brien JJ, Nathanson NM. Retrograde activation of STAT3 by leukemia inhibitory factor in sympathetic neurons. J Neurochem. 2007;103:288–302. doi: 10.1111/j.1471-4159.2007.04736.x. [DOI] [PubMed] [Google Scholar]
- 53.Raivich G, Hellweg R, Kreutzberg GW. NGF receptormediated reduction in axonal NGF uptake and retrograde transport following sciatic nerve injury and during regeneration. Neuron. 1991;7:151–164. doi: 10.1016/0896-6273(91)90083-c. [DOI] [PubMed] [Google Scholar]
- 54.Shadiack A, Sun Y, Zigmond R. Nerve growth factor antiserum induces axotomy-like changes in neuropeptide expression in intact sympathetic and sensory neurons. J Neurosci. 2001;21:363–371. doi: 10.1523/JNEUROSCI.21-02-00363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu C, Cui B, He L, Chen L, Mobley WC. The coming of age of axonal neurotrophin signaling endosomes. J Proteomics. 2009;72:46–55. doi: 10.1016/j.jprot.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Altick AL, Baryshnikova LM, Vu TQ, von Bartheld CS. Quantitative analysis of multivesicular bodies (MVBs) in the hypoglossal nerve: evidence that neurotrophic factors do not use MVBs for retrograde axonal transport. J Comp Neurol. 2009;514:641–657. doi: 10.1002/cne.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaasinen SK, Harvey L, Reynolds AJ, Hendry IA. Autophagy generates retrogradely transported organelles: a hypothesis. Int J Dev Neurosci. 2008;26:625–634. doi: 10.1016/j.ijdevneu.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 58.Weible MW, 2nd, Hendry IA. What is the importance of multivesicular bodies in retrograde axonal transport in vivo? J Neurobiol. 2004;58:230–243. doi: 10.1002/neu.10318. [DOI] [PubMed] [Google Scholar]
- 59.van der Goot FG, Gruenberg J. Intra-endosomal membrane traffic. Trends Cell Biol. 2006;16:514–521. doi: 10.1016/j.tcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Perlson E, et al. A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. J Neurosci. 2009;29:9903–9917. doi: 10.1523/JNEUROSCI.0813-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prager-Khoutorsky M, Spira ME. Neurite retraction and regrowth regulated by membrane retrieval, membrane supply and actin dynamics. Brain Res. 2009;1251:65–79. doi: 10.1016/j.brainres.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 62.Futerman AH, Banker GA. The economics of neurite outgrowth-the addition of new membrane to growing axons. Trends Neurosci. 1996;19:144–149. doi: 10.1016/s0166-2236(96)80025-7. [DOI] [PubMed] [Google Scholar]
- 63.Filbin MT. Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci. 2006;361:1565–1574. doi: 10.1098/rstb.2006.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vance JE, Campenot RB, Vance DE. The synthesis and transport of lipids for axonal growth and nerve regeneration. Biochim Biophys Acta. 2000;1486:84–96. doi: 10.1016/s1388-1981(00)00050-0. [DOI] [PubMed] [Google Scholar]
- 65.Blackmore M, Letourneau PC. Protein synthesis in distal axons is not required for axon growth in the embryonic spinal cord. Dev Neurobiol. 2007;67:976–986. doi: 10.1002/dneu.20395. [DOI] [PubMed] [Google Scholar]
- 66.Craig AM, Wyborski RJ, Banker G. Preferential addition of newly synthesized membrane protein at axonal growth cones. Nature. 1995;375:592–594. doi: 10.1038/375592a0. [DOI] [PubMed] [Google Scholar]
- 67.Popov S, Brown A, Poo MM. Forward plasma membrane flow in growing nerve processes. Science. 1993;259:244–246. doi: 10.1126/science.7678471. [DOI] [PubMed] [Google Scholar]
- 68.Gil GA, et al. c-Fos activated phospholipid synthesis is required for neurite elongation in differentiating PC12 cells. Mol Biol Cell. 2004;15:1881–1894. doi: 10.1091/mbc.E03-09-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crespo PM, et al. c-Fos activates glucosylceramide synthase and glycolipid synthesis in PC12 cells. J Biol Chem. 2008;283:31163–31171. doi: 10.1074/jbc.M709257200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leah JD, Herdegen T, Bravo R. Selective expression of Jun proteins following axotomy and axonal transport block in peripheral nerves in the rat: evidence for a role in the regeneration process. Brain Res. 1991;566:198–207. doi: 10.1016/0006-8993(91)91699-2. [DOI] [PubMed] [Google Scholar]
- 71.Arantes RM, Andrews NW. A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons. J Neurosci. 2006;26:4630–4637. doi: 10.1523/JNEUROSCI.0009-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelkar N, et al. Morphogenesis of the telencephalic commissure requires scaffold protein JNK-interacting protein 3 (JIP3) Proc Natl Acad Sci USA. 2003;100:9843–9848. doi: 10.1073/pnas.1733944100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ha HY, et al. The axon guidance defect of the telencephalic commissures of the JSAP1-deficient brain was partially rescued by the transgenic expression of JIP1. Dev Biol. 2005;277:184–199. doi: 10.1016/j.ydbio.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 74.Dupraz S, et al. The TC10-Exo70 complex is essential for membrane expansion and axonal specification in developing neurons. J Neurosci. 2009;29:13292–13301. doi: 10.1523/JNEUROSCI.3907-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanje M, Skottner A, Sjoberg J, Lundborg G. Insulinlike growth factor I (IGF-I) stimulates regeneration of the rat sciatic nerve. Brain Res. 1989;486:396–398. doi: 10.1016/0006-8993(89)90531-3. [DOI] [PubMed] [Google Scholar]
- 76.Ledeen RW, Golly F, Haley JE. Axon-myelin transfer of phospholipids and phospholipid precursors. Labeling of myelin phosphoinositides through axonal transport. Mol Neurobiol. 1992;6:179–190. doi: 10.1007/BF02780551. [DOI] [PubMed] [Google Scholar]
- 77.Court FA, Hendriks WT, Macgillavry HD, Alvarez J, van Minnen J. Schwann cell to axon transfer of ribosomes: toward a novel understanding of the role of glia in the nervous system. J Neurosci. 2008;28:11024–11029. doi: 10.1523/JNEUROSCI.2429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]