Abstract
Cell-to-cell communication is the basis of all biology in multicellular organisms, allowing evolution of complex forms and viability in dynamic environments. Though biochemical interactions occur over distances, physical continuity remains the most direct means of cellular interactions. Cellular bridging through thin cytoplasmic channels—plasmodesmata in plants and tunneling nanotubes in animals—creates direct routes for transfer of signals and components, even pathogens, between cells. Recently, two new cellular connections, designated epithelial (EP) bridges, were discovered and found to be structurally distinct from other cellular channels. The first EP bridge type facilitates material transport between cells similar to plasmodesmata and tunneling nanotubes, the second EP bridge type mediates migration of cells between EP cell masses representing a novel form of cell migration. Here, we compare the structures and functions of EP bridges with other cellular channels and discuss biochemical and cellular interactions involved in EP bridge formation. Potential roles for EP bridges in health and disease are also presented.
Key words: epithelial bridges, tunneling nanotubes, plasmodesmata, cell migration, intercellular signaling, F-actin, microtubules, reactive oxygen species, NFκB, cyclooxygenase
Communication among cells in multi-cellular systems is facilitated by soluble factors through paracrine and endocrine signaling and by direct contact either through non-cytoplasmic connections (i.e., cytonemes and filopodia bridges)1,2 or cytoplasmic connections (i.e., gap junctions and intercellular bridges).3,4 In both plants and animals, the presence of cell-to-cell cytoplasmic channels creating supracellular assemblies makes the standard version of the cell theory untenable, where cells are postulated to be only building blocks that are structurally and functionally independent entities.4–7 The challenge to this classical version of the cell theory began over 130 years ago when thin membranous channels, termed plasmodesmata, were discovered bridging neighboring plant cells (reviewed in refs. 8–10). Now only in the last decade have animal cell counterparts to plasmodesmata, termed tunneling nanotubes (TNTs), been identified in a variety of cell types (reviewed in refs. 11–14).
Plasmodesmata and TNTs share many structural and functional characteristics, and are the longest cytoplasmic connections between cells in vitro and in vivo.10,11,15 Though plasmodesmata firmly embed in the cell walls of connected plant cells5 and TNTs are unconstrained in the extracellular space between connected animal cells,14 both of these cytoplasmic channels contain F-actin as the prominent cytoskeletal component and most lack microtubules. The diameters of TNTs and plasmodesmata are relatively similar, in the tens to hundreds of nanometers in diameter, but the lengths of TNTs are usually longer (in the tens of microns) as plasmodesmata lengths are limited by the cell wall thickness of the connected plant cells.14 Functionally both types of cytoplasmic connections facilitate transfer of cellular signaling molecules, membrane components, and even pathogens between cells.5,10,11 The striking similarities between plasmodesmata and TNTs have led to speculations on the early evolution of intercellular connectivity in multi-cellular life forms and whether these cytoplasmic channels are derived from a common ancestral structure.12
Recently two novel forms of tubular cellular channels were discovered connecting primary human bronchial epithelial cells (EPs) cultured alone or with other primary human cell types.16 Termed epithelial (EP) bridges (types I and II), these cellular conduits represent the longest direct tubular connections reported to date, and are structurally distinct from plasmodesmata and most TNTs. Functionally, type I EP bridges facilitate cellular material transport between cells similar to other cell-to-cell channels, while type II EP bridges are functionally and structurally distinct from other cellular connections, providing a conduit for whole cells to migrate from one EP cell mass to another. This facilitation of cell transport via type II EP bridges represents a completely new mechanism of cell migration. The formation of EP bridges is likely a natural characteristic of EP biology in vitro regulated by cellular and biochemical interactions that mediate inflammatory pathways. In this mini-review, we discuss the structural, functional and formational traits of EP bridges in relation to other cellular channels and explore the possible physiological relevance of EP bridges in health and disease.
Structure and Function: EP Bridges versus TNTs
As EP bridges and TNTs form connections between animal cells, the focus of comparison in this review will be between these cellular connections, while comparisons to plant cell plasmodesmata will be interjected where appropriate. Since the discovery of TNTs in rat pheochromocytoma PC12 cells,17 TNT-like structures have been characterized forming connections in a variety of cell types. TNTs exist in permanent cell lines and primary cell cultures including human monocytes, human and mouse macrophages, human dendritic cells, rat astrocytes, human glioblastoma cells, human hematopoietic stem and progenitor cells, and even between rat neonatal cardiomyocytes and human endothelial progenitor cells (reviewed in refs. 11 and 14). Properly classifying these structures has proven difficult given their substantial heterogeneity in length, diameter, structural composition and function.
TNTs between PC12 cells measure 50–200 nm in diameter and several cell diameters in length;17,18 nanotubes connecting immune cells—human peripheral blood natural killer cells, macrophages and Epstein Barr Virus-transformed B cells—average 30 µm in length with some measuring over 140 µm;19 and, TNTs connecting rat neonatal cardiomyocytes and human endothelial progenitor cells are up to 800 nm in diameter and 120 µm in length.20 TNTs often contain an F-actin backbone and lack microtubules; however, exceptions do exist. In human macrophages, two distinct nanotubes are present: thin bridges containing only F-actin and thicker bridges 0.7 µm or larger in diameter containing both F-actin and microtubules.21 Intercellular bridges in human prostate cancer cells contain microtubules and range from 100 nm to 5 µm in diameter and a few microns to 100 µm in length.22 Depending on the cellular system, TNTs functionally mediate direct intercellular transfer of a wide range of material including Ca2+ ions, membrane components, small organelles of the endosomal/lysosomal system, and larger organelles such as mitochondria (reviewed in refs. 11–14). The structural and functional heterogeneity among these intercellular bridges indeed requires more detailed study and characterization to properly derive a classification system.
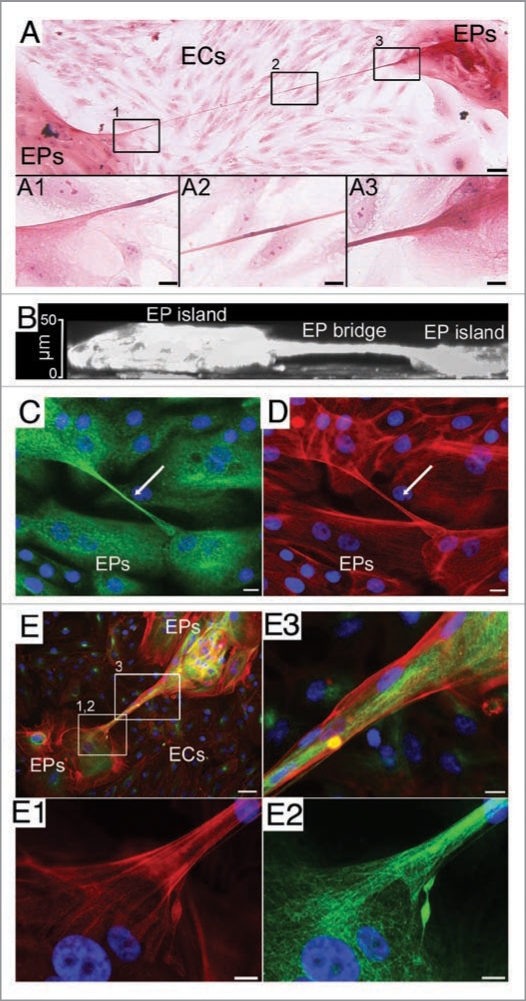
In contrast to the numerous cell types TNTs connect, EP bridges have only been found in primary cultures of human bronchial16 and mammary EPs, and in A549 carcinomic human alveolar basal epithelial cells (Zani BG and Edelman ER, unpublished). These EP connections are observed when EPs are cultured alone or with other primary human cells, such as aortic endothelial cells (ECs) or lung fibroblasts (FBs). In co-cultures, natural segregation occurs where multi-layered EP islands form and become surrounded by seas of ECs or FBs (Fig. 1A). Compared to most TNTs, EP bridge diameters and lengths are greater ranging from 1 to 20 µm in width independent of culture conditions, while EP bridge connections extend from 25 µm to over a millimeter in length depending upon culture conditions. On average EP bridges are longer in co-cultures (∼185 µm) than monocultures (∼100 µm), which likely arises from the greater distances required in making connections with other EPs. An interesting characteristic of both TNTs and EP bridges is that these cellular structures make no contact and hover freely above the underlying substratum (Fig. 1B). Both F-actin and microtubules are components of EP bridges (Fig. 1C–E), unlike the F-actin only backbone constituting most TNTs. The combination of both cytoskeletal components in EP bridges likely conveys structural stability and may be an integral aspect allowing these structures to extend over extreme distances compared to TNTs. It will be interesting to determine if EP bridges are related to TNTs connecting macrophages21 or human prostate cancer cells22 that do possess microtubules. EP bridges also appear to be structurally stable for longer periods of time, remaining intact up to 2 days in culture as opposed to the transient, minutes to several hours, integrity of TNTs. This prolonged survival may reflect reduced sensitivities of EP bridges to light exposure, mechanical stress or chemical fixation that readily disrupts TNTs.11
Figure 1.
EP bridges. (A) Type I EP bridge connects human bronchial EPs and spans 757 µm over underlying ECs in co-culture. (B) An x-z section from confocal 3D reconstruction shows an EP bridge hovering above the substratum and connecting two EP islands of human bronchial EPs.16 (C and D) Type I EP bridge in a monoculture of human mammary EPs connects two cells (nuclei stained blue) and is composed of microtubules (green) and F-actin (red). (E) Type II EP bridge spans over underlying ECs and connects two EP islands of human bronchial EPs in co-culture. Cells (nuclei stained blue) are observed in the EP bridge composed of both microtubules (green) and F-actin (red). Scale bars: (A, B and E), 50 µm; (C and D, A1-A3, E1-E3), 10 µm.
One of the more intriguing aspects of EP bridges is the functional dichotomy between bridge types. Similar in function to TNTs, type I EP bridges facilitate transfer of cellular components between cells. This comparison is based on the seamless transition of lysosomes between cells connected by type I EP bridges and the presence of Golgi complexes within vesicle-like structures contained in these cellular connections.16 Time-lapse microscopy and immunofluorescent studies of type II EP bridges, in contrast, show the mediation of an entirely new form of cell migration where bridges act as tubular conduits for cells to move from one multi-cellular EP island to another. The architecture of type II EP bridges varies in complexity ranging from thin conduits facilitating migration of individual cells to wide conduits facilitating migration of multiple cells. Distinct borders made by the transmembrane protein E-cadherin expressed between type II bridges suggest these complex tubes are derived from multiple cells. Detailed characterization is still required, but the lumenal openings allowing cells to migrate in and out of these conduits appear to vary from being on the surface or embedded within multi-cellular EP islands.
The transfer of cellular components in type I EP bridges may be mediated by similar transport mechanisms employed by TNTs: small cytoplasmic molecules and some membrane components appear to be transported by passive diffusion, while organelles seem to be transferred in a unidirectional manner via F-actin-dependent or an actin-myosin-dependent transport system.11 Since EP bridges contain F-actin and microtubules, organelle transport in type I EP bridges may be more similar to microtubule-containing TNTs in macrophages where transport is bi-directional and microtubule-dependent.21 In the case of type II EP bridges, cell migration may be a combined effort of the cytoskeletal networks of both the EP bridge and the migrating cell. Neither EP bridges nor TNTs have been shown to transport genetic material or entire nuclei alone between animal cells, yet the possibility does exist especially since plasmodesmata transfer nucleic acids and transcription factors between plant cells.8,10 Overall, the elucidation of active and passive mechanisms involved in the dynamics of material transport and cell migration in EP bridges require detailed investigation.
Cellular and Biochemical Roles in EP Bridge Formation
Both EP bridges and TNTs form through similar cellular processes. One mechanism involves a filopodia-like extension that projects from one cell and attaches to a neighboring cell. Once the connection is made the cellular extension is remodeled into an intracellular tube. A second process giving rise to both EP bridges and TNTs arises as cells initially in contact with one another migrate apart while maintaining direct connectivity. The tubes elongate as the cells move farther apart. For these cellular connections actin polymerization is likely an important aspect in both mechanisms of formation, propelling extension of filopodialike structures and stabilizing elongation of cellular bridges.11,14,16 Along with F-actin, microtubules are also present in filopodialike structures involved in EP bridge formation. These EP bridge precursors contain lysosomes and/or nuclei, which raises the possibility these structures are initiating intracellular transport or cell migration even before EP bridge formation is complete. Two processes also seem to be involved in EP bridge deconstruction: first, a bridge can break from one end of the connection and completely retract to the remaining connected cell; second, a bridge can progressively become shorter as connected cells or EP islands moved toward one another and eventually fuse. The fate of EP bridges and TNTs is likely a multi-faceted process involving cell-to-cell interactions, intercellular communication and extracellular signaling.
The formation of lumens within EP bridge types remains undefined. Lumens in type I EP bridges may form in a similar manner to lumens in TNTs: a filopodia-like extension from one cell protrudes the membrane of another cell via supplementation of membrane components and actin remodeling. This process was recently shown to be induced by the protein M-Sec interacting with the octameric protein exocyst and the small GTPase RalA.23,24 However, M-Sec induces F-actin-only TNT formation suggesting TNTs and EP bridges with microtubules may require unknown factors in combination or apart from M-Sec to form.
Compared to TNTs and type I EP bridges, type II EP bridge lumen formation likely requires more extensive cellular and molecular interactions. Possible mechanisms include tube morphogenesis,25 as occurs in capillaries in vertebrates—a process termed cell hollowing—where an intracellular lumen forms within the cell cytoplasm and opens to the external environment.26 Similarly, tube morphogenesis observed in Drosophila heart27 and Madin-Darby canine kidney cultured cells28 can occur via cord hollowing where de novo lumen formation between cells is conducted by the assemblage of multiple cells into a thin cylindrical cord. Determining if specialized organelles, called vacuolar apical compartments that aid in the above processes of tube morphogenesis,25,29 are involved in bridge formation will be of interest in understanding whether type II EP bridge lumens form via tube morphogenesis or unknown processes. The wide range of diameters measured in EP filopodia-like extensions may reflect distinct cellular protrusions for each EP bridge type and/ or morphological transitioning of type I to type II bridges.16
Beyond the involvement of M-Sec, the underlying molecular and biochemical mechanisms propelling TNT formation remain mostly unknown, giving little point of reference for identifying cellular signals and pathways driving EP bridge formation. Interestingly, significantly more EP bridges form in monocultures of EPs versus co-cultures (EPs cultured with ECs or FBs), but the number of type II EP bridges in co-cultures is much greater compared to monocultures.16 These disparate characteristics suggest cellular and biochemical interactions between cell types in co-cultures regulate EP bridges in a manner not observed when EPs are cultured alone, presenting a foundation to compare potential modulators of bridge formation.
Indeed when compared to EP monocultures, levels of inflammatory molecules—interleukin (IL)-6, IL-8 and prostaglandin (PG) E2—are increased in co-cultures. An important transcription factor involved in the regulation of these and other inflammatory and cell migratory gene products is nuclear factor (NF)κB.30,31 When NFκB is pharmacologically inhibited in co-cultures, a ∼2–3 fold increase in the number of both EP bridge types results. Indeed, an inverse correlation was discovered in co-cultures of EPs/ECs and EPs/FBs, where higher levels of PGE2 resulted in lower numbers of EP bridges in the former. Reducing PGE2 levels in co-cultures via pharmacologically blocking cyclooxygenase (COX) activity doubled EP bridge formation.16 Based on these findings, inflammatory molecules appear to downregulate the formation of EP bridges.
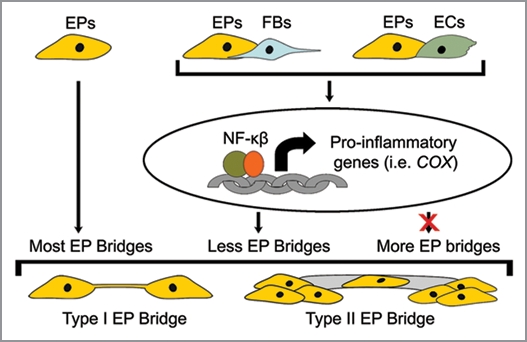
Other factors found to be involved in EP bridge regulation are reactive oxygen species (ROS), which are small molecules derived from molecular oxygen occurring as byproducts of biological reactions with mitochondria, peroxisomes, and other cellular elements. ROS interact with the NFκB and COX pathways, and modulate various cellular functions including inflammation, cell growth and motility.32–34 In one study, exposure to the ROS molecule hydrogen peroxide (H2O2) induced actin polymerization in primary cultures of rat astrocytes resulting in TNT-like connections to form.35 In co-cultures of EPs/ECs and EPs/FBs, exogenous H2O2 increased EP bridge formation ∼2 fold; however, blocking ROS with antioxidants in control co-cultures did not affect the number of EP bridges. These findings imply ROS can but the number of type II EP bridges in co-cultures is much greater compared affect the formation of EP bridges, but under control conditions ROS levels do not play a role. Interestingly though, antioxidants block the increases in EP bridge formation observed in NFκB or COX inhibited co-cultures of EPs/FBs, but not in EPs/ECs.16 These results suggest inflammatory pathway blockade increases ROS levels in EPs/FBs, which in turn induces EP bridge formation. The mediators regulating EP bridge increases in EPs/ECs after NFκB or COX inhibition remain unclear. In support of these findings, NFκB deficiency caused elevation of H2O2 in mouse embryo fibroblast cells and a human EP cell line resulting in morphological and mobility changes including extended cellular protrusions.36,37 Overall, EP bridge biogenesis appears to be dependent on cellular and biochemical interactions, in part through the regulation of inflammatory pathways (Fig. 2). While abrogation of inflammatory mediators induces EP bridge biogenesis via increased morphological and mobility changes. This emerging model supports the formation of EP bridges as a natural feature of EP biology, at least in vitro, which is impeded by inflammation.
Figure 2.
Regulation of EP bridge formation. Monocultures of human EPs produce the highest total number of EP bridges. Morphological and biochemical interactions between EPs and ECs or FBs in co-cultures upregulate the NFκB pathway resulting in increases of inflammatory gene protein products, including COX gene protein products (i.e., PGE2), which impede EP bridge formation. Pharmacological inhibition of these inflammatory pathways in co-cultures causes increased formation of both type I and II EP bridges. More detail provided in ref. 16.
Are EP Bridges Physiologically Relevant?
Cell-cell communication via cellular extensions is crucial for basic physiological processes including embryogenesis, tumorigenesis and the immune response.11,38 It cannot yet be determined if EP bridges exist only in vitro or play functional roles in vivo. This same question was raised when TNTs were first discovered in culture.17 TNTs have since been found in vivo in mouse cornea15 and the breadth of cell types connected by TNTs and the range of cellular cargos transported by these intracellular channels suggest these structures are a general principle of cellular signaling and may participate in processes ranging from development to cancer.13,14 The ability of pathogens, such as prions39 and retroviruses,40–43 to utilize TNTs to spread between cells—just as viral pathogens utilize plasmodesmata to spread within plants8,44—only further strengthens the potential relevance of TNTs in animals.
The functional similarities of type I EP bridges with TNTs in general and the structural similarities with TNTs containing both F-actin and microtubules provide a foundation for possible physiological relevance. Type I EP bridges may even be part of a subclass of TNTs composed of both types of cytoskeletal components. Type II EP bridges may be distinctively novel structures that present intriguing possibilities if in vivo counterparts exist. In embryogenesis, stem or progenitor cells may migrate over more differentiated cells through these tubular channels to initiate development in new areas of the embryo. While tumor cells could use these bridges to spread within a tissue or even initiate metastasis. As both EP bridge types have been identified in cells of the bronchial epithelium, these structures may be involved in inflammatory airway diseases, such as asthma and chronic obstructive pulmonary disorder, where the epithelium undergoes extensive remodeling due in part to the upregulation of various inflammatory molecules.30,45 Since inflammation blocks EP bridge formation, the reduction of intracellular signaling bridges and/or migratory bridges for healthy cells may play a role in the progression of these diseases. If physiologically relevant channels analogous to EP bridges are indeed found in tissues these structures may provide potential targets for disease therapies.
Conclusions and Perspectives
The addition of EP bridges to the growing variety of cell-to-cell conduits brings exciting new routes for exploration in this emerging field of cellular communication. Unfortunately the plethora of cellular extensions being discovered have brought about an amalgam of names for these structures—TNTs, TNT-like structures, EP bridges, filopodia bridges, nanowires and cellular or membrane nanotubes—making previous calls for a proper classification system even more important now.13 Once a proper system is in place, type I EP bridges may eventually be grouped with TNTs composed of both F-actin and microtubules that mediate intracellular signaling, while type II EP bridges may provide the foundation for a new class of tubular conduits facilitating cell migration. At the moment though, the longer lengths both EP bridge types are able to span compared to other known cellular channels provide EP bridges the potential for directly affecting a greater area of the surrounding biological environment. EP bridge traits including processes involved in initiating bridge biogenesis, lumen formation, the variety of cellular cargo transported between cells, the cell types transported between cell masses, and the underlying mechanisms of cargo transport and cell migration through these structures will be key areas to elucidate. Most important, however, will be determining the existence of EP bridges in vivo. Although, if these cellular conduits do not exist within the body, the presence of EP bridges in primary cultures of normal human cells, at the very least, provides the imagination with scientific and philosophical routes to explore given that cells in all human beings have an inherent plasticity to form these fascinating structures.
Acknowledgements
E.R.E. is supported in part by a grant from the National Institutes of Health (R01 GM 49039).
Abbreviations
- EP
bridges, epithelial bridges
- TNTs
tunneling nanotubes
- ROS
reactive oxygen species
- EPs
epithelial cells
- ECs
human aortic endothelial cells
- FBs
human lung fibroblasts
- IL
interleukin
- PGE2
prostaglandin E2
- H2O2
hydrogen peroxide
- COX
cyclooxygenase
- NFκB
nuclear factor-κB
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/11659
References
- 1.Sherer NM, Mothes W. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends Cell Biol. 2008;18:414–420. doi: 10.1016/j.tcb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramirez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- 3.Herve JC, Bourmeyster N, Sarrouilhe D, Duffy HS. Gap junctional complexes: from partners to functions. Prog Biophys Mol Biol. 2007;94:29–65. doi: 10.1016/j.pbiomolbio.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Baluška F, Volkmann D, Barlow PW. Cell-cell channels and their implications for cell theory. In: Baluška F, Volkmann D, Barlow PW, editors. Cell-Cell Channels. Berlin-Heidelberg-New York: Landes Bioscience—Springer Verlag.; 2006. pp. 1–18. [Google Scholar]
- 5.Baluška F, Hlavacka A, Volkmann D, Menzel D. Getting connected: actin-based cell-to-cell channels in plants and animals. Trends Cell Biol. 2004;14:404–408. doi: 10.1016/j.tcb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Baluška F, Volkmann D, Barlow PW. Cell bodies in a cage. Nature. 2004;428:371. doi: 10.1038/428371a. [DOI] [PubMed] [Google Scholar]
- 7.Baluška F. Cell-cell channels, viruses and evolution: via infection, parasitism and symbiosis toward higher levels of biological complexity. Ann N Y Acad Sci. 2009;1178:106–119. doi: 10.1111/j.1749-6632.2009.04995.x. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Medrano R, Xoconostle-Cazares B, Kragler F. The plasmodesmatal transport pathway for homeotic proteins, silencing signals and viruses. Curr Opin Plant Biol. 2004;7:641–650. doi: 10.1016/j.pbi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Carr DJ. Historical perspectives on plasmodesmata. In: Gunning BES, Robards AW, editors. Intercellular Communication in Plants: Studies on Plasmodesmata. Springer-Verlag; 1976. pp. 291–295. [Google Scholar]
- 10.Lucas WJ, Ham BK, Kim JY. Plasmodesmata-bridging the gap between neighboring plant cells. Trends Cell Biol. 2009;19:495–503. doi: 10.1016/j.tcb.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Gurke S, Barroso JF, Gerdes HH. The art of cellular communication: tunneling nanotubes bridge the divide. Histochem Cell Biol. 2008;129:539–550. doi: 10.1007/s00418-008-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rustom A. Hen or egg? Some thoughts on tunneling nanotubes. Ann N Y Acad Sci. 2009;1178:129–136. doi: 10.1111/j.1749-6632.2009.04997.x. [DOI] [PubMed] [Google Scholar]
- 13.Gerdes HH, Carvalho RN. Intercellular transfer mediated by tunneling nanotubes. Curr Opin Cell Biol. 2008;20:470–475. doi: 10.1016/j.ceb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Gerdes HH, Bukoreshtliev NV, Barroso JF. Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett. 2007;581:2194–2201. doi: 10.1016/j.febslet.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 15.Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: Membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180:5779–5783. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zani BG, Indolfi L, Edelman ER. Tubular bridges for bronchial epithelial cell migration and communication. PLoS One. 2010;5:8930. doi: 10.1371/journal.pone.0008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 18.Hodneland E, Lundervold A, Gurke S, Tai XC, Rustom A, Gerdes HH. Automated detection of tunneling nanotubes in 3D images. Cytometry A. 2006;69:961–972. doi: 10.1002/cyto.a.20302. [DOI] [PubMed] [Google Scholar]
- 19.Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: Membrane nanotubes connect immune cells. J Immunol. 2004;173:1511–1513. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- 20.Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–1041. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 21.Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 22.Vidulescu C, Clejan S, O'Connor KC. Vesicle traffic through intercellular bridges in DU 145 human prostate cancer cells. J Cell Mol Med. 2004;8:388–396. doi: 10.1111/j.1582-4934.2004.tb00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, Kitamura H, et al. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat Cell Biol. 2009;11:1427–1432. doi: 10.1038/ncb1990. [DOI] [PubMed] [Google Scholar]
- 24.Ohno H, Hase K, Kimura S. M-Sec. Commun Integr Biol. 2010;3:1–3. doi: 10.4161/cib.3.3.11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- 26.Wolff JR, Moritz A, Guldner FH. ‘Seamless’ endothelia within fenestrated capillaries of duodenal villi (rat) Angiologica. 1972;9:11–14. doi: 10.1159/000157910. [DOI] [PubMed] [Google Scholar]
- 27.Rugendorff A, Younossi-Hartenstein A, Hartenstein V. Embryonic origin and differentiation of the Drosophila heart. Roux's Arch Dev Biol. 1994;203:266–280. doi: 10.1007/BF00360522. [DOI] [PubMed] [Google Scholar]
- 28.Pollack AL, Runyan RB, Mostov KE. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factorinduced tubulogenesis. Dev Biol. 1998;204:64–79. doi: 10.1006/dbio.1998.9091. [DOI] [PubMed] [Google Scholar]
- 29.Chung S, Andrew DJ. The formation of epithelial tubes. J Cell Sci. 2008;121:3501–3504. doi: 10.1242/jcs.037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards MR, Bartlett NW, Clarke D, Birrell M, Belvisi M, Johnston SL. Targeting the NFkappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol Ther. 2009;121:1–13. doi: 10.1016/j.pharmthera.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newton R, Holden NS, Catley MC, Oyelusi W, Leigh R, Proud D, et al. Repression of inflammatory gene expression in human pulmonary epithelial cells by small-molecule IkappaB kinase inhibitors. J Pharmacol Exp Ther. 2007;321:734–742. doi: 10.1124/jpet.106.118125. [DOI] [PubMed] [Google Scholar]
- 32.Bedard K, Krause KH. The NOX family of ROSgenerating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Wahl LM. Oxidative stress augments the production of matrix metalloproteinase-1, cyclooxygenase-2, and prostaglandin E2 through enhancement of NFkappaB activity in lipopolysaccharideactivated human primary monocytes. J Immunol. 2005;175:5423–5429. doi: 10.4049/jimmunol.175.8.5423. [DOI] [PubMed] [Google Scholar]
- 34.Jaspers I, Zhang W, Fraser A, Samet JM, Reed W. Hydrogen peroxide has opposing effects on IKK activity and IkappaBalpha breakdown in airway epithelial cells. Am J Respir Cell Mol Biol. 2001;24:769–777. doi: 10.1165/ajrcmb.24.6.4344. [DOI] [PubMed] [Google Scholar]
- 35.Zhu D, Tan KS, Zhang X, Sun AY, Sun GY, Lee JC. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J Cell Sci. 2005;118:3695–3703. doi: 10.1242/jcs.02507. [DOI] [PubMed] [Google Scholar]
- 36.Chen F, Lu Y, Castranova V, Li Z, Karin M. Loss of Ikkbeta promotes migration and proliferation of mouse embryo fibroblast cells. J Biol Chem. 2006;281:37142–37149. doi: 10.1074/jbc.M603631200. [DOI] [PubMed] [Google Scholar]
- 37.Chen F, Castranova V, Li Z, Karin M, Shi X. Inhibitor of nuclear factor kappaB kinase deficiency enhances oxidative stress and prolongs c-Jun NH2-terminal kinase activation induced by arsenic. Cancer Res. 2003;63:7689–7693. [PubMed] [Google Scholar]
- 38.Onfelt B, Purbhoo MA, Nedvetzki S, Sowinski S, Davis DM. Long-distance calls between cells connected by tunneling nanotubules. Sci STKE. 2005;2005:55. doi: 10.1126/stke.3132005pe55. [DOI] [PubMed] [Google Scholar]
- 39.Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–336. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 40.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 41.Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat Cell Biol. 2007;9:310–315. doi: 10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT): A potential mechanism for intercellular HIV trafficking. Commun Integr Biol. 2009;2:243–244. doi: 10.4161/cib.2.3.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell Immunol. 2009;254:142–148. doi: 10.1016/j.cellimm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oparka KJ. Getting the message across: how do plant cells exchange macromolecular complexes? Trends Plant Sci. 2004;9:33–41. doi: 10.1016/j.tplants.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8:432–446. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]