Abstract
During phagocytosis, neutrophils kill microorganisms by delivering antimicrobial substances to the phagosome. For this, the intracellular targeting and fusion of granules must be strictly regulated and a dependence on the cytosolic concentration of free calcium has been suggested. New evidence show that different mechanisms regulate early and late stages of Fc receptor-mediated phagocytosis. The early fusion events are dependent on calcium but this is not the case for the fusion of azurophilic granules with phagosomes at later stages. Certain pathogens target the granule-phagosome fusion machinery in order to survive intracellularly; a deeper understanding of intracellular membrane traffic processes could allow new approaches for the eradication of pathogens that are harbored inside the cells of our immune system.
Key words: calcium, neutrophil, phagocytosis, phagosome, membrane traffic, azurophilic granules, membrane fusion
The neutrophil is our first line of defense against microorganisms and has an arsenal of cytoplasmic granules at its disposal for this task. Of these, the azurophilic and specific granules contain most of the antimicrobial potency of the neutrophils.1 It is vital for the host to control when and where to release toxic substances, and there exist rigid control mechanisms for this process.2 Phagocytosis is a process where the neutrophil engulfs and internalizes a target and places it in a membrane-bound organelle, termed the phagosome. Having confined its prey in a closed environment, and by fusing the granules with the phagosome, the neutrophil effectively and precisely transforms the intraphagosomal milieu to an extremely inhospitable place. Fusion of granules both with the surface of the cell and with phagosomes have been thought to be calcium dependent.3 With new techniques, we have now re-evaluated this, at least concerning the fusion of azurophilic granules with neutrophil phagosomes.4
We used zymosan particles that are large enough to be easily analyzed with fluorescence microscopy, and Streptococcus pyogenes bacteria, for which we employed a newly developed method for isolating phagosomes. We attached nanometerscale magnetic particles to the surface of the bacteria, allowing magnetic retrieval of bacteria-containing phagosomes.5 The delivery of azurophilic granules to early but not late phagosomes was shown to be calcium dependent. Perhaps also other intracellular fusion events in the neutrophil are calcium-independent.
Our data were obtained using immunoglobulin G (IgG)-opsonized prey, which upon binding activates Fc receptors at the surface of neutrophils. This triggers cytoskeletal re-arrangements necessary for target internalization and formation of a phagosome. Signaling is initiated by phosphorylation of tyrosine residues on the cytoplasmic side of the receptor. Downstream events involve the accumulation of a variety of proteins and lipids on the phagosome, and a release of calcium from intracellular stores (reviewed in ref. 6).
The release of granule contents both to the extracellular environment and to phagosomes is strictly regulated. For instance, the fusion of the different cytoplasmic granules with the plasma membrane have discrete calcium dependencies.7 However, the premises for granule-phagosome fusion are very different from granule delivery to the surface. From the point of view of the approaching granule, the curvature of the receiving membrane (plasma membrane versus phagosomal membrane) is reversed. Since membrane curvature is important for membrane fusion (reviewed in ref. 8) this is likely to affect the requirements for fusion. Also important, the composition of the phagosome progressively changes due to the trafficking of granules and vesicles; this is described as phagosome maturation. This process has been studied in detail in macrophages9 but is not as well characterized in neutrophils. A speedy and efficient delivery of antimicrobial measures to the phagosome is essential to kill pathogens. As this profoundly transforms the phago- somal membrane it will most likely also alter the requirements for fusion.
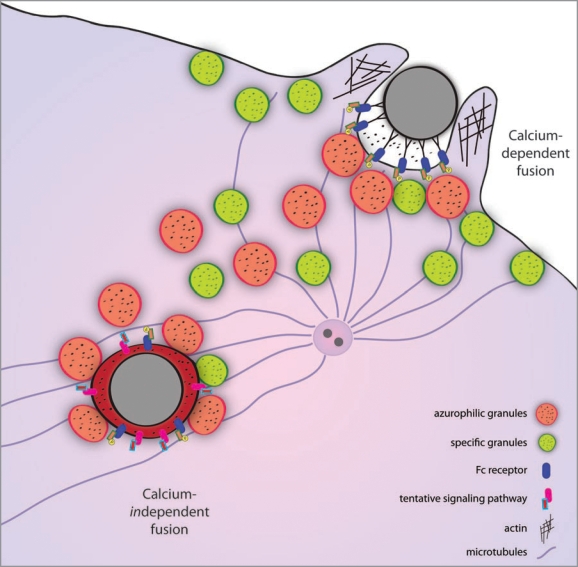
Recently, it has been questioned whether calcium is essential for all types of intracellular fusion processes (reviewed in ref. 10). During phagocytosis, the specific granules have a relatively low calcium threshold for extracellular release and are, besides to phagosomes, also targeted in a diffuse manner to the plasma membrane.11 In contrast, there is a highly localized delivery of azurophilic granules to parts of the plasma membrane and to phagosomes, a process presumably guided by microtubules. It is possible that calcium could regulate targeting and fusion of the different granule types in the neutrophil. However, other signaling events that are only indirectly linked to effects on cytosol calcium could be the regulating signals. Importantly, for late phagosome maturation, the finding of calcium independence of azurophilic granule-phagosome fusion shows that there must exist other signaling pathways that relay signals from the phagosomal lumen to the cytosol (see Fig. 1).
Figure 1.
The role of calcium in neutrophil granule-phagosome fusion. During Fc-receptor-mediated phagocytosis, tyrosine residues on the receptors are phosphorylated and trigger a signaling cascade. Granules are delivered and fuse with the plasma membrane in a calcium-dependent manner already before the phagosome is sealed. The trafficking of azurophilic granules is guided by microtubules, originating from the microtubule-organizing center that re-locates to the site of phagocytosis. Once the phagosome is formed, delivery and fusion of granules continue by unknown calciumindependent mechanisms.
An increase of intracellular free calcium is normally observed immediately after initiation of phagocytosis in neutrophils.12 In a single cell eating a single prey, a spike in calcium is seen followed by a return to base level. Similarly, using synchronized phagocytosis in a whole population of neutrophils, we could only see a single calcium peak. If there is a requirement for an elevated calcium concentration in continuous intracellular fusion, a non-transient calcium peak would be expected instead. It is conceivable that the initial calcium spike could set off a signaling pathway that irreversibly leads to intracellular fusion at a later stage. However, when looking at calcium-depleted neutrophils it is only the early delivery phase that is inhibited; fusion of azurophilic granules with fully internalized phagosomes still occurs. Taken together, our data indicate that an elevated intracellular calcium concentration is not necessary for late phagosome maturation. This process involves fusion with azurophilic as well as with specific granules. However, the calcium dependence of specific granule delivery needs to be verified with experiments that specifically address the intracellular localization and fusion properties of specific granules during Fc-mediated phagocytosis.
Much of what is known about neutrophil phagocytosis has been derived from experiments with macrophages.13 Important in the present context is that intracellular lysosome-phagosome fusion has been demonstrated to be calcium-independent in macrophages. Interestingly, calcium spikes are observed during Fc-receptor mediated phagocytosis but they seem not to be directly related to internalization and microbial killing.14 In the eosinophil, actin polymerization has been shown to be calcium-dependent,15 which is essential for internalization of prey during phagocytosis. The opposite is observed in neutrophils, where such processes are calcium-independent.16 Apparently, regarding calcium-dependence, these fundamental pathways for phagocytosis can be regulated differently in related cell types.
Different mechanisms seem to govern the early delivery of granule content to the plasma membrane vis-à-vis intracellular granule-phagosome fusion. Careful identification and characterization of the mechanisms regulating intracellular fusion is necessary to develop new means to fight pathogens; manipulation of phagosome trafficking is a prime target for pathogens seeking an intracellular refuge where they cannot be reached by our immune system.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/11168
References
- 1.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 3.Jaconi ME, Lew DP, Carpentier JL, Magnusson KE, Sjögren M, Stendahl O. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J Cell Biol. 1990;110:1555–1564. doi: 10.1083/jcb.110.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordenfelt P, Winberg M, Lönnbro P, Rasmusson B, Tapper H. Different requirements for early and late phases of azurophilic granule-phagosome fusion. Traffic. 2009;10:1881–1893. doi: 10.1111/j.1600-0854.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 5.Lönnbro P, Nordenfelt P, Tapper H. Isolation of bacteria-containing phagosomes by magnetic selection. BMC Cell Biol. 2008;9:35. doi: 10.1186/1471-2121-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9:639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lew PD, Monod A, Waldvogel FA, Dewald B, Baggiolini M, Pozzan T. Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 1986;102:2197–2204. doi: 10.1083/jcb.102.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epand RM. Membrane fusion. Biosci Rep. 2000;20:435–441. doi: 10.1023/a:1010498618600. [DOI] [PubMed] [Google Scholar]
- 9.Vieira O, Botelho R, Grinstein S. Phagosome maturation: aging gracefully. Biochem J. 2002. p. 16. [DOI] [PMC free article] [PubMed]
- 10.Hay JC. Calcium: a fundamental regulator of intracellular membrane fusion? EMBO Rep. 2007;8:236–240. doi: 10.1038/sj.embor.7400921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapper H, Furuya W, Grinstein S. Localized exocytosis of primary (lysosomal) granules during phagocytosis: role of Ca2+-dependent tyrosine phosphorylation and microtubules. J Immunol. 2002;168:5287–5296. doi: 10.4049/jimmunol.168.10.5287. [DOI] [PubMed] [Google Scholar]
- 12.Sawyer DW, Sullivan JA, Mandell GL. Intracellular free calcium localization in neutrophils during phagocytosis. Science. 1985;230:663–666. doi: 10.1126/science.4048951. [DOI] [PubMed] [Google Scholar]
- 13.Lee WL, Harrison RE, Grinstein S. Phagocytosis by neutrophils. Microbes Infect. 2003;5:1299–1306. doi: 10.1016/j.micinf.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Myers JT, Swanson JA. Calcium spikes in activated macrophages during Fcgamma receptor-mediated phagocytosis. J Leuk Biol. 2002;72:677–684. [PubMed] [Google Scholar]
- 15.Elsner J, Dichmann S, Dobos GJ, Kapp A. Actin polymerization in human eosinophils, unlike human neutrophils, depends on intracellular calcium mobilization. J Cell Physiol. 1996;167:548–555. doi: 10.1002/(SICI)1097-4652(199606)167:3<548::AID-JCP18>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Downey GP, Chan CK, Trudel S, Grinstein S. Actin assembly in electropermeabilized neutrophils: role of intracellular calcium. J Cell Biol. 1990;110:1975–1982. doi: 10.1083/jcb.110.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]