Abstract
Interleukin-1-Receptor Accessory Protein Like 1 (IL1RAPL1) gene mutations are associated to cognitive impairment ranging from non-syndromic X-linked mental retardation to autism. Functionally IL1RAPL1 belongs to a novel family of Toll/IL-1 Receptors, but its ligand is unknown. In a recent study, we have shown that IL1RAPL1 is present in dendritic spine where it interacts with PSD-95, a major scaffold protein of excitatory post-synaptic density. We demonstrated that IL1RAPL1 regulates the synaptic localization of PSD-95 by controlling JNK (c-Jun terminal Kinase) activity and PSD-95 phosphorylation. Loss of IL1RAPL1 in mouse not only led to a reduction of excitatory synapses but also to specific deficits in hippocampal long-term synaptic plasticity. Here we report that activation of JNK pathway in neurons by Interleukin-1 (IL-1) is mediated by IL1RAPL1. The interaction of IL1RAPL1 with PSD-95 discloses a novel pathophysiological mechanism underlying cognitive impairment associated with alterations of the JNK pathway in response to IL-1 and leading to the mislocalization of PSD-95, that subsequently result in abnormal synaptic organization and function.
Key words: IL1RAPL1, PSD-95, JNK, IL-1 signaling
Mental retardation (MR) is defined by an overall “intelligent quotient” lower than 70, associated with adaptative and behavior problems. Over the past years, about 60 X-linked genes involved in MR disorders have been identified,1 but only few of them give rise to products with well characterized neuronal function.2,3 Mutations in the Interleukin-1-Receptor Accessory Protein Like 1 (IL1RAPL1) gene have been associated with non syndromic forms of MR4 and more recently with autism.5 IL1RAPL1 belongs to the Toll/ IL-1 Receptor family and, as other member of this family, is characterized by three extracellular Ig-like domains, a transmembrane segment, an intracellular TIR domain, plus unlike the others 150 additional amino-acids (aa) at the C-terminal end.4 Through these last aa, IL1RAPL1 interacts with the neuronal calcium sensor NCS-1.6 In neuroendocrine PC12 cells, this interaction between IL1RAPL1 and NCS-1 mediates the regulatory effect of IL1RAPL1 on N-type VDCC activity.7
In our recent study, we found a novel partner of IL1RAPL1, PSD-95, a major scaffold protein of excitatory synapses and showed that IL1RAPL1 regulates dendritic spine number and PSD-95 localization to synapses.8 In vivo, we found that IL1RAPL1 loss of function led to a slight but significant reduction of synapse number in the CA1 region of hippocampus. At the physiological level, IL1RAPL1 deficit led to an altered theta-burst induced long-term potentiation (LTP) at the Schaffer collateral (SC)-CA1 synapses.
It has been previously shown that overexpression of IL1RAPL1 in non-neuronal cells activates the JNK pathway but not the other pathways classically involved in IL-1 signaling9,10 (and personal data). In neurons, the balance between JNK and PP1/PP2A activities modulates the phosphorylation level of PSD-95 on Ser-295 and regulates its synaptic localization.11 These findings led us to test whether the IL1RAPL1 deficit or overexpression were associated with an unbalance between JNK and PP1/PP2A phosphatases activity. In IL1RAPL1 KO neurons, we found a deficit in JNK activity and consequently in phosphorylation level of Ser-295 of PSD-95 leading to a reduction of PSD-95 localization at the synapse.8 Intriguingly, the deficit in JNK activity seems to be independent of neuronal network activity, suggesting that another signal is involved in the activation of the IL1RAPL1/JNK/ PSD-95 pathway.
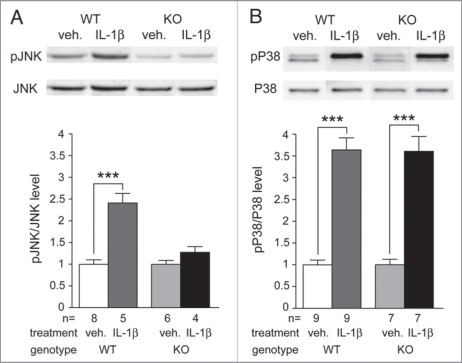
Knowing that IL1RAPL1 is a member of the IL-1 Receptor family and that IL1RAPL1 KO mice present a deficit in neuronal JNK signaling, a pathway activated by IL-1, we tested if this interleukin is involved in the activation of the newly highlighted neuronal IL1RAPL1/JNK/ PSD-95 pathway. To this end, we stimulated cortical differentiated IL1RAPL1 KO or WT neurons with IL-1β or vehicle 10 min prior to protein phosphorylation analysis (Fig. 1). In WT neurons, IL-1β treatment induces about a two fold increase of JNK activity (pJNK/JNK level normalized to vehicle: WTveh = 1.00 ± 0.10, WTIL1β = 2.41 ± 0.22, p < 0.001, t-test) whereas in IL1RAPL1 KO neurons no such effect is observed (pJNK/JNK level normalized to vehicle: KOveh = 1.00 ± 0.09, KOIL1β = 1.28 ± 0.13, p = 0.10, t-test) (Fig. 1A). In order to test the specificity of the deficit in IL-1β effect on JNK pathway, we measured the phosphorylation level of P38, another kinase classically activated by IL-1β. As shown in Figure 1B, in WT neurons, IL-1β stimulation increases the level of P38 phosphorylation (pP38/P38 level normalized to vehicle: WTveh = 1.00 ± 0.11, WTIL1β = 3.64 ± 0.28, p < 0.001, t-test). In IL1RAPL1 KO neurons, we also observed a similar increase in P38 activity (pP38/P38 level normalized to vehicle: KOveh = 1.00 ± 0.12, KOIL1β = 3.61 ± 0.34, p < 0.001, t-test). Thus, these results indicate that IL1RAPL1 is required specifically for IL-1β mediated activation of JNK in neuronal cells.
Figure 1.
IL-1 response is impaired in IL1RAPL1 KO neurons. Mature neurons in culture were treated with IL1β (concentration: 0.01 µg/µL) or with vehicle (veh.) (saline, 0.1% BSA) during 10 min before protein extraction. Protein samples were processed as described in Pavlowsky et al.8 The upper panel shows representative immune-blot of JNK (A) or P38 (B) phosphorylation level in each condition for both genotypes. The lower panel shows quantifications of JNK (A, left) and P38 (B, right) phosphorylation level. Bars show mean of pJNK/JNK or pP38/P38 level ± SEM normalized to vehicle condition. Two-tailed t-test, ***p < 0.001, the number of experiment is indicated as n.
For the first time, we report biological evidences indicating that in neurons IL1RAPL1 might be involved in IL-1 signal transduction in neurons. Moreover, our data strongly suggest that IL1RAPL1 could act as a receptor or a co-receptor of IL-1β, as its closed homolog IL1RacP. A previous works using overexpression approach in COS cells suggested that IL1RAPL1 or the combination of IL1RAPL1 and IL1RacP are not sufficient for IL-1β binding.6 Although binding and affinity of IL-1β to IL1RAPL1 alone or in combination with IL-1 receptor (IL-1R) were not tested in our current work, the apparent difference between the two studies might be likely related to the cell type used and the expression in neurons of specific receptor and/or accessory protein. Interestingly, a specific neuronal isoform of IL1RacP with 140 additional aa at the C-terminal end, IL1RacPb, involved in neuronal response to IL-1, has been recently described,12 though its neurophysiological functions and its implication in cognitive function were not investigated. The discovery of a link between IL-1 and IL1RAPL1, a synaptic protein involved in mental retardation is in agreement with the emerging function of IL-1β in neuronal physiology and not only in immunopathophysiological process. Indeed, at low concentration, i.e., physiological concentration, it has been shown that IL-1β is involved in hippocampal LTP maintenance.13 Moreover, both of the KO mouse model for the IL-1R and the transgenic mouse overexpressing the IL1-Receptor antagonist (IL-1Ra) in the central nervous system have deficit in theta-burst induced LTP in the CA1 area of the hippocampus as well as impairments in spatial memory.14,15 Interestingly, preliminary data of behavioral studies shows that IL1RAPL1 KO male mice show deficits in spatial memory (personal communication, Nosten-Bertrand M), suggesting that the three mouse models share some common features including deficit in synaptic function at the hippocampal SC-CA1 pathway and impairments in spatial memory. Altogether, these data highlight the relevance of IL-1/IL1RAPL1/JNK pathway in cognitive functions and provide a rationale for therapeutic developments targeting this pathway.
Acknowledgements
We like to thank M. Nosten-Bertrand for the preliminary data on IL1RAPL1 KO mice behaviors. M.G. was supported by Telethon Italy (grant GGP05236A) and Compagnia di San Paolo. P.B. was supported by Agence Nationale pour la Recherche (projects ANR-05-Neuro-040-01 and ANR-06-Neuro-003-02), Fondation Jerome Lejeune, and Inserm. C.S. was supported by grants from Telethon Italy (grant GGP06208), Fondazione Cariplo (project2006-0779), Compagnia di San Paolo (project 2005.1964), RSTL-CNR, and PRIN 2007.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/11414
References
- 1.Chelly J, Khelfaoui M, Francis F, Cherif B, Bienvenu T. Genetics and pathophysiology of mental retardation. Eur J Hum Genet. 2006;14:701–713. doi: 10.1038/sj.ejhg.5201595. [DOI] [PubMed] [Google Scholar]
- 2.Humeau Y, Gambino F, Chelly J, Vitale N. X-linked mental retardation: focus on synaptic function and plasticity. J Neurochem. 2009;109:1–14. doi: 10.1111/j.1471-4159.2009.05881.x. [DOI] [PubMed] [Google Scholar]
- 3.Vaillend C, Poirier R, Laroche S. Genes, plasticity and mental retardation. Behav Brain Res. 2008;192:88–105. doi: 10.1016/j.bbr.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Carrie A, Jun L, Bienvenu T, Vinet MC, McDonell N, Couvert P, et al. A new member of the IL-1 receptor family highly expressed in hippocampus and involved in X-linked mental retardation. Nat Genet. 1999;23:25–31. doi: 10.1038/12623. [DOI] [PubMed] [Google Scholar]
- 5.Piton A, Michaud J, Peng H, Aradhya S, Gauthier J, Mottron L, et al. Mutations in the calcium-related gene IL1RAPL1 are associated with autism. Human Molecular Genetics. 2008;17:3965–3974. doi: 10.1093/hmg/ddn300. [DOI] [PubMed] [Google Scholar]
- 6.Bahi N, Friocourt G, Carrie A, Graham ME, Weiss JL, Chafey P, et al. IL1 receptor accessory protein like, a protein involved in X-linked mental retardation, interacts with Neuronal Calcium Sensor-1 and regulates exocytosis. Hum Mol Genet. 2003;12:1415–1425. doi: 10.1093/hmg/ddg147. [DOI] [PubMed] [Google Scholar]
- 7.Gambino F, Pavlowsky A, Begle A, Dupont JL, Bahi N, Courjaret R, et al. IL 1-receptor accessory proteinlike 1 (IL1RAPL1), a protein involved in cognitive functions, regulates N-type Ca2+-channel and neurite elongation. 1 (IL1RAPL1), a protein involved in cognitive functions, regulates N-type Ca2+-channel and neurite elongation. Proc Natl Acad Sci USA. 2007;104:9063–9068. doi: 10.1073/pnas.0701133104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlowsky A, Gianfelice A, Pallotto M, Zanchi A, Vara H, Khelfaoui M, et al. A postsynaptic signaling pathway that may account for the cognitive defect due to IL1RAPL1 mutation. Curr Biol. 2010;20(2):103–115. doi: 10.1016/j.cub.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Born TL, Smith DE, Garka KE, Renshaw BR, Bertles JS, Sims JE. Identification and characterization of two members of a novel class of the interleukin-1 receptor (IL-1R) family. Delineation of a new class of IL-1R-related^proteins based on signaling. J Biol Chem. 2000;275:41528. [PubMed] [Google Scholar]
- 10.Khan JA, Brint EK, O’Neill LA, Tong L. Crystal structure of the Toll/interleukin-1 receptor domain of human IL-1RAPL. J Biol Chem. 2004;279:31664–31670. doi: 10.1074/jbc.M403434200. [DOI] [PubMed] [Google Scholar]
- 11.Kim MJ, Futai K, Jo J, Hayashi Y, Cho K, Sheng M. Synaptic accumulation of PSD-95 and synaptic function regulated by phosphorylation of serine-295 of PSD-95. Neuron. 2007;56:488–502. doi: 10.1016/j.neuron.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Smith DE, Lipsky BP, Russell C, Ketchem RR, Kirchner J, Hensley K, et al. A central nervous system-restricted isoform of the interleukin-1 receptor accessory protein modulates neuronal responses to interleukin-1. Immunity. 2009;30:817–831. doi: 10.1016/j.immuni.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross FM, Allan SM, Rothwell NJ, Verkhratsky A. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J Neuroimmunol. 2003;144:61–67. doi: 10.1016/j.jneuroim.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- 15.Goshen I, Avital A, Kreisel T, Licht T, Segal M, Yirmiya R. Environmental enrichment restores memory functioning in mice with impaired IL-1 signaling via reinstatement of long-term potentiation and spine size enlargement. J Neurosci. 2009;29:3395–3403. doi: 10.1523/JNEUROSCI.5352-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]