Abstract
The fungus-growing termites (Macrotermitinae) have developed an obligate mutualistic symbiosis with fungi (Termitomyces) and, in most cases, the symbiotic partner is collected from the environment upon establishment of a new colony (horizontal transmission). The requirement that partners are able to find and recognize each other after independent reproduction is likely to severely constrain long distance dispersal. In support of this hypothesis, we have recently shown that a single colonisation of Madagascar by fungus-growing termites has occurred. The successful colonizers belong to the genus Microtermes, known to inherit their symbiont from the parental colony (vertical transmission). However, the fungal symbionts of Madagascar were not monophyletic, as expected under strict vertical transmission. Here we further discuss these findings, and we suggest further bottlenecks to dispersion and propose a transient window for horizontal transmission for the otherwise vertically transmitted Termitomyces strains.
Key words: vertical transmission, symbiont transmission mode, long-distance dispersal, mutualism, microtermes, termitomyces
The fungus-growing termites (Macrotermitinae) started cultivating fungi (Termitomyces) for food provisioning about 30 mya.1 The fungus is used as fungal biomass and as an ‘external-rumen’ for plant degradation. The two partners are obligately dependent on each other, since free-living representatives of both insect and fungi have never been found.2–4 The symbiotic fungus grows on a special substrate, the fungus comb, maintained by the termites through continuous addition of pre-digested plant material while the older comb material is consumed.
In most species, the primordial fungus comb (during colony foundation) is inoculated with basidiospores of the right species of fungus, collected by the first foraging workers along with the first forage brought into the nest from the outside environment. 5 This transmission mode—horizontal transmission—has been demonstrated for species of the genera Ancistrotermes, Macrotermes, Pseudacanthotermes and Odontotermes6–9 and is likely to represent the ancestral symbiont transmission mode.2 The Termitomyces symbionts of these macrotermitine genera regularly produce fruiting bodies, so that spores are readily available in the environment. A consequence of this independent partner reproduction is that, at each generation, new combinations of termite and fungal lineages arise10 and obviously this requires that partners are able to find and recognize each other after independent reproduction.
Fungus-growing termites occur throughout the Old-World tropics, but originated in the African rainforest.1 Most of the diversity occurs in Africa, with all genera being present (except for Hypotermes), while only four genera are found in Asia. We have recently shown that a single colonisation has occurred to Madagascar, of termites belonging to the genus Microtermes.11 The species within this genus are believed to belong to one of the only two derived lineages of the Macrotermitinae that have independently evolved vertical transmission of Termitomyces,7,12 i.e., where symbionts are inherited from a parental colony and used to inoculate the fungus comb of the newly founded colonies. This vertical transmission mode has been found in all five studied Microtermes species, where it is uniparental via the female, and in a single species of the genus Macrotermes, M. bellicosus, where it is also uniparental but via the reproductive male. The recent results obtained for the colonisation of Madagascar by fungus-growing termites are compatible with the hypothesis that vertical transmission provides an advantage for long-distance colonisation. By travelling together, the risk of failure in establishment by not finding the right partner in the new environment is minimised.
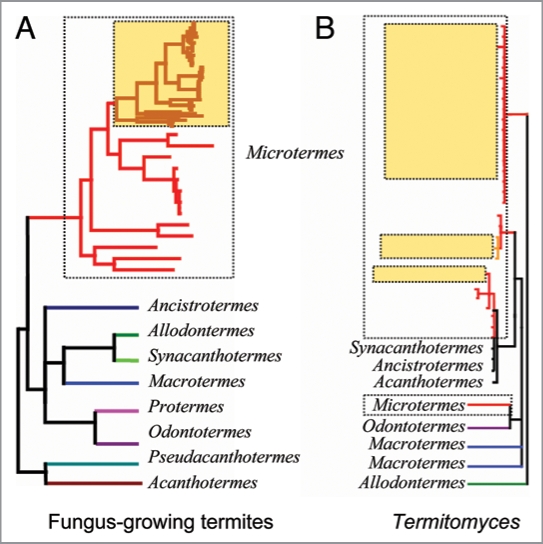
However, we inferred at least three independent colonisation events of Madagascar by the fungal symbionts of the Microtermes clade from Madagascar. This was unexpected: under strict vertical transmission of fungal symbionts, hostswitching would not occur. Therefore, occasional events of horizontal transmission have to be inferred to explain the phylogenetics pattern of the Malagasy fungus-growing termites (Fig. 1). Considering the symbionts’ point of view, selection favors dispersal out of the host and de novo association with new hosts—the Hamilton & May13 effect, which favors individuals to disperse away from close relatives and avoid competition with similar genotypes. Furthermore, at the level of the symbiotic unit, the presence of a certain level of horizontal transmission, associated with sexual reproduction is beneficial on the longer term, as there seems to be a critical level of horizontal transfers below which natural selection is unable to purge deleterious mutations, leading to an expected loss of fitness over time.14
Hence, symbiont vertical transmission seems to be advantageous for long distance dispersion, but might be detrimental in the long term. How (or when) is a new symbiont strain acquired? Within a colony, crop mixing does not occur as the fungal symbionts are always reared as single-strain monocultures regardless of the symbiont transmission mode.2,15–18 It has recently been shown that single-strain monocultures of Termitomyces within single nests are maintained through positive frequency-dependent selection.15 This mechanism thus prevents subsequent occupation of the comb by other fungal strains.
However, the pattern observed on the Malagasy Microtermes implies that the first colonisers arrived to the island and established the fungus comb with inoculum brought from the mainland colony and later host-switching has occurred by horizontal transmission. A transient window for horizontal transmission can be hypothesized for the otherwise vertically transmitted Termitomyces strains. Considering that at the initial stages of the colony formation the symbiotic fungus biomass is still rather low, it will be possible for a new, horizontally transmitted, symbiont strain to establish itself then.
Additional support for occasional horizontal transmission events of the otherwise vertically transmitted Microtermes symbionts is that some Microtermes species share a closely related symbiont with species of the genera Ancistrotermes and Synacanthotermes.2,11 If we compare the number of described species in both symbiotic partners—40 in Termitomyces19 and 330 in fungus-growing termites20—it seems logical to assume that many of these symbionts are shared between termite species. However, Termitomyces taxonomy is largely based on the morphology of sexual fruiting bodies, which are rare and possibly absent in some Termitomyces species, and molecular data indicate that there may also be many morphologically indistinguishable sibling species.21
Therefore, a better understanding of Termitomyces phylogeny is needed. Additionally, closely related Microtermes symbionts have also found between geographical regions,2,11 although a higher level of differentiation seems to have been observed between African and Asiatic Termitomyces.21 It has been shown that fungal symbionts can disperse independently of their hosts,2 but the level of genetic homogenization that we recently reported11 clearly shows regular exchange of fungi between Madagascar and continental Africa.
Further research is needed on the details of symbiont transmission as the current evidence is based on only few species. In fungus-growing termites with vertical symbiont transmission, future experimental studies should focus on the details of symbiont transmission and the frequency of horizontal transmission. In addition, the gathering of Termitomyces spores by fungus-growing termites seems to be a selective process, as suggested by the observed patterns of co-evolution.2,16,22 It remains to be tested how this selectivity occurs.
Figure 1.
Schematic representation of the phylogeny of: (A) fungus-growing termites, showing monophyly of the Malagasy clade and (B) Termitomyces, where symbionts associated with Malagasy Microtermes are shown to belong to three different clades (reviewed in ref. 11); samples from Madagascar are represented by shaded boxes. addition, the gathering of Termitomyces spores by fungus-growing termites seems to be a selective process, as suggested by the observed patterns of co-evolution.2,16,22 It remains to be tested how this selectivity occurs.
Acknowledgements
T.N. is supported by a Marie Curie Intra European Fellowship within the 7th European Community Framework Programme (IEF Project No. 220077).
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/11415
References
- 1.Aanen DK, Eggleton P. Fungus-growing termites originated in African rain forest. Curr Biol. 2005;15:851–855. doi: 10.1016/j.cub.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 2.Aanen DK, Eggleton P, Rouland-Lefevre C, Guldberg-Froslev T, Rosendahl S, Boomsma JJ. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc Natl Acad Sci USA. 2002;99:14887–14892. doi: 10.1073/pnas.222313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Froslev TG, Aanen DK, Laessoe T, Rosendahl S. Phylogenetic relationships of Termitomyces and related taxa. Mycol Res. 2003;107:1277–1286. doi: 10.1017/s0953756203008670. [DOI] [PubMed] [Google Scholar]
- 4.Wood TG, Thomas RJ. The mutualistic association between Macrotermitinae and Termitomyces. In: Wilding N, Collins NM, Hammond PM, Webber JF, editors. Insect—Fungus Interactions. London: Academic Press; 1989. pp. 69–92. [Google Scholar]
- 5.Darlington J. Nutrition and evolution in fungusgrowing termites. In: Hunt JH, Nalepa CA, editors. Nourishment and evolution in insect societies. Boulder,: CO Westview Press; 1994. pp. 105–130. [Google Scholar]
- 6.Grassé P-P, Noirot C. La fondation de nouvelles sociétés par Bellicositermes natalensis Hav. Insect Soc. 1955;2:213–220. (Fre). [Google Scholar]
- 7.Johnson RA, Thomas RJ, Wood TG, Swift MJ. The inoculation of the fungus comb in newly founded colonies of some species of the Macrotermitinae (Isoptera) from Nigeria. J Nat Hist. 1981;15:751–756. [Google Scholar]
- 8.Sands W. The initiation of fungus comb construction in laboratory colonies of Ancistrotermes guineensis (Silvestri) Insectes Sociaux. 1960;7:251–263. [Google Scholar]
- 9.Sieber R. Establishment of fungus comb in laboratory colonies of Macrotermes michaelseni and Odontotermes montanus (Isoptera, Macrotermitinae) Insect Soc. 1983;30:204–209. [Google Scholar]
- 10.Korb J, Aanen DK. The evolution of uniparental transmission of fungal symbionts in fungus-growing termites (Macrotermitinae) Behav Ecol Sociobiol. 2003;53:65–71. [Google Scholar]
- 11.Nobre T, Eggleton P, Aanen DK. Vertical transmission as the key to the colonization of Madagascar by fungus-growing termites? Proc Royal Soc B Biol Sci. 2009. In press. [DOI] [PMC free article] [PubMed]
- 12.Johnson R. Colony development and establishment of the fungus comb in Microtermes sp. nr. usambaricus (Sjöstedt) (Isoptera: Macrotermitinae) from Nigeria. Insect Soc. 1981;28:3–12. [Google Scholar]
- 13.Hamilton WD, May RM. Dispersal in stable habitats. Nature. 1977;269:578–581. [Google Scholar]
- 14.O’Fallon B, Hansen T. Population structure, levels of selection, and the evolution of intracellular symbionts. Evolution. 2008;62:361–373. doi: 10.1111/j.1558-5646.2007.00289.x. [DOI] [PubMed] [Google Scholar]
- 15.Aanen DK, de Fine Licht HH, Debets AJM, Kerstes NAG, Hoekstra RF, Boomsma JJ. High symbiont relatedness stabilizes mutualistic cooperation in fungus-growing termites. Science. 2009;326:1103–1106. doi: 10.1126/science.1173462. [DOI] [PubMed] [Google Scholar]
- 16.Aanen DK, Ros V, de Fine Licht H, Mitchell J, de Beer ZW, Slippers B, et al. Patterns of interaction specificity of fungus-growing termites and Termitomyces symbionts in South Africa. BMC Evol Biol. 2007;7:115. doi: 10.1186/1471-2148-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Fine Licht HH, Andersen A, Aanen DK. Termitomyces sp. associated with the termite Macrotermes natalensis has a heterothallic mating system and multinucleate cells. Mycol Res. 2005;109:314–318. doi: 10.1017/s0953756204001844. [DOI] [PubMed] [Google Scholar]
- 18.Katoh H, Miura T, Maekawa K, Shinzato N, Matsumoto T. Genetic variation of symbiotic fungi cultivated by the macrotermitine termite Odontotermes formosanus (Isoptera: Termitidae) in the Ryukyu Archipelago. Molec Ecol. 2002;11:1565–1572. doi: 10.1046/j.1365-294x.2002.01535.x. [DOI] [PubMed] [Google Scholar]
- 19.Kirk PM, Cannon PF, David JC, Stalpers JA. Ainsworth & Bigby’s Dictionary of the Fungi. Wallingford UK.: CAB International; 2001. [Google Scholar]
- 20.Eggleton P. Global patterns of termite diversity. In: Abe T, Bignell DE, Higashi M, editors. Termites: Evolution, Sociality, Symbioses, Ecology. Dordrecht: Kluwer Academic Publishers; 2000. pp. 25–51. [Google Scholar]
- 21.Frøslev TG, Aanen DK, Læssøe T, Rosendahl S. Phylogenetic relationships of Termitomyces and related taxa. Mycol Res 200. 2003;107:1277–1278. doi: 10.1017/s0953756203008670. [DOI] [PubMed] [Google Scholar]
- 22.Rouland-Lefevre C, Diouf MN, Brauman A, Neyra M. Phylogenetic relationships in Termitomyces (family Agaricaceae) based on the nucleoticle sequence of ITS: A first approach to elucidate the evolutionary history of the symbiosis between fungus-growing termites and their fungi. Molec Phylog Evol. 2002;22:423–429. doi: 10.1006/mpev.2001.1071. [DOI] [PubMed] [Google Scholar]