Abstract
Sexual selection theory posits that females should choose mates in a way that maximizes their reproductive success. But what exactly is the optimal choice? Most empirical research is based on the assumption that females seek a male of the highest possible quality (in terms of the genes or resources he can provide), and hence show directional preferences for indicators of male quality. This implies that attractiveness and quality should be highly correlated. However, females frequently differ in what they find attractive. New theoretical and empirical insights provide mounting evidence that a female’s own quality biases her judgement of male attractiveness, such that male quality and attractiveness do not always coincide. A recent experiment in songbirds demonstrated for the first time that manipulation of female condition can lead to divergent female preferences, with low-quality females actively preferring low-quality males over high-quality males. This result is in line with theory on state-dependent mate choice and is reminiscent of assortative mating preferences in humans. Here we discuss the implications of this work for the study of mate preferences.
Key words: assortative mating, state-dependent mate choice, choosiness, preference function, condition, humans, birds, zebra finch, attractiveness, constrained and unconstrained choice
Birds of a Feather Flock Together: State-Dependent Preferences in Zebra Finches
Holveck & Riebel1 manipulated adult phenotypic quality in zebra finches (Taeniopygia guttata) by rearing nestlings in experimental broods that contained either few or many siblings. This brood-size manipulation is known to have measurable effects on adult physiology, morphology and behavior,1–6 with birds from smaller broods faring better than those from large broods. Instead of showing a uniform preference for males of superior quality, adult females preferred males whose quality matched their own. Quality-matched pairs also showed a much shorter latency to breeding than non-matched pairs, suggesting a reproductive advantage: zebra finches in their natural habitats have to breed quickly if they want to take full advantage of the rare rainfalls.7
Although divergent preference functions (i.e., the order in which prospective mates are ranked8) based on the chooser’s own quality had previously been shown in fish9 and spiders,10 these earlier studies relied on naturally occurring variation in quality, which could be correlated with genetic differences. Furthermore, they measured choice in interactive situations, which complicates interpretation due to the feedback between male and female courtship behavior. The study by Holveck & Riebel1 circumvented both issues, by showing that divergent preferences were a direct response to (experimentally altered) phenotypic quality, and using a non-interactive choice test to measure female preference directly without any confounding influence of the male’s preference. Similar methods had been used by others (e.g., ref. 11) to show that females in poor condition typically have weaker preferences,12 but this was the first demonstration that differences in phenotypic quality can generate preferences in completely opposite directions.
What explains these divergent preferences? Theoretical models of statedependent mate choice point out that individuals in poor condition should be less attracted to high-quality mates when they cannot defend them,13,14 risk being deserted by them15 or are unlikely to be accepted by them in the first place.16 If pursuing these mates is likely to waste time or energy, it may pay to avoid highquality mates altogether and target lowquality partners instead.13 Holveck & Riebel’s work1 provides the first empirical evidence for this prediction, showing that high-quality males are not the most attractive mates for all females. Together with studies on other species9–11 this adds to growing evidence that attractiveness judgments may be closely related to the chooser’s own state.
Beauty is in the Eye of the Beholder in Zebra Finches and Humans
In the unconstrained choice situation used by Holveck & Riebel, females did not experience direct rejection by males or competition with other females, because they chose between pre-recorded songs. Nonetheless, low-quality females unanimously preferred the songs of low-quality males at the first choice opportunity they had as adults.1 This implies the existence of mechanisms that alter females’ mating decisions in relation to their own phenotypic quality.17 Such state-dependent choice is highly reminiscent of mate preferences in humans: people who consider themselves unattractive tend to show weaker preferences for the most soughtafter mates and for traits presumed to indicate mate quality.18–20 Sensitivity to one’s own quality or attractiveness may be useful in species with mutual choice, as a way of deciding which mates are unattainable and therefore not worth courting.1,18,21 But while questionnaire-based studies ask people to reflect consciously on their own attractiveness, in a real choice situation entirely subconscious mechanisms could be at work, in humans as well as in zebra finches. For example, internal physiological monitoring might inform individuals about their body condition6 and lead to adjustment of their preferences. Likewise, individuals may gain feedback about their quality relative to conspecifics from previous non-sexual social experiences1,17 and be sensitive to the level of interest they receive from the opposite sex,16 which could precipitate hormonal changes22,23 and subsequently drive changes in choice. Thus in both species, individuals may change their preferences in response to physiological and social feedback without directly perceiving their own attractiveness (cf. refs. 17, 18).
Assortative Mating in Humans and Birds
The mating systems of humans and passerine birds share important features: both show pronounced plasticity in relation to ecological conditions, but there is a high prevalence of stable pair formation and joint parental care.24 The considerable parental investment by males as well as females means that mutual mate choice is common. In such systems, the time and energy costs of searching for a mate are typically high, and mate-choice decisions can strongly affect reproductive success. Individuals who are too choosy may fail to find a mate and be forced to forego reproduction altogether; while those who are unselective may end up with a mismatched partner, resulting in low parental investment25 and a high risk of desertion or divorce.15 These factors could make it advantageous for individuals to mate assortatively.
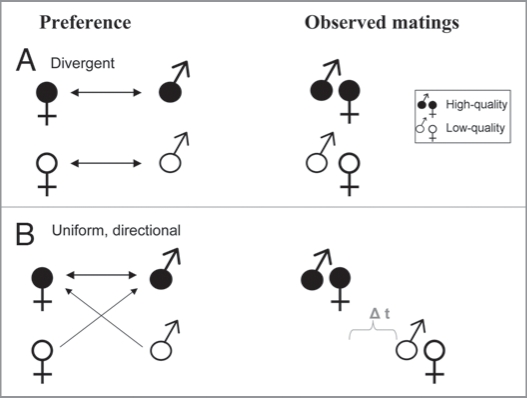
However, positive assortative mating does not necessarily imply that individuals (be they birds or humans) prefer a mate who is similar to themselves.26–28 Figure 1 illustrates how quality-matched pairs could result from divergent, statedependent preferences or from a uniform, directional preference for high quality in both sexes. In the latter case, mutual mate choice will lead to high-quality individuals pairing off first, leaving low-quality individuals as each other’s last resort, perhaps after prolonged search and rejections. This may be compounded by variation in choosiness (i.e., the effort invested in mate assessment8), since directional preferences for high-quality individuals should favor reduced choosiness in low-quality individuals, or at least its gradual decrease with search time.13 Thus, assortative pairing between individuals of the same quality does not imply that preferences are statedependent.
Figure 1.
Different underlying processes can lead to assortative mating. (A) Divergent, state-dependent preferences in both sexes (high-quality individuals prefer high-quality mates, while lowquality individuals prefer low-quality mates). (B) Uniform, directional preference for high-quality mates in both sexes. Due to mutual choice and competition for mates, low-quality individuals are left with only low-quality mates to pair up with. Low-quality individuals will typically spend more time and energy in finding a mate.
Quality and Attractiveness are Not Synonymous
Evidence from several taxa suggests that individual variation in state can favor preferences for quality-matched rather than superior mates,9,10 such that low-quality females actively prefer low-quality males.1 Genetic compatibility (e.g., based on the major histocompatibility complex29), ecological context30 and the particular subset of males available during choice31 are additional factors that can further disrupt the link between male quality and female mate preferences. Consequently, when discussing attractiveness we should be aware that this is a measure of how much a particular male is preferred by a particular female, which may be related in different ways to the quality of both individuals. Highquality males are therefore not always the most attractive.
Viewed in this light, attractiveness is not an inherent property of a particular male phenotype; rather, it is a combined outcome of the male’s phenotype and the female’s response. Females may vary in their responses, in which case the same male is attractive to one female but unattractive to another. Despite this, females may still agree on which male has the highest quality. This may explain why verbally expressed preferences did not match actual choices in human speed dating19 or why female zebra finches consistently laid bigger eggs when mated to high-quality males, despite preferring males of their own quality in choice tests.1
Conclusions
Preferences measured in mate-choice experiments are rarely uniform among the tested subjects; a female’s current state, influenced throughout her development by genetic and non-genetic factors, can affect the direction and strength of her choices. Unraveling the extent to which variation in female preferences can be attributed to state-dependent effects is a major challenge for future studies of sexual selection.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/11557
References
- 1.Holveck M-J, Riebel K. Low quality females prefer low quality males when choosing a mate. Proc R Soc Lond B. 2010;277:18–20. doi: 10.1098/rspb.2009.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holveck M-J, Vieira de Castro AC, Lachlan RF, ten Cate C, Riebel K. Accuracy of song syntax learning and singing consistency signal early condition in zebra finches. Behav Ecol. 2008;19:1267–1281. [Google Scholar]
- 3.de Kogel CH, Prijs HJ. Effects of brood size manipulations on sexual attractiveness of offspring in the zebra finch. Anim Behav. 1996;51:699–708. [Google Scholar]
- 4.de Kogel CH. Long-term effects of brood size manipulation on morphological development and sex-specific mortality of offspring. J Anim Ecol. 1997;66:167–178. [Google Scholar]
- 5.Naguib M, Riebel K, Marzal A, Gil D. Nestling immunocompetence and testosterone covary with brood size in a songbird. Proc R Soc Lond B. 2004;271:833–838. doi: 10.1098/rspb.2003.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verhulst S, Holveck M-J, Riebel K. Long-term effects of manipulated natal brood size on metabolic rate in zebra finches. Biol Lett. 2006;2:478–480. doi: 10.1098/rsbl.2006.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zann RA. The Zebra Finch: a synthesis of field and laboratory studies. Oxford ornithology series. Oxford: Oxford University Press; 1996. [Google Scholar]
- 8.Jennions MD, Petrie M. Variation in mate choice and mating preferences: A review of causes and consequences. Biol Rev. 1997;72:283–327. doi: 10.1017/s0006323196005014. [DOI] [PubMed] [Google Scholar]
- 9.Basolo AL. Variation between and within the sexes in body size preferences. Anim Behav. 2004;68:75–82. [Google Scholar]
- 10.Bel-Venner MC, Dray S, Allaine D, Menu F, Venner S. Unexpected male choosiness for mates in a spider. Proc R Soc Lond B. 2008;275:77–82. doi: 10.1098/rspb.2007.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt J, Broooks R, Jennions MD. Female mate choice as a condition-dependent life-history trait. Am Nat. 2005;166:79–92. doi: 10.1086/430672. [DOI] [PubMed] [Google Scholar]
- 12.Cotton S, Small J, Pomiankowski A. Sexual selection and condition-dependent mate preferences. Curr Biol. 2006;16:755–765. doi: 10.1016/j.cub.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Fawcett TW, Johnstone RA. Mate choice in the face of costly competition. Behav Ecol. 2003;14:771–779. [Google Scholar]
- 14.Härdling R, Kokko H. The evolution of prudent choice. Evol Ecol Res. 2005;7:697–715. [Google Scholar]
- 15.McNamara JM, Forslund P, Lang A. An ESS model for divorce strategies in birds. Phil Trans R Soc Lond B. 1999;354:223–236. [Google Scholar]
- 16.Fawcett TW, Bleay C. Previous experiences shape adaptive mate preferences. Behav Ecol. 2009;20:68–78. [Google Scholar]
- 17.Burley NT, Foster VS. Variation in female choice of mates: condition influences selectivity. Anim Behav. 2006;72:713–719. [Google Scholar]
- 18.Buston PM, Emlen ST. Cognitive processes underlying human mate choice: The relationship between self-perception and mate preference in western society. Proc Natl Acad Sci USA. 2003;100:8805–8810. doi: 10.1073/pnas.1533220100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Todd PM, Penke L, Fasolo B, Lenton AP. Different cognitive processes underlie human mate choices and mate preferences. Proc Natl Acad Sci USA. 2007;104:15011–15016. doi: 10.1073/pnas.0705290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Little AC, Burt DM, Penton-Voak IS, Perrett DI. Self-perceived attractiveness influences human female preferences for sexual dimorphism and symmetry in male faces. Proc R Soc Lond B. 2001;268:39–44. doi: 10.1098/rspb.2000.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Todd PM, Miller GF. From pride and prejudice to persuasion: satisficing in mate search. In: Gigerenzer G, Todd PM, editors. Simple Heuristics That Make Us Smart. New York: Oxford University Press; 1999. pp. 287–308. [Google Scholar]
- 22.Roney JR, Lukaszewski AW, Simmons ZL. Rapid endocrine responses of young men to social interactions with young women. Horm Behav. 2007;52:326–333. doi: 10.1016/j.yhbeh.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Creel S. Social dominance and stress hormones. Trends Ecol Evol. 2001;167:491–549. [Google Scholar]
- 24.Bennett PM, Owens IPF. Evolutionary Ecology of Birds: Life Histories, Mating Systems and Extinction. Oxford: Oxford University Press; 2002. [Google Scholar]
- 25.Cotar C, McNamara JM, Collins EJ, Houston AI. Should females prefer to mate with low-quality males? J Theor Biol. 2008;254:561–567. doi: 10.1016/j.jtbi.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Burley N. The meaning of assortative mating. Ethol Sociobiol. 1983;4:191–203. [Google Scholar]
- 27.Kalick SM, Hamilton TE. The matching hypothesis reexamined. J Pers Soc Psychol. 1986;51:673–682. [Google Scholar]
- 28.Thiessen D, Gregg B. Human assortative mating and genetic equilibrium—an evolutionary perspective. Ethol Sociobiol. 1980;1:111–140. [Google Scholar]
- 29.Mays HL, Hill GE. Choosing mates: good genes versus genes that are a good fit. Trends Ecol Evol. 2004;19:554–559. doi: 10.1016/j.tree.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Qvarnström A, Part T, Sheldon BC. Adaptive plasticity in mate preference linked to differences in reproductive effort. Nature. 2000;405:344–347. doi: 10.1038/35012605. [DOI] [PubMed] [Google Scholar]
- 31.Bateson M, Healy SD. Comparative evaluation and its implications for mate choice. Trends Ecol Evol. 2005;20:659–664. doi: 10.1016/j.tree.2005.08.013. [DOI] [PubMed] [Google Scholar]