Abstract
The incessant tug of war between tyrosine kinases and tyrosine phosphatases regulates critical signaling events during embryogenesis and adulthood. Among these proteins, receptor protein tyrosine phosphatases (RPTPs) have emerged as an important class of neuronal receptors, seemingly capable of mediating cell adhesion and tyrosine dephosphorylation events. Indeed, these proteins combine extracellular domains that resemble those of cell adhesion molecules and tyrosine phosphatase domains that counter the activities of tyrosine kinases. However, the detailed mechanisms underlying RPTP-mediated cell adhesion and RPTP-mediated cell signaling continue to elude our understanding mainly because very few extracellular binding partners of RPTPs have been identified. We have recently characterized biochemically and structurally the interactions between members of the contactin family of neural recognition molecules and the homologous receptor protein tyrosine phosphatase zeta (PTPRZ) and gamma (PTPRG) that are expressed in the nervous system. Here, we present our main findings and we discuss their possible implication for the control of tyrosine dephosphorylation by contactin family members.
Key words: receptor protein tyrosine phosphatase, cell adhesion, cell signaling, crystal structure, protein-protein interactions, nervous system
Receptor protein tyrosine phosphatases (RPTPs) are important neuronal receptors composed of cell adhesion moleculelike extracellular regions and intracellular tyrosine phosphatase domains.1,2 Although it has long been appreciated that these proteins could play significant roles in cell adhesion and cell signaling,3 a molecular understanding of RPTP function during development is still lacking. This can be explained primarily because (a) the nature of the extracellular binding interactions they participate in is uncertain and (b) the relationship between extracellular binding and intracellular signaling is unclear. Because of these gaps we are still unable to grasp how these receptors respond to extracellular cues and regulate phosphotyrosine signaling during neuronal wiring.
Receptor protein tyrosine phosphatase gamma (PTPRG) and receptor protein tyrosine phosphatase zeta (PTPRZ) are two of the ∼20 RPTPs found in humans and are expressed mostly in the developing and adult vertebrate nervous system.4,5 The extracellular regions of these two homologous proteins include a carbonic anhydrase-like (CA-like) domain in which only one the three histidine residues required for catalysis is conserved indicating that these domains are not catalytically active.6,7 Instead, the CA-like domain of PTPRZ mediates binding to the neural recognition molecule contactin1 (CNTN1), which in fact constituted the first identification of a heterophilic binding partner for a RPTP.8 We have undertaken biochemical and structural analyses of PTPRZ and the homologous PTPRG in an effort to shed light on the protein-protein interactions mediated by their CA-like domains.
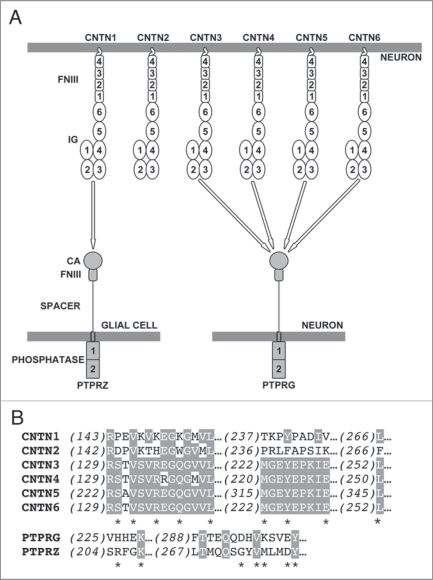
Five CNTN1-like proteins are expressed in multiple regions of the nervous system during embryogenesis and after birth.9 Members of the CNTN family are glycophosphatidyl-anchored proteins that share 40–60% amino acid sequence identity and include six N-terminal immunoglobulin (Ig) repeats and four fibronectin type III (FNIII) repeats (Fig. 1A). Based on the sequence homologies between the CA-like domains of PTPRG and PTPRZ (∼40% amino acid identity) on one hand and CNTNs on the other hand, we thought it plausible that there could be additional interactions between members of these two families. Consistent with this hypothesis, expression of CNTN3, 4, 5 and 6 as well as PTPRG overlaps in space and time in the nervous system of mice.5,10–14 Using affinity-isolation assays, we demonstrated that the CA-like domain of PTPRZ mediates binding to CNTN1, but not to other members of the CNTN family15 (Fig. 1A). In contrast, the CA-like domain of PTPRG associates with CNTN3, 4, 5 and 6, but not with either CNTN1 or 2. These findings constitute, to the best of our knowledge, the first identification of potential binding partners for PTPRG. In addition, we showed that the binding site for PTPRG and PTPRZ in CNTN family members spans their 2nd and 3rd Ig repeats and that neither repeat could mediate binding alone. We determined crystal structures of the CA-like domains of PTPRZ and PTPRG and of a complex between PTPRG and CNTN4 to provide a structural basis for the interactions we identified using affinity-isolation assays. The CA-like domains of PTPRG and PTPRZ are structurally very similar to catalytically active CAs save for the orientation of an extended β-hairpin loop absent in active CAs.15 In the PTPRG·CNTN4 complex, this β-hairpin loop mediates the majority of the contacts with the 2nd and 3rd Ig domains of CNTN4. The amino acid residues found at the interface between PTPRG and CNTN4 are conserved in CNTN3, 5 and 6, which is in line with our observation that PTPRG binds specifically to these four molecules, but not to CNTN1 or CNTN2 (Fig. 1B).
Figure 1.
(A) Overview of the interactions between CNTN family members and the tyrosine phosphatases PTPRG and PTPRZ. CNTN family members consist of six immunoglobulin (IG) domains and four fibronectin type III (FNIII) domains. The U-shaped arrangement of the first four Ig domains in CNTN s reflect the horseshoe-like conformation that these domains are all likely to adopt.15 PTPRG and PTPRZ include two intracellular tyrosine phosphatase repeats, but only the membrane proximal domain is catalytically active. CNTN 2 does not interact with either PTPRG or PTPRZ and mediates homophilic interactions using a binding site localized within its first four Ig domains.20 (B) Sequence alignment of mouse CNTN s and mouse PTPRG and PTPRZ for residues at the interface between PTPRG and CNTN 4. Conserved amino acids are shaded. Asterisks denote contact residues.
A key aspect of RPTP function concerns the regulation of the intracellular phosphatase activity by extracellular interactions. One of the models proposed to explain this relationship is that RPTPs exist in a monomeric active state in the absence of ligands, but transition to a catalytically inactive dimeric state in the presence of their cognate extracellular binding partners.2 For example, binding of the growth factor pleiotrophin to PTPRZ leads to its dimerization and inhibition of its phosphatase activity.16 In addition, dimerization of the tandem phosphatase domains of PTPRG occludes its catalytic site and is thus incompatible with dephosphorylation of tyrosine residues. 17 In contrast, in our biochemical and structural analyses, we found no evidence that CNTN1 or CNTN4 could induce the dimerization of PTPRZ or PTPRG, respectively. Given these observations, what could be the significance of the interactions between CNTN family members and PTPRG/PTPRZ? To begin, if we suppose that CNTN binding induces RPTP dimerization and subsequent inhibition of dephosphorylation activity then the lack of dimers observed in the co-crystal structure of PTPRG and CNTN4 would indicate that additional domains of either PTPRZ, PTPRG and/or CNTNs are necessary to observe a change in oligomeric state and ultimately inhibition of the catalytic activity. This would be a very significant result. Indeed, in the case of PTPRZ, it would imply that two structurally distinct ligands (CNTN1 and pleitrophin, an Ig superfamily protein and a growth factor, respectively) have similar effects on intracellular signaling. This finding would be in stark contrast with the signaling mechanisms of receptor tyrosine kinases, for which kinase activation results from binding of structurally-related ligands.18
In contrast, one may suppose that the binding of CNTN molecules to PTPRZ or PTPRG does not lead to dimerization and inhibition of the phosphatase activity. In this case, CNTN binding could restrict PTPRG and PTPRZ to favored regions of the cell surface to enable a localized control of dephosphorylation.19 Interactions between CNTNs and PTPRG/PTPRZ may also function independently of the phosphatase activity and rather facilitate the formation of cell adhesion complexes that could recruit additional proteins via, for example, the inactive phosphatase domain of PTPRG or PTPRZ. The identification of binding partners and substrates for the phosphatase regions of PTPRG and PTPRZ as well as a detailed analysis of the effect of CNTN binding on the oligomerization of PTPRG and PTPRZ will make it possible to discern between these hypotheses and lead to a better understanding of the significance of these protein-protein interactions during wiring of the brain.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/11656
References
- 1.Alonso A, Sasin J, Bottini N, Friedberg I, Osterman A, Godzik A, et al. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Johnson KG, Van Vactor D. Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev. 2003;83:1–24. doi: 10.1152/physrev.00016.2002. [DOI] [PubMed] [Google Scholar]
- 3.Charbonneau H, Tonks NK, Walsh KA, Fischer EH. The leukocyte common antigen (CD45): a putative receptor-linked protein tyrosine phosphatase. Proc Natl Acad Sci USA. 1988;85:7182–7186. doi: 10.1073/pnas.85.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harroch S, Palmeri M, Rosenbluth J, Custe A, Okigaki M, Shrager P, et al. No obvious abnormality in mice deficient in receptor protein tyrosine phosphatase beta. Mol Cell Biol. 2000;20:7706–7715. doi: 10.1128/mcb.20.20.7706-7715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamprianou S, Vacaresse N, Suzuki Y, Meziane H, Buxbaum JD, Schlessinger J, et al. Receptor protein tyrosine phosphatase gamma is a marker for pyramidal cells and sensory neurons in the nervous system and is not necessary for normal development. Mol Cell Biol. 2006;26:5106–5119. doi: 10.1128/MCB.00101-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnea G, Silvennoinen O, Shaanan B, Honegger AM, Canoll PD, D’Eustachio P, et al. Identification of a carbonic anhydrase-like domain in the extracellular region of RPTPgamma defines a new subfamily of receptor tyrosine phosphatases. Mol Cell Biol. 1993;13:1497–1506. doi: 10.1128/mcb.13.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krueger NX, Saito H. A human transmembrane protein-tyrosine-phosphatase, PTPzeta, is expressed in brain and has an N-terminal receptor domain homologous to carbonic anhydrases. Proc Natl Acad Sci USA. 1992;89:7417–7421. doi: 10.1073/pnas.89.16.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peles E, Nativ M, Campbell PL, Sakurai T, Martinez R, Lev S, et al. The carbonic anhydrase domain of receptor tyrosine phosphatase beta is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–260. doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- 9.Shimoda W. Contactins: Emerging key roles in the development and function of the nervous system. Cell Adh Migr. 2009;3:64–70. doi: 10.4161/cam.3.1.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaneko-Goto T, Yoshihara S, Miyazaki H, Yoshihara Y. BIG-2 mediates olfactory axon convergence to target glomeruli. Neuron. 2008;57:834–846. doi: 10.1016/j.neuron.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Takeda Y, Niki H, Ogawa J, Kobayashi S, Kai N, et al. Aberrant responses to acoustic stimuli in mice deficient for neural recognition molecule NB-2. Eur J Neurosci. 2003;17:929–936. doi: 10.1046/j.1460-9568.2003.02514.x. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa J, Lee S, Itoh K, Nagata S, Machida T, Takeda Y, et al. Neural recognition molecule NB-2 of the contactin/F3 subgroup in rat: Specificity in neurite outgrowth-promoting activity and restricted expression in the brain regions. J Neurosci Res. 2001;65:100–110. doi: 10.1002/jnr.1133. [DOI] [PubMed] [Google Scholar]
- 13.Takeda Y, Akasaka K, Lee S, Kobayashi S, Kawano H, Murayama S, et al. Impaired motor coordination in mice lacking neural recognition molecule NB-3 of the contactin/F3 subgroup. J Neurobiol. 2003;56:252–265. doi: 10.1002/neu.10222. [DOI] [PubMed] [Google Scholar]
- 14.Yoshihara Y, Kawasaki M, Tani A, Tamada A, Nagata S, Kagamiyama H, et al. BIG-1: a new TAG-1/F3-related member of the immunoglobulin superfamily with neurite outgrowth-promoting activity. Neuron. 1994;13:415–426. doi: 10.1016/0896-6273(94)90357-3. [DOI] [PubMed] [Google Scholar]
- 15.Bouyain S, Watkins DJ. The protein tyrosine phosphatases PTPRZ and PTPRG bind to distinct members of the contactin family of neural recognition molecules. Proc Natl Acad Sci USA. 2010;107:2443–2448. doi: 10.1073/pnas.0911235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukada M, Fujikawa A, Chow JP, Ikematsu S, Sakuma S, Noda M. Protein tyrosine phosphatase receptor type Z is inactivated by ligand-induced oligomerization. FEBS Letts. 2006;580:4051–4056. doi: 10.1016/j.febslet.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 17.Barr AJ, Ugochukwu E, Lee WH, King ON, Filippakopoulos P, Alfano I, et al. Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell. 2009;136:352–363. doi: 10.1016/j.cell.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem. 2000;69:373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- 19.Yudushkin IA, Schleifenbaum A, Kinkhabwala A, Neel BG, Schultz C, Bastiaens PI. Live-cell imaging of enzyme-substrate interaction reveals spatial regulation of PTP1B. Science. 2007;315:115–119. doi: 10.1126/science.1134966. [DOI] [PubMed] [Google Scholar]
- 20.Freigang J, Proba K, Leder L, Diederichs K, Sonderegger P, Welte W. The crystal structure of the ligand binding module of axonin-1/TAG-1 suggests a zipper mechanism for neural cell adhesion. Cell. 2000;101:425–433. doi: 10.1016/s0092-8674(00)80852-1. [DOI] [PubMed] [Google Scholar]