Abstract
Purpose
The purpose of this study was to evaluate the prevalence of cardiac autonomic neuropathy (CAN) in a cohort of patients with type 2 diabetes, truly asymptomatic for coronary artery disease (CAD), using heart rate variability (HRV) and 123I-metaiodobenzylguanidine (123I-mIBG) myocardial scintigraphy.
Methods
The study group comprised 88 patients with type 2 diabetes prospectively recruited from an outpatient diabetes clinic. In all patients myocardial perfusion scintigraphy, CAN by HRV and 123I-mIBG myocardial scintigraphy were performed. Two or more abnormal tests were defined as CAN-positive (ECG-based CAN) and one or fewer as CAN-negative. CAN assessed by 123I-mIBG scintigraphy was defined as abnormal if the heart-to-mediastinum ratio was <1.8, the washout rate was >25%, or the total defect score was >13.
Results
The prevalence of CAN in patients asymptomatic for CAD with type 2 diabetes and normal myocardial perfusion assessed by HRV and 123I-mIBG scintigraphy was respectively, 27% and 58%. Furthermore, in almost half of patients with normal HRV, 123I-mIBG scintigraphy showed CAN.
Conclusion
The current study revealed a high prevalence of CAN in patients with type 2 diabetes. Secondly, disagreement between HRV and 123I-mIBG scintigraphy for the assessment of CAN was observed.
Keywords: Diabetes, Cardiac autonomic neuropathy, Heart rate variability, 123I-mIBG scintigraphy
Introduction
Diabetes is an increasing health-care problem [1]. Cardiovascular complications including coronary artery disease (CAD), are the most common causes of mortality and morbidity in patients with type 2 diabetes [2]. Because patients with type 2 diabetes often suffer from CAD without symptoms, risk stratification is needed to identify patients at high risk of CAD. In the DIAD (detection of silent myocardial ischemia in asymptomatic diabetic subjects) study the overall prevalence by SPECT of silent myocardial ischaemia was 22% [3]. Although traditional risk factors failed to predict silent myocardial ischaemia, cardiac autonomic neuropathy (CAN) assessed by heart rate variability (HRV), was a strong predictor of silent myocardial ischaemia in this study. Furthermore, CAN is one of the most common complications associated with diabetes and has been associated with an increased risk of mortality and major cardiovascular events [4]. CAN assessed by HRV is a sensitive method for detecting neuropathy of the vagal nerve, characterized by resting tachycardia and reduced HRV [5]. In addition, sympathetic cardiac innervation can nowadays be assessed directly and noninvasively with 123I-mIBG myocardial scintigraphy in many clinical disorders, including diabetes [6]. Several previous studies have evaluated the relationship between HRV and 123I-mIBG myocardial scintigraphy in patients with diabetes [7–9]. However, the current observational study specifically evaluated the prevalence of CAN in patients with type 2 diabetes and without symptoms of CAD comparing HRV with 123I-mIBG myocardial scintigraphy.
Methods
Patient population
A total of 155 patients were referred from an outpatient diabetic clinic to our institution for comprehensive clinical risk stratification. This comprehensive evaluation included adenosine stress SPECT myocardial perfusion imaging to assess myocardial ischaemia, and123I-mIBG myocardial scintigraphy and HRV to assess CAN. The diagnosis of diabetes was established by the referring physician and confirmed by patient history or the use of medication such as insulin or oral hypoglycaemic agents [10]. Inclusion criteria consisted of confirmed type 2 diabetes in combination with complete absence of angina or angina-equivalent symptoms. Asymptomatic status was confirmed using the Rose questionnaire for angina [11]. Exclusion criteria were: known CAD, treatment with antianginal medication, electrocardiographic evidence of Q wave myocardial infarction, ischaemic ST segment or T wave changes or complete left bundle branch block and active bronchospasm, excluding the use of adenosine as stressor for SPECT myocardial perfusion imaging. For the current analysis, the relationship between sympathetic dysfunction as assessed by 123I-mIBG myocardial scintigraphy and the prevalence of CAN as assessed by HRV was evaluated only in patients with normal myocardial perfusion (stress and rest).
Adenosine stress SPECT myocardial perfusion imaging
Data acquisition and analysis were performed as described before [12]. Briefly, ECG-gated SPECT imaging was performed using a 2-day protocol (adenosine and rest) with technetium-99m sestamibi (99mTc-MIBI). Twelve-lead electrocardiography (ECG) was recorded each minute and continuously monitored (leads aVF, V1 and V5) for the development of arrhythmia or ST-segment deviation. Imaging was performed as a standardized protocol 120 min after tracer injection, using a triple-head SPECT camera (GCA 9300/HG; Toshiba, Tokyo, Japan) using low-energy, high-resolution collimators. Images were acquired using a circular 360° orbit, 60 projections and 40 s per projection [13]. No attenuation correction was applied. The cardiac images were processed and short-axis, horizontal and vertical long-axis views were reconstructed. The adenosine stress SPECT myocardial perfusion imaging studies were clinically read and considered normal under the following conditions: (1) if both the summed rest score and the summed difference score were <2, (2) if the resting LVEF was ≥45%, and (3) in the absence of increased lung uptake, transient ischaemic dilation or flat or down-sloping ST-segment depression of ≥1 mm at 80 ms after the J-point in two or more leads on the ECG during adenosine infusion [14].
Cardiovascular autonomic reflex tests
Data acquisition
To assess CAN, HRV was measured during the following four cardiovascular reflex tests and recorded with the aid of a Holter ECG [15, 16]:
HRV at rest over 150 consecutive beats
HRV during deep breathing at six cycles per minute over 120 consecutive beats
HRV during Valsalva manoeuvre
Immediate heart rate response to standing from the recumbent position
A final test measured the difference in systolic blood pressure between supine and upright positions. No patients were receiving drugs influencing HRV, such as neuroleptic drugs or clozapine. Antihypertensive medications such as angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) were stopped 1 week before HRV measurements [17].
Data analysis
All Holter ECG recordings were analysed by two experienced observers unaware of the clinical history of the patients. For the evaluation of HRV at rest, the coefficient of variation was used [18]. HRV during deep breathing was evaluated by calculating the E-I ratio (ratio of the mean longest to shortest R-R interval during inspiration and expiration, respectively) [19]. The ‘Valsalva ratio’ was calculated as the ratio of the longest R-R interval following release to the shortest R-R interval during the manoeuvre [20]. Immediate heart rate response to standing up was calculated as the ‘30-15 ratio’, defined as the longest R-R interval of beats 20–40 divided by the shortest R-R interval of beats 5–25 with beat one being the first one during the process of getting up. The shortest R-R interval came always prior to the longest R-R interval in the calculation of the 30-15 ratio. Age-related normal ranges were used for evaluation of these four cardiovascular reflex tests [21]. Patients were excluded from analysis when either one or more recordings of HRV during Holter monitoring consisted of too few consecutive beats for interpretation or if extra systoles impaired interpretation. A decrease in systolic blood pressure equal to or greater than 30 mmHg within 2 min of standing was defined as an abnormal heart rate response [22].
Thus, CAN assessed by HRV was classified according to the number of abnormal cardiovascular reflex tests. Two or more abnormal tests were defined as CAN-positive (ECG-based CAN) and one or fewer as CAN-negative [23, 24].
123I-mIBG myocardial scintigraphy imaging protocol
Data acquisition
Tricyclic antidepressant drugs, sympathetic agents, and other drugs known to interfere with mIBG uptake were withdrawn 1 week before data acquisition. Thyroid uptake of unbound 123I was blocked with sodium iodide solution given orally, and 60 min later 185 MBq of 123I-mIBG (GE Healthcare) was injected intravenously over 60 s at rest. Anterior planar images of the chest were acquired 15 min after injection and stored in a 256x256 matrix. Thereafter, a 360° SPECT study was acquired using a double-head gamma camera (GCA 9300/HG; Toshiba, Tokyo, Japan) (4° per step, 35 s per projection, 128 × 128 matrix). Repeat planar and SPECT studies were acquired approximately 4 h after injection. All camera heads were equipped with low-energy high-resolution collimators and all acquisitions were performed with a 15% energy window centred at the 159 keV photopeak of 123I.
Data analysis and image interpretation
Planar images
Early (15 min after injection) and late (4 h delayed) planar images were processed to determine the heart-to-mediastinum ratio (HMR). The planar 123I-mIBG images were analysed using regions of interest to calculate the uptake ratios and washout percentages. A region of interest was drawn manually over the left ventricle (LV). The HMR of average counts per pixel was calculated for the early and delayed images. After correcting for the physical decay of 123I, early and delayed HMR values were then used to compute the myocardial washout rate (WR) of 123I-mIBG as follows:
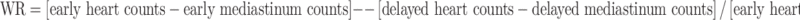 |
Since most available evidence supports the use of the delayed 123I-mIBG (and the derived HMR and WR) for risk stratification, abnormal delayed HMR and WR were defined as those values lower than 1.8 and higher than 25%, respectively [6, 25–28].
SPECT images
Polar map formats were used for semiquantitative interpretation. The delayed myocardial SPECT images were divided into 17 segments and each segment was evaluated in consensus by two expert observers, using a four-point scoring system (0 >70% tracer uptake, 1 50–70% tracer uptake, 2 30–50% tracer uptake, 3 <30% tracer uptake) [14]. As a result, the summed score for each study could theoretically range from 0 to 51 (17 × 3). The total defect score (TDS) was calculated as the sum of all defect scores. The TDS was converted to percentage of the total denervated myocardium. The percentage denervation in the LV was calculated as follows: 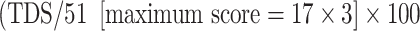 . Based on the study by Kasama et al., a TDS of >18 was considered abnormal, and in terms of the four-point scoring system used in the current study, a TDS of >13 would be considered abnormal [26].
. Based on the study by Kasama et al., a TDS of >18 was considered abnormal, and in terms of the four-point scoring system used in the current study, a TDS of >13 would be considered abnormal [26].
Next, the location of defects was evaluated, and categorized as located inferior, apical, lateral, anterior or septal. In addition, patients were categorized as having one defect allocated to one region (see above) or having defects in two or more regions. Diffusely reduced 123I-mIBG uptake was defined as abnormal HMR and no clear defect on the SPECT polar map.
A composite endpoint (HMR <1.8, WR >25%, or TDS >13) was used to define an abnormal 123I-mIBG study.
Statistical analysis
Categorical baseline characteristics were expressed as numbers and percentages. Continuous variables were expressed as means (±standard deviation) and compared with the two-tailed t test for independent samples.
Statistical analyses were performed using SPSS software, version 12.0 (SPSS, Chicago, IL) and SAS software (The SAS system, release 6.12; SAS, Cary, NC). A p-value<0.05 was considered as statistically significant.
Results
Patient characteristics
Initially 155 patients were referred for risk stratification including adenosine stress SPECT myocardial perfusion imaging to assess myocardial ischaemia, and123I-mIBG myocardial scintigraphy and HRV to assess CAN. Of these 155 patients, 110 (71%) with normal SPECT perfusion studies were identified, and of these, 22 (20%) were excluded because of incomplete datasets (13) or poor 123I-mIBG image quality (9). Thus, the final patient population consisted of 88 patients (56 men, age 53 ± 10 years). The patient characteristics are shown in Table 1. The mean duration of diabetes was 9.8 ± 8.2 years. Most of the patients were on oral antidiabetic therapy (61%), 35% were using ACE inhibitors, and 21% ARB. Complications of diabetes, such as peripheral neuropathy and/or peripheral vascular disease, were seen 28 patients.
Table 1.
Characteristics of 88 patients with diabetes type 2. Values are number (%) of patients or means±SD
| Characteristic | Value |
|---|---|
| Male | 56 (64) |
| Age (years) | 53 ± 10 |
| Diabetes-related risk factors | |
| Duration (years) | 9.8 ± 8.2 |
| Age at time of diagnosis of diabetes (years) | 44 ± 12 |
| HbA1C (%) | 7.4 ± 1.6 |
| Treatment | |
| Oral | 54 (61) |
| Insulin | 21 (24) |
| Oral and insulin | 11 (13) |
| Peripheral vascular disease | 9 (10) |
| Peripheral neuropathy | 19 (22) |
| Peripheral vascular and neuropathy | 11(13) |
| Body mass index (kg/m2) | 29.2 ± 5.7 |
| Waist circumference (cm) | 103 ± 15.0 |
| Hypertension | 47 (53) |
| Hypercholesterolaemia | 46 (52) |
| Family history of CAD | 52 (59) |
| Current smoking | 12 (14) |
| Medication | |
| Aspirin | 18 (21) |
| ACE inhibitors | 31 (35) |
| ARB | 18 (21) |
| Statins | 45 (51) |
| Total cholesterol (mmol/l) | 4.9 ± 1.1 |
| Low-density lipoprotein (mmol/l) | 3.2 ± .1.1 |
| High-density lipoprotein (mmol/l) | 1.4 ± 0.6 |
| Cholesterol/HDL ratio | 3.8 ± 1.3 |
| Triglycerides (mmol/l) | 2.0 ± 1.2 |
| Creatinine (mmol/l) | 78.5 ± 20.0 |
| Urine albumin/creatinine ratio | 12.4 ± 32.8 |
| Chain reactive protein (mg/l) | 8.3 ± 5.8 |
| Apolipoprotein A1 (g/l) | 1.4 ± 0.30 |
| Apolipoprotein B (g/l) | 0.9 ± 0.30 |
| Fibrinogen (g/l) | 3.9 ± 0.90 |
Cardiac autonomic neuropathy assessed by HRV
CAN was classified according to the number of abnormal cardiovascular reflex tests. Of the 88 patients, 30 (34%) had one or more abnormal cardiovascular reflex tests, and 24 (27%) had two or more abnormal tests and were categorized as CAN-positive.
Cardiac autonomic dysfunction assessed by 123I-mIBG
Of the 88 patients, 37 (42%) had a normal 123I-mIBG study. In the remaining 51 patients (58%), the 123I-mIBG study was abnormal. Of these 51 patients, 22 (43%) had one criterion for an abnormal 123I-mIBG study, and the remaining 29 (57%) had two or more criteria. Of the total 88 patients, 36 (41%) had a HMR of <1.8 (mean 1.62 ± 0.13) and 32 (36%) had a WR of >25% (mean 34 ± 8). Finally, 25 patients (28%) had a TDS of >13 (mean 43 ± 18).
Location of 123I-mIBG defects on SPECT
Among the total study population of 88 patients, 49 patients (56%) had defects on SPECT. The distribution of the location of the defects in the total study population was as follows: inferior 25%, apex 8%, anterior 3%, diffuse diminished uptake 9% and two or more regions 10%.
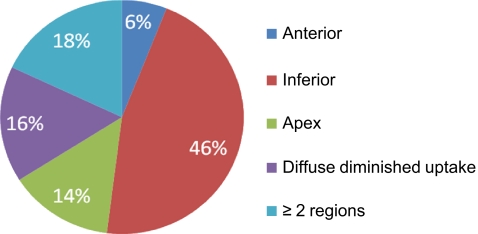
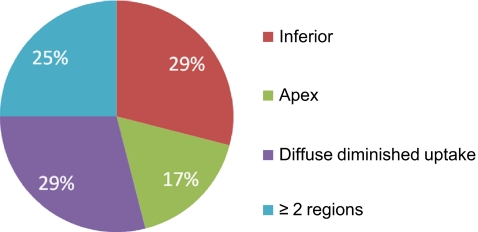
Figures 1 and 2 show the distribution of the location of the innervation defects in all patients with abnormalities and in patients with a TDS of >13, respectively. The majority of the innervation defects (in 22 patients) were located in the inferior wall. Seven patients showed defects in the apex, whereas three patients had defects in the anterior wall. Eight patients had diffuse diminished tracer uptake over the entire LV and nine patients had defects in two or more regions. In the 25 patients with a TDS of >13, the locations of the defects was as follows: inferior wall in seven patients, apex in four, and two or more regions in six. Diffused diminished uptake was seen in seven patients.
Fig. 1.
Pie chart showing the location of the innervation defects on 123I-mIBG myocardial scintigraphy
Fig. 2.
Pie chart showing the location of the innervation defects on 123I-mIBG myocardial scintigraphy in patients with a TDS of >13
Cardiac autonomic dysfunction assessed by 123I-mIBG versus cardiac autonomic neuropathy by HRV
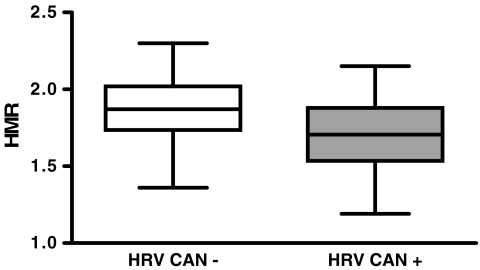
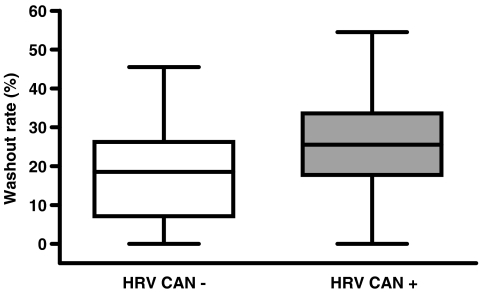
Results of the individual comparisons of HMR, WR and TDS values between patients with and without CAN as determined by HRV are presented in Figs. 3, 4 and 5, respectively. Among patients with normal HRV, 21 (72%) had a HMR of <1.8 (mean 1.66 ± 0.10), 18 (62%) had a WR of >25% (mean 34 ± 7) and 13 (45%) had a TDS of >13 (mean 26 ± 11). In 15 of these patients (52%) two or more variables were abnormal.
Fig. 3.
Box plot showing the distribution of HMR values in patients with and without CAN as determined by HRV. Average HMR was significantly higher in patients without CAN (1.89 ± 0.21) than in patients with CAN (1.72 ± 0.23, p=0.001)
Fig. 4.
Box plot showing the distribution of WR values in patients with and without CAN as determined by HRV. Average WR was significantly lower in patients without CAN (18.5% ± 12.7%) than in patients with CAN (25.9% ± 13.5%, p=0.019)
Fig. 5.
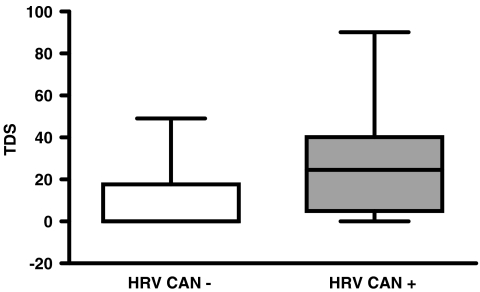
Box plot showing the distribution of TDS values in patients with and without CAN as determined by HRV. Average TDS was significantly lower in patients without CAN (11.2 ± 4.7) than in patients with CAN (28.8 ± 28.3, p=0.002)
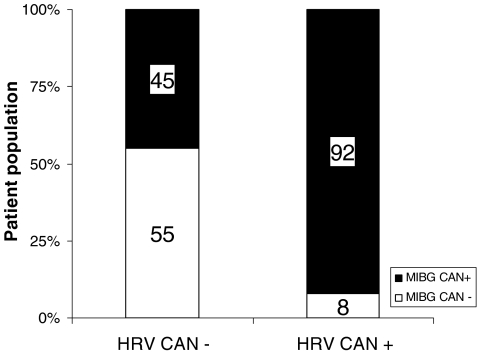
The agreement and disagreement between HRV and 123I-mIBG for the assessment of CAN is shown in Fig. 6. Of the 64 patients without CAN by HRV, only 35 (55%) had a normal 123I-mIBG study. In contrast, almost all patients (22, 92%) with CAN by HRV also had an abnormal 123I-mIBG.
Fig. 6.
Agreement and disagreement between HRV and 123I-mIBG scintigraphy for the assessment of CAN
Discussion
In the present study, the prevalence of CAN in patients with type 2 diabetes, asymptomatic for CAD and with normal myocardial perfusion, was assessed by HRV and 123I-mIBG scintigraphy; the prevalence of CAN was 27% by HRV and 58% on 123I-mIBG scintigraphy. Importantly, in almost 50% of patients with normal HRV, 123I-mIBG scintigraphy showed CAN, suggesting that 123I-mIBG imaging may be more sensitive for the assessment of CAN than HRV.
CAN is an important complication of diabetes. CAN results from damage to the autonomic nerve fibres innervating the heart and blood vessels, which causes abnormalities in heart rate control and impairs vascular dynamics. Clinical manifestations of CAN include exercise intolerance, intraoperative cardiovascular instability, orthostatic hypotension, asymptomatic ischaemia and painless myocardial infarction [29]. Furthermore, CAN is associated with poor outcome related to ventricular arrhythmias and sudden cardiac death [4, 30]. In clinical practice, it is possible to assess CAN by measuring HRV. When CAN has progressed significantly, it may result in a reduction in HRV [31]. Measuring HRV has been shown to be a simple, noninvasive method to evaluate the sympatheticovagal balance at the sinoatrial level, and can identify patients at risk of cardiac arrhythmias and sudden death [32, 33].
Therefore, assessing HRV is potentially important in the management of patients with diabetes. In the current study, a series of simple bedside tests for detecting CAN were used [34]. As early as 1976 Ewing et al. reported the results in 37 diabetic patients with symptoms and clinical features suggestive of autonomic neuropathy who were followed for 33 months [35]. The authors concluded that simple autonomic function tests provided significant prognostic information, with abnormal tests being associated with a high mortality. These results were confirmed in a larger population of 605 patients (the Hoorn study) with a follow-up period of 9 years [4]. Mortality during the follow-up was 17% (101 patients); patients with diabetes and impaired autonomic function had a twofold mortality risk.
123I-mIBG is an analogue of the false neurotransmitter guanethidine. It is taken up by adrenergic neurons in a similar fashion to norepinephrine and does not undergo intracellular metabolism. Imaging with this agent reflects uptake in presynaptic neurons as modulated by the norepinephrine transporter and the status of intraneuronal storage of norepinephrine [36]. Recently, Nagamachi et al. investigated retrospectively the long-term (mean 7.2 ± 3.2 years) prognostic value of 123I-mIBG scintigraphy for both cardiac events and mortality in 144 patients with type 2 diabetes and normal myocardial perfusion [6]. A decreased delayed HMR was an independent predictor of long-term mortality. A combination of 123I-mIBG scintigraphy parameters and HRV was independently predictive of cardiac events.
Previous data have suggested that 123I-mIBG scintigraphy may be more sensitive than HRV for the detection of CAN in diabetic subjects [16, 37]. The role of CAN in asymptomatic diabetic patients has been described by Valensi et al. [38]. In that study, 75 patients with at least two cardiovascular risk factors, were evaluated for silent myocardial ischaemia and CAN with a follow-up period of 3–7 years. Of these patients, 11 (15%) had a major cardiovascular event, and multivariate analysis demonstrated that CAN was a better predictor of major cardiac events than silent myocardial ischaemia. Furthermore, Langer et al. investigated CAN in relation to HRV and 123I-mIBG scintigraphy in 23 normal subjects and 65 asymptomatic patients with diabetes type 2 and silent myocardial ischaemia [39]. The authors found that 123I-mIBG uptake was largely diminished in diabetic patients, especially in those with clinically detectable CAN; moreover, diffuse abnormalities in 123I-mIBG uptake were observed in patients with silent myocardial ischaemia.
In the present study, we performed a systematic, head-to-head comparison between HRV and 123I-mIBG scintigraphy in 88 patients with type 2 diabetes asymptomatic for CAD to evaluate the presence of CAN. We included many more patients than previous studies, but although the inclusion criteria were different, our results are in line with those of previous studies showing that 123I-mIBG scintigraphy identified a significantly higher proportion of patients with CAN than HRV [16, 40, 41]. The fact that more patients exhibit abnormalities in 123I-mIBG imaging as compared to HRV underscores the suggestion that abnormalities in cardiac sympathetic innervation occur prior to ECG-based (HRV) cardiac autonomic dysfunction [16]. An alternative explanation is that 123I-mIBG scintigraphy mainly reflects sympathetic innervation, whereas HRV may be more related to parasympathetic function [42]. It remains to be determined which of these two parameters is more useful in predicting long-term outcome.
While HRV and other traditional parameters provide an impression of global innervation abnormalities, 123I-mIBG scintigraphy with SPECT provides information on regional innervation. The findings of the current study indicate that regional abnormalities occur often in patients with asymptomatic diabetes. Other studies using 123I-mIBG scintigraphy in populations with various cardiovascular diseases have also shown regional innervation abnormalities [16, 40, 41, 43]. For example, Langer et al. evaluated 65 diabetic patients and noted significantly impaired 123I-mIBG uptake in the inferior wall and apex [39]. Additional studies have shown that abnormalities in CAN tend to occur first in the inferior regions of the myocardium and then progressively spread to adjacent segments [44, 45].
Clinical implications
Of all established diabetes-related and cardiac risk factors in patients with asymptomatic diabetes, poor glycaemic control is of great importance in the development and progression of CAN [37, 44]. Accordingly, early detection of CAN is of the utmost importance. As observed in the current study, 123I-mIBG scintigraphy appears more sensitive than HRV for the detection of CAN in asymptomatic diabetic patients without myocardial perfusion abnormalities. Identification of these patients may permit risk factor modification and intensive medical treatment aiming at better glycaemic control, which in turn may favourably affect outcome [46–49]. Indeed, several studies have shown that improvement in CAN (as evidenced by HRV) can be achieved by weight loss and regular exercise [50, 51]. Furthermore, progression of CAN can also be delayed by intensive medical therapy, thereby reducing the risk of premature mortality [52].
Limitations
The current study included only diabetic patients, and no control population was available. Precise threshold values for diabetic patients and normal controls need to be further explored for the individual 123I-mIBG variables (HMR, WR and TDS) to define CAN, and these cut-off values may be dependent on patient demographics and acquisition. Moreover, the variation in the individual values appears to be substantial and the use of a composite endpoint to define an abnormal 123I-mIBG study may be preferred.
Conclusion
The poor long-term prognosis in asymptomatic diabetic patients with CAN justifies early risk stratification. In the current head-to-head comparison between 123I-mIBG scintigraphy and HRV, 123I-mIBG scintigraphy identified significantly more patients with CAN than HRV. These findings suggest that 123I-mIBG myocardial scintigraphy may be suited to early detection of CAN. However, the current limitations in defining imaging abnormalities in diabetic patients need to be considered and more data are clearly needed.
Acknowledgments
Conflicts of interest
Jeroen J. Bax has received research grants from BMS Medical Imaging, GE Healthcare, St Jude, Medtronic, Boston Scientific, Biotronik, and Edwards Lifesciences. The other authors have no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Wackers FJ, Young LH, Inzucchi SE, Chyun DA, Davey JA, Barrett EJ, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27:1954–1961. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 4.Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, et al. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care. 2001;24:1793–1798. doi: 10.2337/diacare.24.10.1793. [DOI] [PubMed] [Google Scholar]
- 5.Schonauer M, Thomas A, Morbach S, Niebauer J, Schonauer U, Thiele H. Cardiac autonomic diabetic neuropathy. Diab Vasc Dis Res. 2008;5:336–344. doi: 10.3132/dvdr.2008.047. [DOI] [PubMed] [Google Scholar]
- 6.Nagamachi S, Fujita S, Nishii R, Futami S, Tamura S, Mizuta M, et al. Prognostic value of cardiac I-123 metaiodobenzylguanidine imaging in patients with non-insulin-dependent diabetes mellitus. J Nucl Cardiol. 2006;13:34–42. doi: 10.1016/j.nuclcard.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Yufu K, Takahashi N, Nakagawa M, Hara M, Saikawa T, Yoshimatsu H. Brain natriuretic peptide and cardiac autonomic function in type 2 diabetic patients. Diabetes Res Clin Pract. 2006;72:12–19. doi: 10.1016/j.diabres.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Uehara A, Kurata C, Sugi T, Mikami T, Shouda S. Diabetic cardiac autonomic dysfunction: parasympathetic versus sympathetic. Ann Nucl Med. 1999;13:95–100. doi: 10.1007/BF03164884. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi N, Nakagawa M, Saikawa T, Ooie T, Yufu K, Shigematsu S, et al. Effect of essential hypertension on cardiac autonomic function in type 2 diabetic patients. J Am Coll Cardiol. 2001;38:232–237. doi: 10.1016/S0735-1097(01)01363-8. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31(Suppl 1):S55–S60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 11.Rose G, McCartney P, Reid DD. Self-administration of a questionnaire on chest pain and intermittent claudication. Br J Prev Soc Med. 1977;31:42–48. doi: 10.1136/jech.31.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholte AJ, Schuijf JD, Kharagjitsingh AV, bbets-Schneider P, Stokkel MP, Jukema JW, et al. Different manifestations of coronary artery disease by stress SPECT myocardial perfusion imaging, coronary calcium scoring, and multislice CT coronary angiography in asymptomatic patients with type 2 diabetes mellitus. J Nucl Cardiol. 2008;15:503–509. doi: 10.1016/j.nuclcard.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 13.American Society of Nuclear Cardiology. Imaging guidelines for nuclear cardiology procedures. doi: http://www.asnc.org/section_73.cfm.
- 14.Schinkel AF, Bax JJ, Sozzi FB, Boersma E, Valkema R, Elhendy A, et al. Prevalence of myocardial viability assessed by single photon emission computed tomography in patients with chronic ischaemic left ventricular dysfunction. Heart. 2002;88:125–130. doi: 10.1136/heart.88.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewing DJ, Campbell IW, Clarke BF. Assessment of cardiovascular effects in diabetic autonomic neuropathy and prognostic implications. Ann Intern Med. 1980;92:308–311. doi: 10.7326/0003-4819-92-2-308. [DOI] [PubMed] [Google Scholar]
- 16.Schnell O, Hammer K, Muhr-Becker D, Ziegler A, Weiss M, Tatsch K, et al. Cardiac sympathetic dysinnervation in type 2 diabetes mellitus with and without ECG-based cardiac autonomic neuropathy. J Diabetes Complications. 2002;16:220–227. doi: 10.1016/S1056-8727(01)00180-5. [DOI] [PubMed] [Google Scholar]
- 17.Athyros VG, Didangelos TP, Karamitsos DT, Papageorgiou AA, Boudoulas H, Kontopoulos AG. Long-term effect of converting enzyme inhibition on circadian sympathetic and parasympathetic modulation in patients with diabetic autonomic neuropathy. Acta Cardiol. 1998;53:201–209. [PubMed] [Google Scholar]
- 18.Morguet A, Springer HJ. Microcomputer-based measurement of beat-to-beat intervals and analysis of heart rate variability. Med Prog Technol. 1981;8:77–82. [PubMed] [Google Scholar]
- 19.Sundkvist G, Almer L, Lilja B. Respiratory influence on heart rate in diabetes mellitus. Br Med J. 1979;1:924–925. doi: 10.1136/bmj.1.6168.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin AB. A simple test of cardiac function based upon the heart rate changes induced by the Valsalva maneuver. Am J Cardiol. 1966;18:90–99. doi: 10.1016/0002-9149(66)90200-1. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler D, Laux G, Dannehl K, Spuler M, Muhlen H, Mayer P, et al. Assessment of cardiovascular autonomic function: age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet Med. 1992;9:166–175. doi: 10.1111/j.1464-5491.1992.tb01754.x. [DOI] [PubMed] [Google Scholar]
- 22.Clarke BF, Ewing DJ, Campbell IW. Diabetic autonomic neuropathy. Diabetologia. 1979;17:195–212. doi: 10.1007/BF01235856. [DOI] [PubMed] [Google Scholar]
- 23.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ. 1968;56:1–188. [PubMed] [Google Scholar]
- 24.Turpeinen AK, Vanninen E, Kuikka JT, Uusitupa MI. Demonstration of regional sympathetic denervation of the heart in diabetes. Comparison between patients with NIDDM and IDDM. Diabetes Care. 1996;19:1083–1090. doi: 10.2337/diacare.19.10.1083. [DOI] [PubMed] [Google Scholar]
- 25.Estorch M, Carrio I, Berna L, Lopez-Pousa J, Torres G. Myocardial iodine-labeled metaiodobenzylguanidine 123 uptake relates to age. J Nucl Cardiol. 1995;2:126–132. doi: 10.1016/s1071-3581(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 26.Kasama S, Toyama T, Sumino H, Nakazawa M, Matsumoto N, Sato Y, et al. Prognostic value of serial cardiac 123I-MIBG imaging in patients with stabilized chronic heart failure and reduced left ventricular ejection fraction. J Nucl Med. 2008;49:907–914. doi: 10.2967/jnumed.107.047548. [DOI] [PubMed] [Google Scholar]
- 27.Ohshima S, Isobe S, Izawa H, Nanasato M, Ando A, Yamada A, et al. Cardiac sympathetic dysfunction correlates with abnormal myocardial contractile reserve in dilated cardiomyopathy patients. J Am Coll Cardiol. 2005;46:2061–2068. doi: 10.1016/j.jacc.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 28.Somsen GA, Verberne HJ, Fleury E, Righetti A. Normal values and within-subject variability of cardiac I-123 MIBG scintigraphy in healthy individuals: implications for clinical studies. J Nucl Cardiol. 2004;11:126–133. doi: 10.1016/j.nuclcard.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 30.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 31.Maser RE, Lenhard MJ. Cardiovascular autonomic neuropathy due to diabetes mellitus: clinical manifestations, consequences, and treatment. J Clin Endocrinol Metab. 2005;90:5896–5903. doi: 10.1210/jc.2005-0754. [DOI] [PubMed] [Google Scholar]
- 32.Sztajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly. 2004;134:514–522. doi: 10.4414/smw.2004.10321. [DOI] [PubMed] [Google Scholar]
- 33.Kataoka M, Ito C, Sasaki H, Yamane K, Kohno N. Low heart rate variability is a risk factor for sudden cardiac death in type 2 diabetes. Diabetes Res Clin Pract. 2004;64:51–58. doi: 10.1016/j.diabres.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 35.Ewing DJ, Campbell IW, Clarke BF. Mortality in diabetic autonomic neuropathy. Lancet. 1976;1:601–603. doi: 10.1016/S0140-6736(76)90413-X. [DOI] [PubMed] [Google Scholar]
- 36.Patel AD, Iskandrian AE. MIBG imaging. J Nucl Cardiol. 2002;9:75–94. doi: 10.1067/mnc.2002.121471. [DOI] [PubMed] [Google Scholar]
- 37.Ziegler D, Weise F, Langen KJ, Piolot R, Boy C, Hubinger A, et al. Effect of glycaemic control on myocardial sympathetic innervation assessed by [123I]metaiodobenzylguanidine scintigraphy: a 4-year prospective study in IDDM patients. Diabetologia. 1998;41:443–451. doi: 10.1007/s001250050928. [DOI] [PubMed] [Google Scholar]
- 38.Valensi P, Sachs RN, Harfouche B, Lormeau B, Paries J, Cosson E, et al. Predictive value of cardiac autonomic neuropathy in diabetic patients with or without silent myocardial ischemia. Diabetes Care. 2001;24:339–343. doi: 10.2337/diacare.24.2.339. [DOI] [PubMed] [Google Scholar]
- 39.Langer A, Freeman MR, Josse RG, Armstrong PW. Metaiodobenzylguanidine imaging in diabetes mellitus: assessment of cardiac sympathetic denervation and its relation to autonomic dysfunction and silent myocardial ischemia. J Am Coll Cardiol. 1995;25:610–618. doi: 10.1016/0735-1097(94)00459-4. [DOI] [PubMed] [Google Scholar]
- 40.Schnell O, Kirsch CM, Stemplinger J, Haslbeck M, Standl E. Scintigraphic evidence for cardiac sympathetic dysinnervation in long-term IDDM patients with and without ECG-based autonomic neuropathy. Diabetologia. 1995;38:1345–1352. doi: 10.1007/BF00401768. [DOI] [PubMed] [Google Scholar]
- 41.Schnell O, Muhr D, Weiss M, Dresel S, Haslbeck M, Standl E. Reduced myocardial 123I-metaiodobenzylguanidine uptake in newly diagnosed IDDM patients. Diabetes. 1996;45:801–805. doi: 10.2337/diabetes.45.6.801. [DOI] [PubMed] [Google Scholar]
- 42.Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 1982;285:916–918. doi: 10.1136/bmj.285.6346.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott LA, Kench PL. Cardiac autonomic neuropathy in the diabetic patient: does 123I-MIBG imaging have a role to play in early diagnosis? J Nucl Med Technol. 2004;32:66–71. [PubMed] [Google Scholar]
- 44.Schnell O, Muhr D, Dresel S, Weiss M, Haslbeck M, Standl E. Partial restoration of scintigraphically assessed cardiac sympathetic denervation in newly diagnosed patients with insulin-dependent (type 1) diabetes mellitus at one-year follow-up. Diabet Med. 1997;14:57–62. doi: 10.1002/(SICI)1096-9136(199701)14:1<57::AID-DIA297>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 45.Hattori N, Tamaki N, Hayashi T, Masuda I, Kudoh T, Tateno M, et al. Regional abnormality of iodine-123-MIBG in diabetic hearts. J Nucl Med. 1996;37:1985–1990. [PubMed] [Google Scholar]
- 46.Anan F, Takahashi N, Nakagawa M, Ooie T, Saikawa T, Yoshimatsu H. High-sensitivity C-reactive protein is associated with insulin resistance and cardiovascular autonomic dysfunction in type 2 diabetic patients. Metabolism. 2005;54:552–558. doi: 10.1016/j.metabol.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Anan F, Takahashi N, Shinohara T, Nakagawa M, Masaki T, Katsuragi I, et al. Smoking is associated with insulin resistance and cardiovascular autonomic dysfunction in type 2 diabetic patients. Eur J Clin Invest. 2006;36:459–465. doi: 10.1111/j.1365-2362.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- 48.Anan F, Masaki T, Yonemochi H, Takahashi N, Nakagawa M, Eshima N, et al. Abdominal visceral fat accumulation is associated with the results of (123)I-metaiodobenzylguanidine myocardial scintigraphy in type 2 diabetic patients. Eur J Nucl Med Mol Imaging. 2007;34:1189–1197. doi: 10.1007/s00259-007-0421-6. [DOI] [PubMed] [Google Scholar]
- 49.Anan F, Yonemochi H, Masaki T, Takahashi N, Nakagawa M, Eshima N, et al. Homocysteine levels are associated with the results of 123I-metaiodobenzylguanidine myocardial scintigraphy in type 2 diabetic patients. Eur J Nucl Med Mol Imaging. 2007;34:28–35. doi: 10.1007/s00259-006-0200-9. [DOI] [PubMed] [Google Scholar]
- 50.Howorka K, Pumprla J, Haber P, Koller-Strametz J, Mondrzyk J, Schabmann A. Effects of physical training on heart rate variability in diabetic patients with various degrees of cardiovascular autonomic neuropathy. Cardiovasc Res. 1997;34:206–214. doi: 10.1016/S0008-6363(97)00040-0. [DOI] [PubMed] [Google Scholar]
- 51.Kontopoulos AG, Athyros VG, Didangelos TP, Papageorgiou AA, Avramidis MJ, Mayroudi MC, et al. Effect of chronic quinapril administration on heart rate variability in patients with diabetic autonomic neuropathy. Diabetes Care. 1997;20:355–361. doi: 10.2337/diacare.20.3.355. [DOI] [PubMed] [Google Scholar]
- 52.Ziegler D. Diabetic cardiovascular autonomic neuropathy: prognosis, diagnosis and treatment. Diabetes Metab Rev. 1994;10:339–383. doi: 10.1002/dmr.5610100403. [DOI] [PubMed] [Google Scholar]