Abstract
The development of acute myeloid leukaemia after low-dose radioiodine therapy and its presentation as a myeloid sarcoma of the uterine cervix are both rare events. We report a case of acute myeloid leukaemia revealed by a myeloid sarcoma of the uterine cervix in a 48-year-old woman, 17 months after receiving a total dose of 100 mCi 131I for papillary thyroid cancer. A strict hematological follow-up of patients treated with any dose of 131I is recommended to accurately detect any hematological complications which might have been underestimated. Unusual presentations, such as chloroma of the uterine cervix, may reveal myeloid malignancy and should be kept in mind.
Key Words: Acute myeloid leukaemia, Myeloid sarcoma of the uterine cervix, Radioiodine therapy
Introduction
Myeloid sarcoma (MS) are solid tumours composed of immature myeloid cells involving an extramedullary site [1]. These tumours are also named chloroma in view of their green coloration due to high myeloperoxydase content [1]. MS occurs mainly in patients with known acute myeloid leukaemia (AML), myeloproliferative disorder or myelodysplastic syndrome [1, 2]. In few cases, MS may be the presenting feature of AML, and the most common sites of involvement are bones, soft tissue, lymph nodes, skin, gastrointestinal tract and body cavities [2]. Presentations in the female genital tract (FGT) are uncommon [2].
Radioiodine (131I) is used in the treatment of thyroid cancer in order to eliminate residual thyroid tissue following total thyroidectomy and to treat metastatic disease [3, 4]. It is known that ionizing radiations, including 131I, are highly effective in producing chromosomal aberrations that sometimes are linked to the occurrence of secondary leukaemia [5]. AML is an uncommon complication of exposure to 131I, occurring mostly after a cumulative dose higher than 800 mCi [3, 5]. Although cases of patients developing leukaemia after low-dose radioactive iodine are reported [3,4,5,6,7,8,9,10], the link with the treatment is still a matter of debate. We report a case of acute myeloid leukaemia revealed by a MS of the uterine cervix in a 48-year-old woman, 17 months after receiving a total dose of 100 mCi 131I for papillary thyroid cancer. We also present a review of the literature.
Case Presentation
A 48-year-old woman, gravida 3, para 3, was referred to our unit in April 2007 for a large uterine cervical mass detected during an intrauterine device change. Her medical history was marked by a total thyroidectomy for papillary carcinoma performed 17 months before. Surgical intervention was followed by radioiodine therapy. She received a single dose of 100 mCi radioiodine (131I) and a suppressive dose of thyroid hormone. Six months later she had a negative whole-body 131I scan.
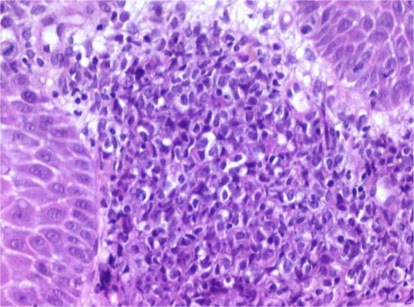
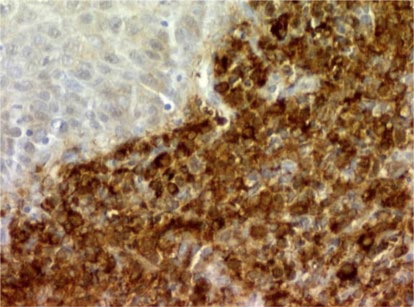
On admission, besides pallor and moderate asthenia, the physical examination revealed a large gray-green-tinged cervical mass extended to both parametriae. Her last gynecological examination and PAP smear (one year before) were normal, as were the last laboratory examinations. Cervical smear performed by the gynecologist revealed the presence of atypical squamous cells of undetermined significance (ASCUS-Bethesda System 2001). Imaging confirmed the presence of a homogeneous cervical tumour measuring 6 cm, extending to both parametriae. Large periaortic and iliac lymph nodes were detected. Cervical biopsy showed cervical stroma being infiltrated by sheets of medium sized immature cells, many of which had clear cytoplasm (fig. 1; hematoxylin and eosin stain; original magnification × 200). Immunohistochemical analysis showed that the neoplastic cells were strongly positive for myeloperoxydase (fig. 2; original magnification × 200), CD 117 and CD 34 antigens, leading to the diagnosis of MS. Laboratory examinations revealed the following values: hemoglobin 5.4 g/dl, hematocrit 16%, white blood cell count 5.1 × 103/mm3 with 75% of blast cells and a platelet count of 19 × 103/mm3. A bone marrow biopsy confirmed the diagnosis of acute AML type M2 according to the French-American-British Classification Scheme. Cytogenetic studies on bone marrow cells failed to show any translocation or inversion, but demonstrated a trisomy 3 confirmed by FISH.
Fig. 1.
Hematoxylin and eosin stain of the cervical biopsy showed cervical stroma being infiltrated by sheets of medium sized immature cells, many of which had clear cytoplasm (original magnification × 200).
Fig. 2.
Immunohistochemical analysis showed that the neoplastic cells were strongly positive for myeloperoxydase, CD 117 and CD 34 antigens (original magnification × 200).
Induction treatment was carried out using high-dose cytarabine and daunorubicin according to LAM 2006 protocol. As the medullogram showed 66% pathological blasts 15 days after the beginning of the treatment, the patient underwent reinduction therapy using the same drugs. The patient's course was complicated by febrile neutropenia and staphylococcal sepsis which was successfully treated. A post-chemotherapy medullogram done prior to discharge in May 2007 showed a hypocellular marrow with 3% blasts (1% pathological blasts). Biopsies of the female genital tract showed no leukaemic blasts. One month later, a bone marrow biopsy showed the persistence of 11% pathological blasts. A compensation treatment according to the LAM 2006 protocol (high doses of amsacrine and aracytine) was performed. The patient achieved complete remission. Consolidation courses are being administered.
Discussion
MS are solid tumours composed of immature myeloid cells involving an extramedullary site [1]. They occur mainly in patients with known AML, myeloproliferative disorder or myelodysplastic syndrome. In very few cases, MS may be the presenting feature of AML [1, 2]. MS of the uterine cervix as an initial clinical presentation of acute myeloid leukaemia is uncommon [1, 2]. Radioiodine is used in the treatment of thyroid cancer in order to eliminate residual thyroid tissue following total thyroidectomy and to treat metastatic disease [3, 4]. AML is an uncommon complication of exposure to 131I, which when it occurs, does so mostly after cumulative doses higher than 800 mCi [3, 5]. Although cases of patients developing leukaemia after low-dose radioactive iodine are reported [3,4,5,6,7,8,9,10], the link with the treatment is still a matter of debate. In this report, a routine gynecological examination led to the diagnosis of chloroma which revealed AML. The patient had received a single dose of 100 mCi 131I for the treatment of a thyroid cancer 17 months before the diagnosis.
MS occurs most commonly in bone, periosteum, soft tissue, lymph nodes and skin, whereas the FGT is rarely involved [1, 2]. The ovaries and breasts deserve special mention as sites of involvement [1], but Garcia et al. [2] suggest that the uterine cervix and ovary are involved with equal frequency. Only 23 cases of MS of the uterine cervix have been reported before [2]. The majority of patients with MS of the cervix present vaginal or postcoital bleeding. Other presenting features are abdominal pain or discomfort or a combination of these symptoms [2]. MS of the FGT are usually large and green coloured [2]. Diagnosis is especially difficult when the tumour mass is isolated with no evidence of systemic AML. In these cases, MS are frequently misdiagnosed in biopsies and the most common incorrect diagnosis cited is large cell lymphoma. Cytochemical and immunohistochemical studies are extremely helpful for establishing the correct diagnosis in such cases. Prognosis of MS of the uterine cervix is poor, ranging from an average survival of 9 months in patients without manifest leukaemia to 3.2 months for patients with manifest AML at presentation [1]. In our case the patient had no history of hematologic disorder. She was asymptomatic and an examination of peripheral blood and bone marrow after the diagnosis of FGT involvement revealed AML M2. She is still alive 6 months after the diagnosis.
The development of AML is a rare but known complication of radioiodine therapy usually occurring after a cumulative dose of more than 800 mCi. However, a few cases of AML after a lower dose of 131I have already been reported. Table 1 summarizes a review of published reports of acute myeloid leukaemia in patients treated with 131I for thyroid disorders [3,4,5,6,7,8,9,10].
Table 1.
Incidence of acute myeloid leukaemia after low-dose radioiodine treatment for thyroid disorders
| Case | Age years | Dose of 131I (mCi) | Time in months from 131I to AML onset | Type of myeloid leukaemia | Karyo-type | Reference |
|---|---|---|---|---|---|---|
| 1 | NA | 100 | 12 | Acute granulocytic | NA | Beierwaltes, 1981 |
| 2 | 19 | 230 | 24 | Acute myeloblastic | NA | Siemsen, 1970 |
| 3 | 28 | 300 | 14 | AML M4 | NA | Bitton, 1993 |
| 4 | 34 | 150 | 24 | AML M2 | NA | Roldan Schilling, 1998 |
| 5 | 43 | 150 | 60 | AML M3 | NA | Roldan Schilling, 1998 |
| 6 | 48 | 23 | 14 | AML M2 | Trisomy 8 | Laurenti, 1998 |
| 7 | 47 | 118 | 14 | AML M3 | t(15,17) | Grudeva-Popova, 2007 |
| 8 | 55 | 90 | 144 | AML M4 | Iso 7q | Focosi, 2007 |
| 9 | 51 | 22.1 | 27 | AML M3 | NA | Kolade, 2005 |
| 10 | 48 | 100 | 17 | AML M2 | Trisomy 3 | Our case, 2007 |
mCi = Millicurie; Iso = isochromosome; NA = not available.
Most cases of AML described were in patients younger than 50 years of age. Total doses of 131I were included between 23 and 300 mCi. Two groups can clearly be distinguished: patients who developed leukaemia 12 to 24 months after the radioiodine therapy, and those whose disease occurred after a period longer than 2 years. Cytogenetic analysis of bone marrow showed described chromosomal rearrangement in cases 6 and 7 (trisomy 8 and t(15,17), respectively) [6]. In our patient a trisomy 3 was highlighted. We do not know whether these cases of AML can be considered as a second neoplasia or as a secondary effect of radioactive treatment. Nevertheless, ionizing radiation, including 131I, is known to be highly involved in producing chromosome aberrations that could be linked to the occurrence of secondary leukaemia [8, 9]. It has actually been shown that the frequency of complex abnormalities in karyotypes is higher in radioiodine treatment-related AML than in de novo AML [6].
It is unclear whether treatment with low-dose radioactive iodine contributed to the development of leukaemia in the patients described. The development of AML after low-dose radioiodine could represent a therapy-induced complication. Alternatively, we could also hypothesize that the bone marrow of these patients shows individual susceptibilities that put them at greater risk for the potential damaging effect of 131I therapy. Finally, these patients could represent a part of the population that would have developed AML without any 131I therapy.
As the incidence of leukaemia after low-dose radioiodine therapy is unclear, a strict hematological follow-up of patients treated with any dose of 131I therapy is recommended to accurately detect any hematological complication which might have been underestimated. Unusual presentations, such as chloroma of the uterine cervix, may reveal myeloid malignancy and should therefore be kept in mind.
Acknowledgments
The authors thank Pr Patrick Dufour and Ms. Liane Acito-Khan for their expert assistance for the preparation of the manuscript.
References
- 1.Friedman HD, Adelson MD, Elder RC, Lemke SM. Granulocytic sarcoma of the uterine cervix - literature review of granulocytic sarcoma of the female genital tract. Gynecol Oncol. 1992;46:128–137. doi: 10.1016/0090-8258(92)90210-a. [DOI] [PubMed] [Google Scholar]
- 2.Garcia MG, Deavers MT, Knobloch RJ, Chen W, Tsimberidou AM, Manning JT, Jr, Medeiros LJ. Myeloid sarcoma involving the gynecologic tract: a report of 11 cases and review of the literature. Am J Clin Pathol. 2006;125:783–790. doi: 10.1309/H9MM-21FP-T7YB-L3PW. [DOI] [PubMed] [Google Scholar]
- 3.Roldan Schilling V, Fernandez Abellan P, Dominguez Escribano JR, Rivas Gonzalez C, Mut Barbera E, Calatayud Cendra R. Acute leukemias after treatment with radioiodine for thyroid cancer. Haematologica. 1998;83:767–768. [PubMed] [Google Scholar]
- 4.Bitton R, Sachmechi I, Benegalrao Y, Schneider BS. Leukemia after a small dose of radioiodine for metastatic thyroid cancer. J Clin Endocrinol Metab. 1993;77:1423–1426. doi: 10.1210/jcem.77.5.8077344. [DOI] [PubMed] [Google Scholar]
- 5.Laurenti L, Salutari P, Sica S, Piccirillo N, Zini G, Zollino M, Leone G. Acute myeloid leukemia after iodine-131 treatment for thyroid disorders. Ann Hematol. 1998;76:271–272. doi: 10.1007/s002770050400. [DOI] [PubMed] [Google Scholar]
- 6.Focosi D, Galimberti S, Petrini M. Acute myeloid leukemia and follicular lymphoma after very low dose radioiodine therapy for thyroid diseases. Haematologica. 2007;92:e96–e97. doi: 10.3324/haematol.11981. [DOI] [PubMed] [Google Scholar]
- 7.Grudeva-Popova J, Yaneva M, Zisov K, Ananoshtev N. Therapy-related acute promyelocytic leukemia after treatment with radioiodine for thyroid cancer: case report with literature review. J BUON. 2007;12:129–132. [PubMed] [Google Scholar]
- 8.Beierwaltes WH. New horizons for therapeutic nuclear medicine in 1981. J Nucl Med. 1981;22:549–554. [PubMed] [Google Scholar]
- 9.Siemsen JK. Leukemia following cancericidal doses of radioiodine (Abstract) J Nucl Med. 1970;11:400. [Google Scholar]
- 10.Kolade VO, Bosinski TJ, Ruffy EL. Acute promyelocytic leukemia after iodine-131 therapy for Graves' disease. Pharmacotherapy. 2005;25:1017–1020. doi: 10.1592/phco.2005.25.7.1017. [DOI] [PubMed] [Google Scholar]