Abstract
Epithelioid cell tumors presenting in the gastrointestinal tract are uncommon, but when they arise, arriving at a correct diagnosis is important. We report a case of anal malignant melanoma in an 82-year-old man who microscopically showed an epithelioid malignant tumor simulating a gastrointestinal stromal tumor. C-kit stain and Melan-A were diffusely and strongly positive, while HMB-45 was focally positive. This case illustrates the potential pitfall of relying on a single antibody or inadequate panel of immunohistochemical stains to confirm the diagnosis. We recommend to apply an adequate immunohistochemical panel which includes S-100 protein, HMB-45 and Melan-A in order to make an accurate diagnosis, and discuss the differential diagnosis and surgical treatment modalities.
Key Words: Malignant melanoma, Rectum, Anus, Immunohistochemistry, Gastrointestinal stromal tumor
Introduction
Anorectal melanomas (ARM) are uncommon neoplasias, and most of them are metastases from a cutaneous primary [1]. Primary ARM represents 0.4 to 1.6% of all melanoma manifestations [2, 3]. Nevertheless, the anorectum is the most common site of primary melanoma in the alimentary tract [4].
Malignant melanoma (MM) occurs in up to 15% of primary malignant tumors of the anus. The tumor originates from melanocytes that are normally found in the transitional zone above the dentate line. In early stages, it can mimic clinically thrombosed hemorrhoids [5]. Grossly ARM may display a polypoid mass covered by a smooth surface which may eventually be ulcerated and usually extends into the lower rectum. The epidemiology of ARM has not been studied, although its putative relevance and prognostic studies have been based on referred cases [6]. Some epidemiologic data suggest that immunodeficiency virus infection increases the risk of ARM [3, 7]. Due to its low incidence, treatment of ARM is not well standardized since it was implemented based on retrospective studies referring to a short series of cases from data collected for up to 64 years [7].
The most frequent presentation is rectal bleeding, followed by palpable mass and pain [8]. ARM presents as a large protuberant or ulcerating mass which usually extends into the lower rectum. The mass is pigmented in only one third of cases. Most patients present with lesions >2 mm thick. At the time of diagnosis about 30% of cases present distant metastases, and metastases eventually develop in 60% [6, 7, 9, 10].
Case Report
An 82-year-old man complained of rectal bleeding. Physical examination showed a polypoid and firm tumor mass which was thought to be clinically malignant. No endoscopic study was performed. Chest X-ray and whole-body computed tomography failed to show any tumor mass. The patient underwent a local transrectal resection of the anorectal mass.
Pathologic Findings
The gross specimen was a segmental anorectal resection measuring 2.6 × 1.7 × 0.7 cm. In the center, a polypoid well-delimitated lesion, measuring 1.7 cm in diameter, which was whitish and elastic, was observed. Microscopically, the tumor was located in the submucosa and muscularis mucosae and extended to rectal mucosa infiltrating lamina propria. Extensive areas of ulceration were present. No junctional activity or connection was observed with the lining squamous upper mucosa. The tumor cells were epithelioid with abundant cytoplasms and paranuclear clear spaces. The nuclei were round and showed prominent nucleoli. Mitotic figures were frequent and atypical, averaged 16 per 10 high-power fields. Vascular or perineural invasion was not observed. The surgical margins were free of tumor. The tumor cells showed strong positivity for vimentin (V9), CD117 (C-kit), S-100 protein, and MART-1 (Melan-A). HMB-45 stain was focally positive at the inner portion of the tumor (20% of the cells). Stains for cytokeratins, smooth muscle actin, desmin, h-caldesmon, chromogranin, synaptophysin and CD34 (QBEN-10) were negative. With ki67 the proliferative activity was 60%. A diagnosis of submucosal amelanotic malignant melanoma, ulcerated of the anus, Breslow 2.8 mm thick, was made. After the diagnosis, the patient was examined by a dermatologist and no skin lesion suggestive of cutaneous melanoma was observed. The patient denied any complementary surgical treatment. Currently, 5 months after the diagnosis, the patient is well and free of tumor.
Discussion
Anal melanomas exhibit the same immunohistochemical and ultrastructural features as their cutaneous counterparts [11]. However, similar to melanomas arising in other mucous membranes, they are typical of the acrolentiginous type. Invasive melanomas in this region are commonly epithelioid, although spindle cell and desmoplastic variants have been described.
Differentiation of anal malignant amelanotic epithelioid melanoma from Paget's disease, lymphoma, undifferentiated carcinoma and gastrointestinal stromal tumor (GIST) can be difficult on the basis of histologic criteria alone. Immunostains are of invaluable help to make the diagnosis accurately. In Paget's disease, the tumor cells should stain with cytokeratins. Lymphoma should stain for CD45 and CDs. Undifferentiated carcinoma may stain with chromogranin and synaptophysin. The most challenging diagnosis in this particular case were GIST and epithelioid leiomyosarcomas. The microscopic appearance should indicate a malignant epithelioid GIST, with high risk of metastasis because of the presence of nuclear atypia and large number of mitosis/HPF. Although it is infrequent to find a high-grade GIST measuring less than 2 cm, it could be possible. Rectal GISTs are nearly always positive for CD34 [12]. Unlike leiomyomas and leiomyosarcomas, colorectal GISTs rarely present as mucosal polyps because they arise within the muscularis propria [12, 13]. Besides, those muscular tumors stain positively with smooth muscle antigens (desmin and caldesmon), all of which were negative in our case. It should be kept in mind that c-kit positivity is not specific of GIST. In fact it is well-known that leukemias, some lymphomas, germinal cell tumors, and melanomas show positive results with this antibody, and awareness of this is important in order to prevent a diagnostic error with treatment and outcome consequences [14,15,16]. Further, its application should be interpreted in an appropriate context. Chute el al. studied a series of 17 cases of ARM and reported that c-kit is frequently expressed (75%), although specific melanoma markers HMB-45 and Melan-A are not always expressed in this tumor [17]. On the other hand, melanomas may not always uniformly stain for HMB-45. It should be realized that MART-1 (Melan-A) may be positive in cases of melanomas which completely lack reactivity or show a heterogeneous pattern of reactivity with HMB-45 stain, as in the case discussed here. Recently, Seya et al. [18] reported a rectal malignant melanoma that was misdiagnosed in the endoscopic biopsy as neuroendocrine carcinoma or GIST because of the reactivity with CD56, S100 and CD117, and a malignant melanoma was ruled out because HMB-45 was negative. However, the study of the surgical specimen showed HMB-45 and Mart-1 reactivity in both biopsy and surgical specimen [18]. The phenotypic variability is most likely due to tumor heterogeneity or suboptimal fixation. Similarly, if our case had been endoscopically biopsied and if a limited immunohistochemical panel of antibodies would have been employed, such as cytokeratins-, CD34-/+, Ckit+ and HMB-45- (in the main part of the tumor mass, nearly 80%), a misdiagnosis of GIST would have been more likely. Therefore, we favor the application of an adequate immunohistochemical panel in epithelioid tumors of the anorectal region in order to rule out leiomyosarcoma, GIST or MM, which pursue different biological behaviors. In the case of suspected malignant melanoma, this panel should include S-100, vimentin and both specific antibodies HMB-45 and Mart-1. Once a diagnosis of malignant melanoma has been established, it must be determined whether the tumor is primary or metastatic. The presence of junctional activity beneath the squamous epithelium favors a primary malignant melanoma, although it may be obscured by ulceration. In fact, our case showed large areas of ulceration which could miss this important key feature. However, the absence of the obviously helpful history of primary melanoma elsewhere should it exist; we assumed confidently that the tumor was primarily from the anorectal region.
The prognosis of anal melanoma is poor, regardless of the type of treatment [7, 10, 19], with a mean 5-year survival of approximately 15% [3, 20], ranging from 6 to 31% [21, 22]. Thus, ARM may represent a systemic disease at the time of diagnosis in most cases, and the choice of surgical or other local procedures to amend the primary tumor may not have great influence on the systemic course of the disease. Most recurrences occur within the first 30 months, and common sites are the inguinal lymph nodes, lung, liver and bones. Local recurrence is almost always associated with or followed by regional or disseminated disease. The thickness of the tumor measured from the top of the overlying intact mucosa or ulcerated tumor correlates with the outcome. Anal melanomas of less than 2.0 mm in thickness have better prognosis than those greater than 2.0 mm in thickness.
Regarding the surgical treatment of ARM, it is a matter of debate between wide local excision (WLE) and abdominoperineal resection (APR). A meta-analysis which included compiled data of 426 patients failed to show the advantage of either approach with respect to the overall survival [19]. It should be noted, however, that in most studies it was difficult to compare patients’ prognostic factors due to the fact that the information provided was insufficient. Nevertheless, there was a trend for local disease to be more effectively controlled by APR than WLE [4, 10, 19, 23]. Ballo et al. [22] reported that adjuvant radiation of the pelvis and inguinal lymph nodes after WLE showed similar rates of local tumor control as APR. WLE should be the procedure of choice for other investigators, because the alternative of APR may have a high mortality rate and lack of survival advantages [24].
In ARM treatment, therapeutic strategies should be adjusted to the prognosis of the disease. Prognostic parameters still remain to be defined. Only a few studies have addressed this question. Goldman et al. [9] reported a correlation between overall survival and the size of the tumor. Among 33 patients, only 2 were long-term survivors and the tumor was less than 2 cm in diameter. The tumor thickness was below 2.5 mm. Unfortunately, the histopathologic data were not provided for all patients. On the other hand, Slingluff et al. [21] suggested that the only significant value for survival was the stage of disease at the time of diagnosis, whereas Ballo et al. reported a favorable disease-free survival with less than 4 mm of tumor thickness [22]. More recently, a series of 19 patients with ARM were studied [25]. The median tumor thickness was 10 mm (range 0.6-40 mm). 9 out of 19 patients presented either regional or distant metastases at the time of diagnosis. The remaining 13 patients were treated with curative intent (APR or WLE). There were no significant differences in overall survival. The incidence of local recurrences was lower following APR even for patients with less favorable tumors. The authors concluded that WLE alone is not sufficient for local tumor control in thick ARM.
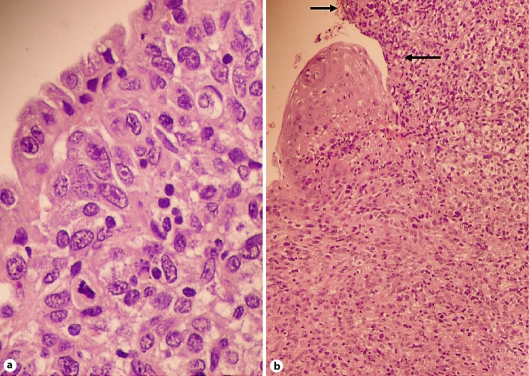
Fig. 1.
a Anal malignant melanoma lined by epithelial mucinous cells. HE. ×400. b Rectal malignant melanoma lined by squamous rectal epithelium. Note the large ulcerated areas (arrows). HE. ×400.
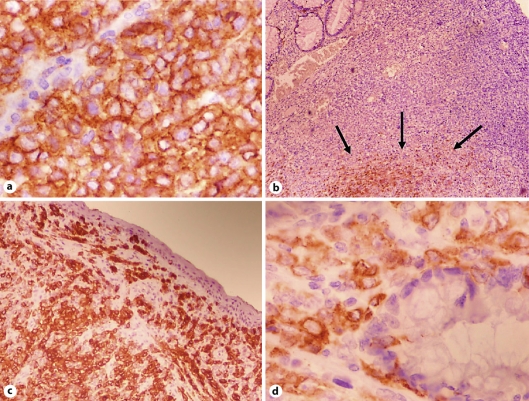
Fig. 2.
a Tumor cells showed strong and diffuse reactivity with C-kit. CD117 stain ×400. b Only 20% of tumor cells showed positivity with HMB-45, in the deeper portion (arrows). HMB-45 stain ×100. c The tumor showed diffuse and strong positivity with Melan-A. Note the absence of junctional features. Mart-1 ×100. d Epithelioid atypical cells infiltrating rectal glands. Mart-1 ×400.
References
- 1.McDermott VG, Low VH, Keogan MT, et al. Malignant melanoma metastatic to the gastrointestinal tract. AJR Am J Roentgenol. 1996;166:809–813. doi: 10.2214/ajr.166.4.8610555. [DOI] [PubMed] [Google Scholar]
- 2.Wanebo HJ, Woodruff JM, Farr GH, Quan SH. Anorectal melanoma. Cancer. 1981;47:1891–1900. doi: 10.1002/1097-0142(19810401)47:7<1891::aid-cncr2820470730>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 3.Cagir B, Whiteford MH, Topham A, et al. Changing epidemiology of anorectal melanoma. Dis Colon Rectum. 1999;42:1203–1208. doi: 10.1007/BF02238576. [DOI] [PubMed] [Google Scholar]
- 4.Roumen RM. Anorectal melanoma in The Netherlands: a report of 63 patients. Eur J Surg Oncol. 1996;22:598–601. doi: 10.1016/s0748-7983(96)92346-x. [DOI] [PubMed] [Google Scholar]
- 5.Felz MW, Winburn GB, Kallab AM, Lee JR. Anal melanoma: an aggressive malignancy masquerading as hemorrhoids. South Med J. 2001;94:880–885. [PubMed] [Google Scholar]
- 6.Weinstock MA. Epidemiology and prognosis of anorectal melanoma. Gastroenterology. 1993;104:174–178. doi: 10.1016/0016-5085(93)90849-8. [DOI] [PubMed] [Google Scholar]
- 7.Brady MS, Kavolius JP, Quan SH. Anorectal melanoma. A 64-year experience at Memorial Sloan-Kettering Cancer Center. Dis Colon Rectum. 1995;38:146–151. doi: 10.1007/BF02052442. [DOI] [PubMed] [Google Scholar]
- 8.Angeras U, Jönsson N, Jönsson PE. Primary anorectal malignant melanoma. J Surg Oncol. 1983;22:261–264. doi: 10.1002/jso.2930220411. [DOI] [PubMed] [Google Scholar]
- 9.Goldman S, Glimelius B, Pahlman L. Anorectal malignant melanoma in Sweden. Report of 49 patients. Dis Colon Rectum. 1990;33:874–877. doi: 10.1007/BF02051925. [DOI] [PubMed] [Google Scholar]
- 10.Ross M, Pezzi C, Pezzi T, et al. Patterns of failure in anorectal melanoma. A guide to surgical therapy. Arch Surg. 1990;125:313–316. doi: 10.1001/archsurg.1990.01410150035007. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Izhak O, Levy R, Weill S, et al. Anorectal malignant melanoma. A clinicopathologic study, including immunohistochemistry and DNA flow cytometry. Cancer. 1997;79:18–25. doi: 10.1002/(sici)1097-0142(19970101)79:1<18::aid-cncr4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen M, Sarlomo-Rikala M, Sobin LH. Mesenchymal tumors of muscularis mucosae of colon and rectum are benign leiomyomas that should be separated from gastrointestinal stromal tumors - a clinicopathologic and immunohistochemical study of eighty-eight cases. Mod Pathol. 2001;14:950–956. doi: 10.1038/modpathol.3880417. [DOI] [PubMed] [Google Scholar]
- 13.Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Gastrointestinal stromal tumors and leiomyosarcomas in the colon: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases. Am J Surg Pathol. 2000;24:1339–1352. doi: 10.1097/00000478-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Furitsu T, Tsujimura T, Tono T, et al. Identification of mutations in the coding sequence of the proto-oncogene c-Kit in a human mast cell leukemia cell line causing ligand-independent activation of c-Kit product. J Clin Invest. 1993;92:1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longley BJ, Tyrrell L, Lu SZ, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in human mast cell neoplasm. Nat Genet. 1996;12:312–314. doi: 10.1038/ng0396-312. [DOI] [PubMed] [Google Scholar]
- 16.Palumbo C, van Roozendaal K, Gillis AJ, van Gurp RH, de Munnik H, Oosterhuis JW, van Zoelen EJ, Looijenga LH. Expression of the PDGF alpha-receptor 1.5 kb transcript, OCT-4, and c-KIT in human normal and malignant tissues. Implications for the early diagnosis of testicular germ tumours and for our understanding of regulatory mechanisms. J Pathol. 2002;196:467–477. doi: 10.1002/path.1064. [DOI] [PubMed] [Google Scholar]
- 17.Chute DJ, Cousar JB, Mills SE. Anorectal malignant melanoma: morphologic and immunohistochemical features. Am J Clin Pathol. 2006;126:93–100. doi: 10.1309/DVWL-TV8F-FKC3-L80H. [DOI] [PubMed] [Google Scholar]
- 18.Seya T, Tanaka N, Shinji S, Shinji E, Yokoi K, Horiba K, Kanazawa Y, Yamada T, Oaki Y, Tajiri T. Case of rectal malignant melanoma showing immunohistochemical variability in a tumor. J Nippon Med Sch. 2007;74:377–381. doi: 10.1272/jnms.74.377. [DOI] [PubMed] [Google Scholar]
- 19.Thibault C, Sagar P, Nivatvongs S, et al. Anorectal melanoma - an incurable disease? Dis Colon Rectum. 1997;40:661–668. doi: 10.1007/BF02140894. [DOI] [PubMed] [Google Scholar]
- 20.Rossetti C, Koukouras D, Eboli M, et al. Primary anorectal melanomas: an institutional experience. J Exp Clin Cancer Res. 1997;16:81–85. [PubMed] [Google Scholar]
- 21.Slingluff CL, Jr, Vollmer RT, Seigler HF. Anorectal melanoma: clinical characteristics and results of surgical management in twenty-four patients. Surgery. 1990;107:1–9. [PubMed] [Google Scholar]
- 22.Ballo MT, Gershenwald JE, Zagars GK, et al. Sphincter-sparing local excision and adjuvant radiation for anal-rectal melanoma. J Clin Oncol. 2002;20:4555–4558. doi: 10.1200/JCO.2002.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Abbas JS, Karakousis CP, Holyoke ED. Anorectal melanoma: clinical features, recurrence and patient survival. Int Surg. 1980;65:423–426. [PubMed] [Google Scholar]
- 24.Droesch JT, Flum DR, Mann GN. Wide local excision or abdominoperineal resection as the initial treatment for anorectal melanoma? Am J Surg. 2005;189:446–449. doi: 10.1016/j.amjsurg.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Weyandt GH, Eggert AO, Houf M, Raulf F, Bröcker EB, Becker JC. Anorectal melanoma: surgical management guidelines according to tumour thickness. Br J Cancer. 2003;89:2019–2022. doi: 10.1038/sj.bjc.6601409. [DOI] [PMC free article] [PubMed] [Google Scholar]