Abstract
We report on a patient with carboplatin-induced bilateral papilledema, as it was described in the 1970s for cisplatin. Loss of visual accuracy up to full blindness, often loss of color vision and scotomas can be seen as a result of cortical blindness, macula degeneration, retrobulbar neuritis and papilledema. These symptoms are mostly unilateral and initially mild, so that more chemotherapy is given before the diagnosis is made. The symptoms are usually reversible within weeks to months after cessation of the platinum treatment. The therapeutic strategy is stopping the platinum treatment. In addition the empiric use of corticosteroids is suggested.
Key Words: Carboplatin, Cisplatin, Papilledema, Visual impairment
Introduction
Mild visual impairment is common during chemotherapy. Occasionally, however, it can be a sign of a more serious, often unrecognized condition. We report on a patient with carboplatin-induced bilateral papilledema resulting in only partially reversible visual impairment. Despite the fact that carboplatin is widely used in the treatment of a variety of common tumors (including cancer of the lung, ovary, testis), this is the first report of carboplatin-induced papilledema following regular AUC-determined dose. Reported cases of papilledema after carboplatin administration include two cases after high-dose carboplatin (AUC12) [1], one report using carboplatin and cisplatin simultaneously [2], and two patients to whom carboplatin was given in a fixed standard dose (400 mg/m2) [3] rather than in a creatinine-clearance dependent dose (AUC). Papilledema after intracarotid carboplatin application [4] have also been observed.
Materials and Methods
A 70-year-old woman with stage IIIc (FIGO) serous-papillary ovarian carcinoma started postoperative chemotherapy 5 weeks after optimal surgical debulking (TAH+BSO) in September 2005. Concurrent medical conditions included stable coronary heart disease, controlled arterial hypertension and mild urinary retention as sole sequel of a previous injury to the lower back. Baseline renal function was normal with a calculated creatinine clearance of 100 ml/min (Cockroft formula). Chemotherapy consisted of a 3-weekly intravenous administration of carboplatin (AUC 5 mg/ml × min) over 30 min and was well tolerated, grade 1 nausea, fatigue and grade 2 thrombocytopenia (53 × 109/l) being the only adverse effects. On day 8 of the 4th cycle, however, the patient was admitted because of nausea, constipation and acute urinary retention. Post-renal acute renal failure (calculated creatinine clearance 40 ml/min) was completely reversible after catheterization. At this time, she first complained about visual impairment in her right eye (lack of focus and scattered blind spots). Other neurological signs were absent.
Investigations and Differential Diagnosis
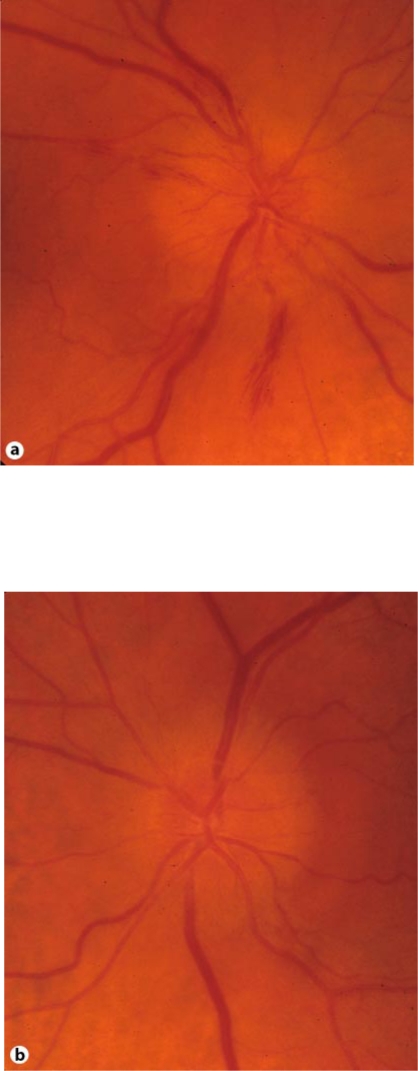
An ophthalmologic examination revealed a bilateral papillary edema (fig. 1a, b). The optic nerve head was at that time more prominent in the right eye than in the left eye, and there were some hemorrhages within the nerve fiber layer. Visual acuity was slightly reduced in both eyes. Differential diagnosis of increased intracranial pressure due to meningeosis carcinomatosa or cerebral metastasis and anterior ischemic optic neuropathy (AION) due to anemia were discussed. An MRI of the brain showed no signs of intracranial pressure, leptomeningeal carcinomatosis or pathology of the optic nerve.
Fig. 1.
a, b Bilateral papillary edema after 4th cycle.
The serum tumor marker CA-125 had normalized (from 1,541 U/ml pre-, and 114 U/ml postoperatively) and complete remission was confirmed by abdominal ultrasound and chest X-ray. Chemotherapy was therefore continued. However, after the 5th cycle the visual acuity decreased further, and new visual field losses in the left eye appeared. At the same time an increase of the bilateral papilledema was observed, particularly in the left eye. In contrast, the hemorrhages in the right eye had almost disappeared, and some signs of ischemia were now noted. Visual acuity had dropped to 20/100 in the left eye and there was a left relative afferent pupillary defect. Diagnostic lumbar puncture was performed and intracranial pressure was measured at 10 cm H2O. Based on the normal intracranial pressure, the differential diagnosis of pseudotumor cerebri, a condition characterized by ophthalmopathy, papilledema and elevated intracranial pressure, was dismissed. Biochemical (protein, glucose, LDH) and cytological examination of the cerebral fluid was normal, without malignant cells. Tests for specific antibodies were not diagnostic: anti-CNS and anti-Yo antibodies titers were (marginally) elevated in the serum, but not in the cerebral fluid. Serum titers of anti-Hu, anti-Ri, anti-Amphiphysin, anti-CV2, anti-Tr, anti-Ma-antibodies and antibodies against retinal rods, cones, Mueller cells as well as plexiform structures were all negative. In view of these results, an autoimmune syndrome - unusual in the context of ovarian cancer - seemed very unlikely.
Diagnosis
Based on the history, the clinical picture and the exclusion of other conditions via MRI, cerebral fluid examination and serum antibody-titers, the diagnosis of bilateral carboplatin-induced papilledema was made.
Management and Follow-Up
Chemotherapy was stopped after the 5th cycle and Prednisolone was initiated at a dose of 100 mg/d for 2 weeks, then tapered over 10 weeks. While the visual acuity in the right eye was stable, the left eye improved over 2 years. At this follow-up examination (2 years after cessation of chemotherapy), the optic nerve head showed some signs of optic atrophy with increased pallor and reduced visibility of the nerve fiber layer. This atrophy was more pronounced in the left eye.
Discussion
In our case report, we postulate a direct toxic effect of carboplatin, as it was described in the 1970s for cisplatin [5,6,7,8,9,10,11,12,13]. In the case of cisplatin cortical blindness, macula degeneration, retrobulbar neuritis and papilledema were described. Clinically, a loss of visual accuracy up to full blindness, often loss of color vision and scotomas were seen. Usually these symptoms are unilateral and initially mild, so that more chemotherapy is given before the diagnosis is made. A correlation with renal insufficiency and arterial hypertension was reported [1, 2, 3]. The symptoms are usually reversible within weeks to months after cessation of the platinum treatment [2, 3], and the restoration of color vision can take up to 1 year [13]. It seems to be a cumulative toxicity, as all cases occurred after several cycles of chemotherapy. Furthermore, pharmacokinetic studies of cisplatin show a continual rise of the drug concentration in the cerebrospinal fluid with each cycle [14]. It is possible that the cerebrospinal fluid concentration is important for the occurrence of this toxicity: in one patient with blindness and a seizure equally high concentrations of cisplatin in the cerebrospinal fluid and the serum were measured [15]. There are no proven therapeutic interventions; corticosteroids are used empirically.
The early recognition of this potentially severe side effect is paramount, since stopping chemotherapy before irreversible damage occurs is crucial.
Conclusion
Clinically relevant papilledema is a rare, but important side effect of carboplatin. It can occur at standard doses using creatinine-clearance dependent dose calculations (AUC) and it can be partly irreversible. Since carboplatin is extensively used in a variety of common tumors in the palliative as well as the curative setting, this potentially severe side effect has to be kept in mind. Early recognition followed by cessation of further carboplatin administration is crucial.
Acknowledgment
Written consent for publication was obtained from the patient.
References
- 1.O'Brien ME, et al. Blindness associated with high-dose carboplatin. Lancet. 1992;339:558. doi: 10.1016/0140-6736(92)90384-f. [DOI] [PubMed] [Google Scholar]
- 2.Caraceni A, et al. Recovering optic neuritis during systemic cisplatin and carboplatin chemotherapy. Acta Neurol Scand. 1997;96:260–261. doi: 10.1111/j.1600-0404.1997.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 3.Rankin EM, Pitts JF. Ophthalmic toxicity during carboplatin therapy. Ann Oncol. 1993;4:337–338. doi: 10.1093/oxfordjournals.annonc.a058497. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe W, et al. Severe ocular and orbital toxicity after intracarotid injection of carboplatin for recurrent glioblastomas. Graefes Arch Clin Exp Ophthalmol. 2002;240:1033–1035. doi: 10.1007/s00417-002-0573-9. [DOI] [PubMed] [Google Scholar]
- 5.Al-Tweigeri T, Magliocco AM, DeCoteau JF. Cortical blindness as a manifestation of hypomagnesemia secondary to cisplatin therapy: case report and review of literature. Gynecol Oncol. 1999;72:120–122. doi: 10.1006/gyno.1998.5211. [DOI] [PubMed] [Google Scholar]
- 6.Becher R, et al. Peripheral neuropathy and ophthalmologic toxicity after treatment with cis-dichlorodiaminoplatinum II. J Cancer Res Clin Oncol. 1980;96:219–222. doi: 10.1007/BF00405506. [DOI] [PubMed] [Google Scholar]
- 7.Cohen RJ, et al. Transient left homonymous hemianopsia and encephalopathy following treatment of testicular carcinoma with cisplatinum, vinblastine, and bleomycin. J Clin Oncol. 1983;1:392–393. doi: 10.1200/JCO.1983.1.6.392. [DOI] [PubMed] [Google Scholar]
- 8.Gregg RW, et al. Cisplatin neurotoxicity: the relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. J Clin Oncol. 1992;10:795–803. doi: 10.1200/JCO.1992.10.5.795. [DOI] [PubMed] [Google Scholar]
- 9.Martin M, Weber-Varszegi J, Flammer J. [Toxic optic neuropathy due to cisplatin therapy: a case report] Klin Monatsbl Augenheilkd. 2005;222:244–247. doi: 10.1055/s-2005-858020. [DOI] [PubMed] [Google Scholar]
- 10.Ostrow S, et al. Cis-Dichlorodiammine platinum and adriamycin therapy for advanced gynecological and genitourinary neoplasms. Cancer. 1980;46:1715–1721. doi: 10.1002/1097-0142(19801015)46:8<1715::aid-cncr2820460802>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 11.Ostrow S, et al. Ophthalmologic toxicity after cis-dichlorodiammineplatinum(II) therapy. Cancer Treat Rep. 1978;62:1591–1594. [PubMed] [Google Scholar]
- 12.Walsh TJ, et al. Neurotoxic effects of cisplatin therapy. Arch Neurol. 1982;39:719–720. doi: 10.1001/archneur.1982.00510230045013. [DOI] [PubMed] [Google Scholar]
- 13.Wilding G, et al. Retinal toxicity after high-dose cisplatin therapy. J Clin Oncol. 1985;3:1683–1689. doi: 10.1200/JCO.1985.3.12.1683. [DOI] [PubMed] [Google Scholar]
- 14.Hill JM, et al. Clinical studies of Platinum Coordination compounds in the treatment of various malignant diseases. Cancer Chemother Rep. 1975;59:647–659. [PubMed] [Google Scholar]
- 15.Berman IJ, Mann MP. Seizures and transient cortical blindness associated with cis-platinum (II) diamminedichloride (PDD) therapy in a thirty-year-old man. Cancer. 1980;45:764–766. doi: 10.1002/1097-0142(19800215)45:4<764::aid-cncr2820450425>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]