Abstract
Metastatic prostate cancer is an incurable disease. After a period of hormone sensitivity that allows for the use of antiandrogens, the disease invariably progresses to a situation of androgen-independent growth, which deserves the consideration or the use of chemotherapy. As many of these patients are elderly and fragile, treatment with chemotherapy is challenging. Therefore, new drugs are required. Preclinical evidence supports the role of estrogen receptor (ER) signaling in prostate cancer. In this paper, we report the first published evidence of PSA control in a patient with metastatic prostate cancer treated with fulvestrant acetate.
Key Words: Fulvestrant acetate, Prostate cancer, PSA decrease
Introduction
In the United States, prostate cancer (PCa) is the second leading cause of cancer death in men. Approximately 50% of men with PCa have locally advanced or metastatic disease [1], and 30% of patients with apparent localized disease have biochemical relapse after the first line of treatment [2]. Androgen ablation therapy is the mainstay treatment for metastatic PCa [3]; however, most neoplasms ultimately become androgen refractory, at which time virtually no effective therapies are available. Therefore, there is a strong demand for alternatives to the treatment of androgen-independent PCa (AIPC).
Fulvestrant belongs to the SERD class of estrogen receptor (ER) antagonists and has shown no estrogen-agonist activity in either preclinical or clinical studies [4, 5]. Fulvestrant binds competitively to the ER, inhibits receptor dimerization [6], and reduces the receptor's half-life by increasing protein turnover [7]. Thus, fulvestrant's mechanism of action is different from that of tamoxifen. In fact fulvestrant is recommended for the treatment of ER-positive metastatic breast cancer in postmenopausal women with disease progression following acquired tamoxifen resistance [8].
Recently, interest in using estrogenic therapies for advanced PCa has reemerged, primarily in response to the published results [9].
Estrogen receptor beta (ER-β) is the predominant ER subtype expressed in normal basal epithelial cells of the prostate, in local PCa, and in PCa metastasized to the lymph nodes and bones [10]. It has been shown that it is expressed in abundance in most established PCa cell lines, including DU145, which we found to express only the ER-β subtype of ER [11, 12]. Collectively, these data suggest that ER-β may confer survival advantages to PCa cells [9]. Thus, targeted activation or blockade of ER-β action with selective ligands may present an attractive strategy for the therapeutic intervention of PCa. Cell growth inhibition of the DU145 prostate cancer cell line by the effect of fulvestrant by an ER-β-dependent mechanism has been reported [11].
Androgen receptor (AR) expression is retained in a significant proportion of AIPC [13, 14]. AR seems to be a key protein involved in many cases of AR-dependent PCa and is critical for promoting prostate cancer cell growth. Therefore, targeting the AR for down-regulation or degradation could be a useful approach for decreasing AR-dependent prostate cancer cell growth and for treating AIPC [15].
Bhattacharyya et al. [16] published interesting results treating prostate cancer cell lines with fulvestrant. After 6 days of fulvestrant treatment, a 70% growth inhibition was seen in androgen-stimulated LNCaP cells, showing that fulvestrant is a potent AR downregulator that can produce significant inhibition of prostate cancer cell growth and suggesting a strategy for treating prostate cancer with fulvestrant [16]. Very interesting results about the potential insulin growth factor receptor-1 (IGR-1) inhibition with the combination of fulvestrant and finasteride have also been published [17].
A phase II trial was recently published on the use of fulvestrant in castration-resistant prostate cancer patients, and no evidence of activity was detected [18].
Clinical Case
A 83-year-old male patient was diagnosed with a Gleason 6 metastatic prostate cancer to the bones in October 2006. Initially, he was treated with goserelin acetate (GA) 10.4 mg-depot quarterly, and oral bicalutamide 50 mg on a daily basis and 4 mg of monthly intravenous Zoledronia Acid.
The initial prostate-specific antigen (PSA) level was 89 ng/ml (<4 ng/ml). After 3 months of treatment, the PSA level was reduced to 1.5 ng/ml. In May 2007, the PSA level was raised to 17 ng/ml; subsequently, GA was discontinued and the daily dose of bicalutamide was increased to 150 mg. In May 2008, the PSA level reached the level of 120 ng/ml, and the patient began to suffer from bone pain. Bicalutamide was stopped and, as the patient refused the possibility of chemotherapy with docetaxel 75 mg/m2 every 3 weeks and oral prednisone 5 mg twice daily, he was offered treatment continuation with oral cyproterone acetate 50 mg every 8 h. In September 2008, the PSA level was 334 ng/ml, and the patient began to suffer from bone pain. We discussed different treatment possibilities with the patient and decided to request authorization of the Spanish Health Authorities for treatment with fulvestrant under compassionate use, based on our previous experience with 2 other patients.
After we had received authorization, we began treatment with a loading dose strategy, namely 500 mg intramuscularly (IM) every 2 weeks during the first month, and 250 mg IM monthly thereafter. This strategy follows the CONFIRM study, as a dose-response existence is supposed for fulvestrant [19, 20].
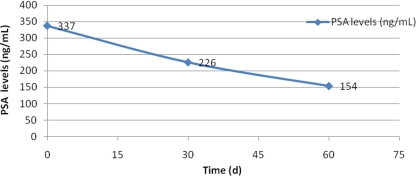
The first dose was administered in October 2008. After completion of the first month, the PSA level fell to 226 ng/ml, and in January 2008, the PSA level was 154 ng/ml (fig. 1). The patient's analgesia requirements also diminished. Fulvestrant has not been combined with any other drug. No side effect has been documented.
Fig. 1.
PSA level dynamics.
Discussion
Treatment of AIPC is a current challenge in oncology. Docetaxel-based chemotherapy has demonstrated capacity for increasing overall survival in AIPC [21], but its side effects are relevant. Many of our patients are elderly and fragile men with significant comorbidities. It is urgent to have new agents with new mechanisms of action available in the clinic for the management of metastatic PCa that do not bear a potentially high cost in terms of side effects for these patients. Fulvestrant is an interesting drug to be studied in this context because of its novel mechanism of action and its low side-effect profile.
The present case report is our third PSA response to fulvestrant in PCa. The observed activity is impressive. Any relevant side effect has been recorded following the adverse effects reported by Chadha et al. [18] in their phase II trial. This group did not describe any relevant toxicity in any of their recruited patients.
In view of this reported activity we consider it justified to continue with the investigation of fulvestrant in patients with advanced PCa and with the inclusion of fulvestrant in the design of future trials for the treatment of PCa. We are beginning to treat similar patients with fulvestrant, and we will communicate our results in future papers.
References
- 1.Bott SR. Management of recurrent disease after radical prostatectomy. Prostate Cancer Prostatic Dis. 2004;7:211–216. doi: 10.1038/sj.pcan.4500732. [DOI] [PubMed] [Google Scholar]
- 2.Loberg RD, Fielhauer JR, Pienta BA, et al. Prostate-specific antigen doubling time and survival in patients with advanced metastatic prostate cancer. Urology. 2003;62(suppl 1):128–133. doi: 10.1016/j.urology.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Taplin ME, Ho SM. Clinical review 134: the endocrinology of prostate cancer. J Clin Endocrinol Metab. 2001;86:3467–3477. doi: 10.1210/jcem.86.8.7782. [DOI] [PubMed] [Google Scholar]
- 4.Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SG, et al. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst. 1995;87:746–750. doi: 10.1093/jnci/87.10.746. [DOI] [PubMed] [Google Scholar]
- 5.Nuttall ME, Pendrak I, Emery JG, Nadeau DP, et al. Antagonism of oestrogen action in human breast and endometrial cells in vitro: potential novel antitumour agents. Cancer Chemother Pharmacol. 2001;47:437–443. doi: 10.1007/s002800000259. [DOI] [PubMed] [Google Scholar]
- 6.Fawell SE, White R, Hoare S, Sydenham M, Page M, Parker MG. Inhibition of estrogen receptor-DNA binding by the ‘pure’ antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci USA. 1990;87:6883–6887. doi: 10.1073/pnas.87.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dauvois S, Danielian PS, White R, Parker MG. Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proc Natl Acad Sci USA. 1992;89:4037–4041. doi: 10.1073/pnas.89.9.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howell A, Robertson JF, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20:3396–3403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 9.Ho SM. Estrogens and anti-estrogens: key mediators of prostate carcinogenesis and new therapeutic candidates. J Cell Biochem. 2004;91:491–503. doi: 10.1002/jcb.10759. [DOI] [PubMed] [Google Scholar]
- 10.Leav I, Lau KM, Adams JY, et al. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159:79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau KM, LaSpina M, Long J, Ho SM. Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res. 2000;60:3175–3182. [PubMed] [Google Scholar]
- 12.Mobley JA, L'Esperance JO, Wu M, Friel CJ, Hanson RH, Ho SM. The novel estrogen 17alpha-20Z-21-[(4-amino)phenyl]-19-norpregna-1,3,5(10),20-tetraene-3,17beta-diol induces apoptosis in prostate cancer cell lines at nanomolar concentrations in vitro. Mol Cancer Ther. 2004;3:587–595. [PubMed] [Google Scholar]
- 13.Culig Z, Bartsch G. Androgen axis in prostate cancer. J Cell Biochem. 2006;99:373–381. doi: 10.1002/jcb.20898. [DOI] [PubMed] [Google Scholar]
- 14.Santos AF, Huang H, Tindall DJ. The androgen receptor: a potential target for therapy of prostate cancer. Steroids. 2004;69:79–85. doi: 10.1016/j.steroids.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Scher HI, Buchanan G, Gerald W, Butler LM, Tilley WD. Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr Relat Cancer. 2004;11:459–476. doi: 10.1677/erc.1.00525. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharyya RS, Krishnan AV, Swami S, Feldman D. Fulvestrant (ICI 182,780) down-regulates androgen receptor expression and diminishes androgenic responses in LNCaP human prostate cancer cells. Mol Cancer Ther. 2006;5:1539–1549. doi: 10.1158/1535-7163.MCT-06-0065. [DOI] [PubMed] [Google Scholar]
- 17.Huynh H, Alpert L, Alaoui-Jamali MA, Ng CY, Chan TW. Co-administration of finasteride and the pure anti-oestrogen ICI 182,780 act synergistically in modulating the IGF system in rat prostate. J Endocrinol. 2001;171:109–118. doi: 10.1677/joe.0.1710109. [DOI] [PubMed] [Google Scholar]
- 18.Chadha MK, Ashraf U, Lawrence D, et al. Phase II study of fulvestrant (Faslodex®) in castration resistant prostate cancer. Prostate. 2008;68:1461–1466. doi: 10.1002/pros.20813. [DOI] [PubMed] [Google Scholar]
- 19.Robertson JF, Nicholson RI, Bundred NJ, et al. Comparison of the short-term biological effects of 7alpha-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)-nonyl]estra-1,3,5,(10)-triene-3,17beta-diol (Faslodex) versus tamoxifen in postmenopausal women with primary breast cancer. Cancer Res. 2001;61:6739–6746. [PubMed] [Google Scholar]
- 20.Gutteridge E, Robertson JFR, Cheung KL, et al. Effects of fulvestrant on estrogen receptor levels during long-term treatment of patients with advanced breast cancer - final results. Breast Cancer Res Treat. 2004;88(suppl 1):S177. [Google Scholar]
- 21.Tannock IF, de Wit R, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]