Abstract
Background
Spinal metastases of patients with advanced stage lung cancer are an important target for palliative therapy, because their incidence is high, and they often cause severe symptoms and worsen the quality of life. Surgery is one of the most effective treatment options, but the indication of surgery is unclear as the procedure is invasive and patients with spinal metastasis have a rather short life expectancy. Furthermore, there have been few studies that have focused on lung cancer with poor prognosis.
Methods
We reviewed all of the cases of lung cancer from January 1999 to July 2007 in the Department of Respiratory Medicine, Kyoto University Hospital, Japan. Thirteen patients with metastatic spinal tumor of lung cancer underwent surgery, and all of them had a poor performance status score (3 or 4).
Results
Neurological improvement by at least 1 Frankel grade was seen in 10 of 14 cases (71%). Improvement of the movement capacity was noted in 9 of 14 cases (64%), and pain improvement was noted in 12 of 14 (86%). Median postoperative survival was 5 months (1–25 months). In particular, the group with a good postoperative performance status score (0–2) was shown to have a better median postoperative survival of 13 months.
Conclusions
Surgical treatment for symptomatic metastatic spinal tumor of lung cancer can improve quality of life in a substantially high percentage of patients. Surgery should be considered even if preoperative performance status is poor.
Key Words: Lung cancer, Metastatic vertebra bone tumor, Surgical treatment
Introduction
Worldwide, lung cancer remains the leading cause of cancer death in both men and women. Fifty percent of the patients have distant metastasis at the time of diagnosis. Spinal metastasis in patients with all types of cancer combined varies between 30 and 70%, and is most frequent in patients with lung cancer (60%). All these patients are at risk of developing symptomatic spinal cord compression [1] with an incidence of 5-14% [2]. Spinal metastasis often causes severe pain and neurologic dysfunction, and may exacerbate the prognosis and quality of life of patients. Thus, metastatic spinal disease is one of the important therapeutic targets in the management of patients with advanced cancer.
Metastatic spinal tumor is treated by surgery, radiotherapy or chemotherapy as well as more conservative palliative treatments such as opioids, non-steroid anti-inflammatory agents or corticosteroid. Surgical procedures often contribute to pain relief, neurological recovery and mechanical stability of the site of metastasis. It can stop rapidly progressive paralysis and recover neurological symptoms in some cases in which such recovery is not attainable with other kinds of interventions. However, the criteria for surgical indication are not clear. Because of its invasiveness, surgery is indicated only if a relatively long survival is expected and if the anticipated improvement in quality of life outweighs the risks.
There have been some studies on the outcome of surgical treatment of metastatic spinal disease from various cancers [3,4]. The prognosis of patients with spinal metastasis and surgical treatment is relatively good, when all kinds of cancers are considered together. Tatsui et al. [5] reported that survival rates were the lowest in patients with lung cancer. Moreover, it was reported that patients with lung cancer and melanoma had the poorest prognosis in a series of 76 patients with various cancers [1]. However, there have been a few studies that have focused on lung cancer. Chen et al. [6] reported good symptom relief for metastatic spinal disease of lung cancer, and 18 patients (58.1%) had a relatively good Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0 or 1. In the present study, including patients with poor performance status, we studied the usefulness of surgery in symptomatic spinal metastasis of lung cancer.
Patients and Methods
We retrospectively reviewed all records of patients with lung cancer in the Department of Respiratory Medicine, Kyoto University Hospital, Japan, from January 1999 to July 2007. There were 14 patients who underwent surgery for metastatic spinal disease of lung cancer. Information was obtained from patient records, including age, pathological diagnosis, findings of spinal MRI and CT from the neck to the pelvis, ECOG PS, symptoms (paralysis, pain and movement capacity), pre- and postoperative movement capability, operative method, location of operation, pre- and postoperative prescription, duration from symptom appearance to operation, and postoperative complications and prognosis.
To evaluate neurological status, we used the Frankel grading system [7] in the preoperative and postoperative periods. Activities of daily living (ADL) were classified into 3 categories: can walk independently, can move with wheel chair, and can not move. Pain was expressed as grade of prescription necessary to control pain according to the World Health Organization Pain Relief Ladder (WHO Ladder). This evaluation was performed 1 month after operation.
Results
A total of 772 lung cancer patients were diagnosed or referred to the Department of Respiratory Medicine, Kyoto University Hospital, from January 1999 to July 2007, and 14 (1.7%) underwent surgery for metastatic spinal disease. The indications for surgery consisted of intractable pain or neurological deterioration. Surgery was not considered if the extent of the metastatic disease precluded adequate stabilization with segmental instrumentation and if the expected survival was <3 months.
The baseline characteristics of all patients at surgery are summarized in table 1. The median age was 63.8 years (range 54-78 years). There were 8 male and 5 female patients. The most common histological type was adenocarcinoma. The most common site of involvement, in 9 patients, was the thoracic spine. Eleven patients underwent spinal decompression by laminectomy and posterior spinal fusion, and 1 underwent spinal decompression and anterior spinal fusion. There was 1 postoperative complication and recovery without reoperation. The maximum recovery lasted until the end of life in all patients. Thirteen of 14 cases were treated with chemotherapy and/or radiotherapy before or after surgery.
Table 1.
Patient characteristics and treatments
| Case | Age (sex) | Pathology | Location | Surgical approach | Other treatment |
Other metastases | |
|---|---|---|---|---|---|---|---|
| pre-operative | post-operative | ||||||
| 1 | 57 (F) | Adenocarcinoma | Th5, 8 | Posterior | CT | RT, gefitinib | Th11, L1, liver, ribs |
| 2 | 75 (F) | Adenocarcinoma | Th7–11 | Posterior | − | RT | Liver, adrenal gland |
| 3 | 50 (F) | Adenocarcinoma | L1, 3 | Posterior | RT | CT | Femoral bone |
| 4 | 63 (M) | Adenocarcinoma | Th9–11 | Posterior | − | CRT | Th1, brain, pericardial effusion |
| 5 | 59 (M) | Adenocarcinoma | C5, 6 | Laminectomy | RT | CT, gefitinib | Th1, 2, ribs, femoral bone |
| 6 | 60 (M) | Adenocarcinoma | Th4, 5 | Posterior | CRT | Gefitinib | Adrenal gland |
| 7 | 65 (M) | Adenocarcinoma | Th6–8 | Posterior | CRT | CT | Esophagus |
| 8 | 65 (M) | Adenocarcinoma | L1 | Posterior | − | RT | − |
| 9 | 62 (M) | NSCLC | Th4 | Posterior | CRT | − | Brain |
| 10 | 54 (M) | Squamous cell carcinoma | Th2 | Posterior | − | CRT | Pleural effusion |
| 11 | 73 (F) | Squamous cell carcinoma | Th11, 12 | Posterior | − | RT | Th2 |
| 12 | 72 (M) | SCLC | Th11 | Posterior | − | CRT | Th8–L3, pleural effusion |
| 13 | 75 (F) | SCLC | C4–6 | Anterior | − | − | Pleural effusion |
| 14 | 78 (M) | SCLC | Th2–8 | Anterior | CT | CT | Brain |
The table shows the baseline characteristics of all patients who underwent surgery for metastatic spinal disease of lung cancer. The most common histological type was adenocarcinoma, and the most common site of involvement was the thoracic spine. CT = Chemotherapy; RT = radiotherapy; CRT = chemo-radiotherapy; SCLC = small cell lung cancer; NSCLC = non-small cell lung cancer.
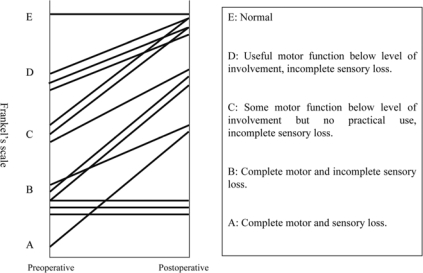
The neurological outcome after surgery is shown in fig. 1 and table 2. More than 1 grade of improvement in Frankel's scale was noted in 10 of 14 cases (71%). Three cases graded B did not show improvement after surgery. None became worse after surgery.
Fig. 1.
Neurological status: preoperative and postoperative neurological evaluation using the Frankel classification. More than one grade of improvement in Frankel's scale was noted in 10 of 14 cases (71%).
Table 2.
Preoperative and postoperative symptoms and outcomes
| Case | PS |
Paralysis (Frankel grade) |
Improvement |
Post-operative complication | Post-operative survival (months) | |||
|---|---|---|---|---|---|---|---|---|
| pre | post | pre | post | pain | ADL | |||
| 1 | 4 | 2 | C | D | + | − | − | 4 |
| 2 | 4 | 3 | A | C | + | − | − | 5 |
| 3 | 4 | 1 | D | E | + | + | − | 24 |
| 4 | 3 | 2 | B | D | + | + | − | 13 |
| 5 | 3 | 1 | C | E | + | + | − | 16 |
| 6 | 3 | 2 | D | E | + | + | − | 4 |
| 7 | 3 | 2 | E | E | + | + | − | 3 |
| 8 | 4 | 1 | D | E | + | + | − | >3 |
| 9 | 4 | 4 | B | B | + | − | + | 3 |
| 10 | 3 | 1 | C | E | + | + | − | 25 |
| 11 | 4 | 3 | B | B | − | − | − | 2 |
| 12 | 4 | 2 | B | C | + | + | − | 5 |
| 13 | 4 | 4 | B | B | − | − | − | 1 |
| 14 | 3 | 1 | C | D | + | + | − | 8 |
Preoperative PS was immediately before surgery. The explanation of the Frankel classification is shown in figure 1. There was 1 postoperative complication (wound infection). Improvements of at least 1 symptom were noted in 12 of 14 cases (86%).
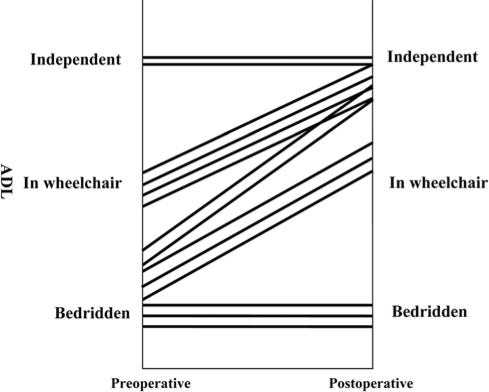
We also estimated ADL as indicated in the Patients and Methods section. Improvement in ADL was noted in 9 of 14 cases (64%) (fig. 2). ECOG PS before the symptoms caused by spinal metastasis was a mean of 2.29 (PS1, 2; PS2, 6; PS3, 6). PS immediately before surgery was a mean of 3.61 (PS3, 5; PS4, 8). After surgery, PS recovered to a mean of 1.29 (PS1, 5; PS2, 5; PS3, 2; PS4, 2). PS of 2 patients with PS4 did not change, and none of the cases worsened after surgery (table 2).
Fig. 2.
Preoperative and postoperative movement capacity evaluation. We estimated ADL with movement capacity classified into 3 categories: can walk independently, can move with wheel chair, and can not move. Improvement in ADL was noted in 9 of 14 cases (64%).
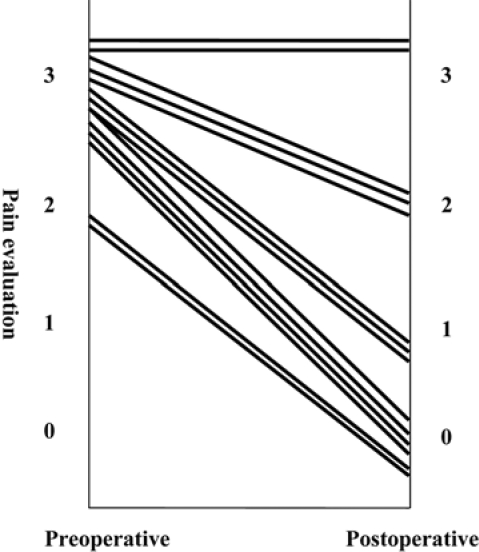
Symptoms of spinal pain were reported by all patients before surgery, and pain grade was 2 or 3 on the WHO Ladder. After surgery, improvements of more than 1 grade were noted in 12 of 14 cases (86%), and 6 of these became grade 0, with complete relief of pain (fig. 3).
Fig. 3.
Preoperative and postoperative pain evaluation using the WHO classification. Improvements of more than 1 grade were noted in 12 of 14 cases (86%).
Median postoperative survival was 5 months (1-24 months). One patient was still alive at the time of this study. The group with a good postoperative PS (0-2) was shown to have better median postoperative survival than that with a poor postoperative PS (3-4) (table 3).
Table 3.
Summary of patient outcomes
| Postoperative survival (mean), months | 1–25 (5) | |
| Preoperative PS (immediately before surgery) | 0–2 | 0 case |
| 3–4 | 14 cases | |
| Postoperative PS (mean survival months) | 0–2 | 9 cases (13 months) |
| 3–4 | 5 cases (3 months) | |
| Improvement | pain | 12/14 cases (86%) |
| movement capacity | 9/14 cases (64%) | |
| paralysis | 10/14 cases (71%) | |
| Postoperative complication | wound infection | 1 case |
The group with a good postoperative PS (0–2) was shown to have a better median postoperative survival than that with a poor postoperative PS (3–4).
Discussion
Prognosis of patients with extensive lung cancer is still poor even with the development of chemotherapeutic drugs. Thus, symptom relief and maintenance of quality of life are important aims of medical intervention in patients with advanced-stage lung cancer, especially after failure of the initial therapy. Lung cancer frequently develops metastasis to the spine, and it often leads to compression fracture or spinal dysfunction. Once compression fracture or spinal dysfunction has occurred, the quality of life of the patient deteriorates severely. Therefore, spinal metastasis is an important target in supportive care. In recent years, surgical treatment has been indicated for patients with symptoms of metastatic spinal tumors. Surgical procedures can immediately improve pain, neurological dysfunction, mechanical stability, and quality of life, although it is the most invasive treatment for patients with advanced lung cancer, and the criteria for surgical treatment of metastatic spinal tumor are still not clear. We reviewed our series of patients to investigate the efficacy of surgical treatment.
The indication of surgery for metastatic spinal disease is still controversial. The life expectancy is one of the important factors to select the treatment modality [4,8], and the prognosis of the patients with metastatic spine tumors is related to the primary site of malignancy [9]. Tokuhashi et al. [8] studied 246 patients with metastatic spinal tumors and proposed scoring systems to evaluate prognosis and the suitability of the subsequent treatment strategy [2,8]. In their series, lung cancer belonged to the poor prognosis groups, and they concluded that lung cancer is a negative factor for indication of surgery. Weigel et al. [1] also stated that survival of patients with lung cancer is the poorest (2.1 months) among all patients with solid cancer in their series. These reports may indicate that surgical indication of patients with lung cancer needs to be discussed separately from other solid cancers with a relatively longer life expectancy.
According to the past few reports which studied patients with lung cancer, an expected survival time in a range of 3 to 6 months has been used as a criterion for choosing surgical treatment. In 1995, Sundaresan et al. [10] showed that the median survival was 6 months after surgical treatment for spinal metastasis. In the study of Chen et al. [10] in 2007, median survival after surgery was 8.8 months, and 10 of 31 patients survived more than 1 year. The survival in other series ranged from 1.5 to 9.9 months [1, 10, 11, 12]. Ogihara et al. [15] found that the prognostic factors of patients with spinal metastases from non-small cell lung cancer were PS, serum calcium, and serum albumin, and that PS was a significant factor for survival in the postoperative period among the operated patients. The PS of patients involved in the current study was 3 or 4, which is poorer than in previous reports. However, postoperative median survival in the current study was 5 months (1-25 months), and it was compatible with those of previous reports.
Tanaka et al. [13] studied 100 cases of metastatic spinal tumors with various origins, and they found that preoperative pain and paralysis improved by 88.0 and 53%, respectively, and that quality of life score improved by 53%. Bach et al. [9] reported 102 cases of metastatic spinal cord compression secondary to lung cancer. Ninety-five percent of patients were able to walk after surgery. Sundaresan et al. [10] reported that 19 of 25 patients regained the ability to walk. Chen et al. [6] studied 31 patients with lung cancer and spinal metastasis and showed that 23 regained the ability to walk after surgery and that neurological improvement was noted in 25. In our study, after surgical treatment, neurological improvement, improvement of movement capacity and improvement in pain was 69, 62 and 84%, respectively. Performance status scores also improved in 9 of 13 patients. These results show that, in advanced lung cancer with spinal metastases, improvement of symptoms by spinal surgery can be expected in a substantially high percentage of patients.
In the current study, the group with a good postoperative PS (1 or 2) was shown to have a better median postoperative survival of 13 months, compared with 3 months in the group with a poor PS (3 or 4). Recovery of PS almost correlated with improvement of paralysis and ambulation. Commonly, it takes about 1 month for the patient to recover to the best postoperative condition and the patient suffers from degradation of the general condition and limitation of ADL for 1 month or so until death. Median postoperative survival was 5 months (1-24 months) in this series. It is suspected that the beneficial period to the patients was less than 3 months in this series. Some authors have suggested that symptoms have no impact on survival in patients with spinal metastases [4,14], and Hosono et al. [11] reported that walking ability is not a prognostic factor in patients with spinal metastases. However, other authors [12, 15, 16] have suggested that postoperative ambulation ability is associated with longer survival after surgery, even in patients with lung cancer. In the present study, postoperative but not preoperative PS was significant for postoperative survival time.
Postoperative complications are another important factor in selecting treatment modality. In our study, 1 (7.7%) patient had postoperative infection. None of the patients died in the immediate postoperative period. Previous reports showed that complications occurred in a range from 13 to 25.8% [1, 3, 7]. Chen et al. [6] and Weigel et al. [1] reported that operation-related death occurred in 1 (3.2%) and 2 (2.3%) patients, respectively. Other minor complications reported were wound infection, wound dehiscence, hoarseness and respiratory insufficiency. In recent studies, there was no case of intraoperative mortality.
The limitations of our study are a relatively small number of patients studied and lack of a control group. However, lung cancer is often thought to be one of the poorest prognosis groups among solid cancers. Surgical treatment of metastatic spinal disease might be considered to improve quality of life and recovery of independent ambulation. Further prospective studies are needed on metastatic spinal tumor of lung cancer to confirm the utility of the surgical treatment of metastatic spinal disease especially compared with radiation therapy.
Conclusion
Surgical treatment for symptomatic metastatic spinal tumor of lung cancer can improve quality of life in a substantially high percentage of patients. Surgery should be considered even if preoperative performance status is poor.
References
- 1.Weigel B, Maghsudi M, Neumann C, Kretschmer R, Muller FJ, Nerlich M. Surgical management of symptomatic spinal metastases. Postoperative outcome and quality of life. Spine. 1999;24:2240–2246. doi: 10.1097/00007632-199911010-00012. [DOI] [PubMed] [Google Scholar]
- 2.Aebi M. Spinal metastasis in the elderly. Eur Spine J. 2003;12(suppl 2):S202–S213. doi: 10.1007/s00586-003-0609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wai EK, Finkelstein JA, Tangente RP, Holden L, Chow E, Ford M, Yee A. Quality of life in surgical treatment of metastatic spine disease. Spine. 2003;28:508–512. doi: 10.1097/01.BRS.0000048646.26222.FA. [DOI] [PubMed] [Google Scholar]
- 4.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine. 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 5.Tatsui H, Onomura T, Morishita S, Oketa M, Inoue T. Survival rates of patients with metastatic spinal cancer after scintigraphic detection of abnormal radioactive accumulation. Spine. 1996;21:2143–2148. doi: 10.1097/00007632-199609150-00017. [DOI] [PubMed] [Google Scholar]
- 6.Chen YJ, Chang GC, Chen HT, Yang TY, Kuo BI, Hsu HC, Yang HW, Lee TS. Surgical results of metastatic spinal cord compression secondary to non-small cell lung cancer. Spine. 2007;32:E413–E418. doi: 10.1097/BRS.0b013e318074d6c7. [DOI] [PubMed] [Google Scholar]
- 7.Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, Vernon JD, Walsh JJ. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 8.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 9.Bach F, Larsen BH, Rohde K, Borgesen SE, Gjerris F, Boge-Rasmussen T, Agerlin N, Rasmusson B, Stjernholm P, Sorensen PS. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien) 1990;107:37–43. doi: 10.1007/BF01402610. [DOI] [PubMed] [Google Scholar]
- 10.Sundaresan N, Sachdev VP, Holland JF, Moore F, Sung M, Paciucci PA, Wu LT, Kelligher K, Hough L. Surgical treatment of spinal cord compression from epidural metastasis. J Clin Oncol. 1995;13:2330–2335. doi: 10.1200/JCO.1995.13.9.2330. [DOI] [PubMed] [Google Scholar]
- 11.Hosono N, Ueda T, Tamura D, Aoki Y, Yoshikawa H. Prognostic relevance of clinical symptoms in patients with spinal metastases. Clin Orthop Relat Res. 2005:196–201. doi: 10.1097/01.blo.0000160003.70673.2a. [DOI] [PubMed] [Google Scholar]
- 12.Hirabayashi H, Ebara S, Kinoshita T, Yuzawa Y, Nakamura I, Takahashi J, Kamimura M, Ohtsuka K, Takaoka K. Clinical outcome and survival after palliative surgery for spinal metastases: palliative surgery in spinal metastases. Cancer. 2003;97:476–484. doi: 10.1002/cncr.11039. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, Nakahara S, Ito Y, Kunisada T, Misawa H, Koshimune K, Ozaki T. Surgical treatment of metastatic vertebral tumors. Acta Med Okayama. 2009;63:145–150. doi: 10.18926/AMO/31849. [DOI] [PubMed] [Google Scholar]
- 14.Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop Scand. 1995;66:143–146. doi: 10.3109/17453679508995508. [DOI] [PubMed] [Google Scholar]
- 15.Ogihara S, Seichi A, Hozumi T, Oka H, Ieki R, Nakamura K, Kondoh T. Prognostic factors for patients with spinal metastases from lung cancer. Spine. 2006;31:1585–1590. doi: 10.1097/01.brs.0000222146.91398.c9. [DOI] [PubMed] [Google Scholar]
- 16.Siegal T, Tiqva P, Siegal T. Vertebral body resection for epidural compression by malignant tumors. Results of forty-seven consecutive operative procedures. J Bone Joint Surg Am. 1985;67:375–382. [PubMed] [Google Scholar]