Abstract
Hemangiopericytoma (HPC) is a rare sarcomatous tumor arising from pericytes, a support cell found in blood vessels. These tumors can occur throughout the body, particularly in the lower extremities and retroperitoneum. In rare circumstances, HPCs can arise from the meninges. In these cases, they behave similar to meningiomas, in particular angiomatous meningiomas, but tend to be more aggressive and are likely to recur. Treatment usually focuses on surgical resection and radiotherapy with possible inclusion of chemotherapy for control of recurrent disease. We describe a case of recurrent right temporal HPC that first manifested as a paraneoplastic syndrome of oncogenic osteomalacia. Despite maximum therapy, this patient experienced multiple recurrences of the tumor, and immunohistochemical analysis revealed overexpression of platelet-derived growth factor receptor, a member of the SRC-related tyrosine kinases. After multiple recurrences, the patient's tumor has been stable with treatment with monotherapy utilizing molecularly targeted therapy to SRC-related tyrosine kinases. This is the first case report of the treatment of recurrent meningeal HPC with molecularly targeted therapy to SRC-related tyrosine kinases.
Key Words: Hemangiopericytoma, SRC-related tyrosine kinase, Dasatinib
Introduction
Hemangiopericytomas (HPCs) were first categorized by Stout as malignant tumors arising from the blood vessels and described as a sarcoma [1]. The cell of origin is thought to be the pericyte which interacts with endothelial cells and is critical for blood vessel maintenance [2]. HPCs are extremely rare and occur usually outside the central nervous system (CNS) with the most common sites being the lower extremities or retroperitoneum [3]. In rare instances, HPCs can arise from the meninges in the CNS and can be confused with solitary fibrous tumors [1,4]. In the case series by Guthrie and et al. [4], most patients with meningeal HPCs are adults, usually in the 5th decade. Furthermore, presentation of the HPC was primarily related to location of the sarcoma. The primary mode of treatment included surgical resection, followed by radiotherapy, but, unfortunately, HPCs can both recur and metastasize.
Paraneoplastic syndromes have been reported as the initial presentation or part of the diagnosis of HPC. The paraneoplastic syndrome of oncogenic osteomalacia, defined by hypophosphatemia, hyperphosphaturia, and osteomalacia, has been seen in soft tissue sarcomas, including HPCs [5]. Treatment of HPC is often difficult as the tumors tend to recur locally. While the roles of surgical resection and radiotherapy are well understood, the role of chemotherapy in the treatment of multiple recurrences of HPC has not been well established [6]. We present a case of a male with a right sphenoid wing HPC who presented initially with the paraneoplastic syndrome of oncogenic osteomalacia 9 years before his HPC diagnosis. After multiple recurrences, he has been stabilized with molecularly targeted monotherapy utilizing dasatinib, a small molecule that inhibits SRC-related tyrosine kinases.
Case Report
A 22-year-old healthy male presented with diffuse bone pain. On clinical laboratory testing, serum phosphorus was extremely low at 0.8 mg/dl. The patient was later found to have hyperphosphaturia and hypercalcemia and was diagnosed with hypophosphatemic osteomalacia. Etiology of the hypophosphatemic osteomalacia was unclear at that time, but the patient was treated with neutral sodium phosphate and dihydrotachysterol, which improved his pain. He also began to experience sharp pain at the right retro-orbital region and right-sided hearing loss. Later, placement of a right tympanostomy was attempted because of presumed chronic otitis media and a vascular tumor was visualized in his right middle ear. Initial biopsy was only remarkable for granular tissue. At 30 years of age, he developed numbness at the right temple and a head CT revealed a mass arising from the right sphenoid wing that infiltrated into the floor of the middle cranial fossa. Embolization was performed which was followed by a partial resection of the mass via right temporal craniotomy. Pathology showed HPC (WHO grade II) (fig. 1). The previous diagnosis of hypophosphatemic osteomalacia was attributed to a paraneoplastic presentation of oncogenic osteomalacia due to HPC. Within 4 years from initial tumor diagnosis, the tumor recurred, and the patient was found to have a right seventh nerve palsy. A second embolization was performed for this recurrent tumor, and the patient was followed with serial scans. Approximately 3 years after this resection, the tumor increased in size. Despite partial resection, the patient continued to have osteomalacia and hypophosphatemia. He was also noted to have brown tumors associated with hyperparathyroidism involving the mandible and the right hand. A third embolization was performed followed by a second partial resection with craniotomy. Pathology was consistent with HPC (WHO grade II). Octreoscan (indium-labeled pentetreotide scan) showed active uptake by the remaining tumor. He received octreotide injections for the next 3 years. After identification of the brown tumors, the patient underwent a total parathyroidectomy with reimplantation of one parathyroid into the left arm to address the tertiary hyperparathyroidism. After 3 years of octreotide treatment, he developed déjà vu episodes (consistent with possible seizure activity) and magnetic resonance imaging (MRI) revealed progression of his tumor. He underwent a gamma knife procedure and was followed with serial scans. When the mass recurred approximately 2 years later, he had fractionated stereotactic radiotherapy to the right temporal mass. Eleven months after fractionated radiotherapy, he participated in a clinical trial for 5 months using somatostatin receptor radiopharmaceutical called (90) Y-dodecane-tetrateaacetic acid-Phe1-Tyr3-octreotide. After completion of 3 six-week cycles of this therapy, he was followed with serial MRI scans. Approximately 1 year later, he developed worsening right facial weakness and was found to have recurrent tumor extending into the middle ear cavity, external auditory canal, intratemporal fossa, inferior orbital fissure, and the anterior aspect of the cavernous sinus. There was no change in his treatment course and he continued to be followed with serial scans until 4 years later. At that time (age 56 years), he underwent a right temporal craniotomy with partial resection for recurrent tumor (fig. 2a and b). Testing of his tumor from the second resection showed high immunoreactivity to platelet-derived growth factor receptor (PDGFR), therefore, dasatinib was considered for monotherapy. After the third resection, he was placed on dasatinib at 70 mg orally once a day and has been followed with serial scans. Currently, he has been on monotherapy with dasatinib for 2 years and continues to be both clinically and radiographically stable by MRI an dFDG-PET (fig. 2c).
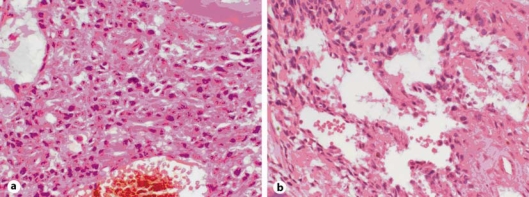
Fig. 1.
a Initial biopsy of HPC composed of elongated spindle- to carrot-shaped cells with pleomorphic nuclei and eosinophilic cytoplasm. Dilated vascular channels lined by flattened endothelium are evident. A mitotic figure is noted centrally (HE, ×10). b HPC is characterized by large dilated ‘staghorn’ vascular channels (HE, ×10).
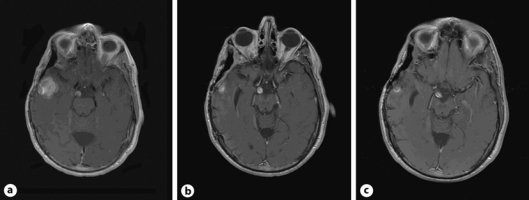
Fig. 2.
Contrast-enhancing right temporal lobe mass. a Recurrent mass before third partial resection and craniotomy. Pathology was consistent with HPC. b After third craniotomy and partial resection. c After 1 year of monotherapy with dasatinib (axial T1 with gadolinium enhancement MRI images).
Discussion
HPC is a rare malignancy that is believed to arise from pericytes, a support cell of the vasculature that interacts with endothelial cells. Usually, HPCs present in the lower extremities or retroperitoneum, but in some circumstances, they can occur within the CNS. When they involved the CNS, HPCs were previously described as angioblastic meningiomas, as the tumors were uniquely vascular and arose from the meninges [7]. Now, these two entities are considered to be separate, but similar, malignancies. Two case series of meningeal HPCs have described the presentation, course, and treatment of these rare tumors [4,8]. In both case series, patients present in adulthood, with a median occurrence of HPC in the fifth decade. The initial presentation was usually focal neurological signs, and headache was also a prominent symptom that led to the diagnosis of meningeal HPCs. A majority of patients experienced recurrences of their tumors within a range of 60.6-76%. In rare circumstances, clinicians have documented that HPCs can metastasize with sites including the bone, liver, lung, and peritoneum, with a 5-year metastasis rate at 33% [4].
Treatment of meningeal HPCs is similar to meningiomas in that surgical resection is utilized at the initial presentation if amenable to surgery. Total resection is often hindered by the extensive vasculature of the tumor, and pre-embolization of the tumor before resection can prevent blood loss [9]. Radiotherapy is used post-operatively to prevent recurrence, but in a study by Dufour et al. [10], radiotherapy did not change the rate of metastases. The utility of chemotherapy in the treatment of meningeal HPCs is unclear. Doxorubin-based regimens have been used, but have yet to establish a clear role in the treatment of HPC [6]. With the recurrent nature of HPCs as seen in our current case report, there is a vital need for better treatments of persistently recurrent HPCs. One possible therapy could be the use of molecularly targeted therapies based on the pattern of expression of growth factors in HPCs. A recent report showed that angiomatous meningiomas and HPCs often overexpress PDGFR as detected by immunohistochemistry [11]. Therefore, it is provocative that this overexpression of PDGFR could be utilized to treat recurrent angiomatous meningiomas and HPCs.
Dasatinib, at nanomolar concentrations, inhibits the following kinases: BCR-ABL, SRC family (SRC, LCK, YES, FYN), c-KIT, EPHA2, and PDGFRβ [12,13]. Dasatinib is approved by the Federal Drug Administration for the treatment of chronic myelogenous leukemia or Philadelphia-chromosome positive acute lymphoblastic leukemia [14,15]. In our case presentation, the patient's tumor tissue showed overexpression of PDGFR based on immunohistochemical analysis. Because of dasatinib's ability to inhibit PDGFR, we initiated therapy with this agent. Since initiation of therapy, the patient has tolerated that therapy well with little toxicity and has continued on monotherapy for over 2 years. MRI and FDG-PET imaging reveal no evidence of recurrent disease at this time. Thus, this suggests that there may be a role for targeted therapy in the treatment of HPCs. While this tumor is extremely rare and it is thus difficult to evaluate the drug's effectiveness in clinical trials, the further use of dasatinib in the treatment of recurrent HPC may be warranted.
In conclusion, our case report provides a first example of treatment of recurrent HPC with molecular targeted monotherapy.
References
- 1.Koch M, Nielsen GP, Yoon SS. Malignant tumors of blood vessels: angiosarcomas, hemangioendotheliomas, and hemangioperictyomas. J Surg Oncol. 2008;97:321–329. doi: 10.1002/jso.20973. [DOI] [PubMed] [Google Scholar]
- 2.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espat NJ, Lewis JJ, Leung D, Woodruff JM, Antonescu CR, Shia J, Brennan MF. Conventional hemangiopericytoma: modern analysis of outcome. Cancer. 2002;95:1746–1751. doi: 10.1002/cncr.10867. [DOI] [PubMed] [Google Scholar]
- 4.Guthrie BL, Ebersold MJ, Scheithauer BW, Shaw EG. Meningeal hemangiopericytoma: histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery. 1989;25:514–522. [PubMed] [Google Scholar]
- 5.Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28:1–30. doi: 10.1097/00000478-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Galanis E, Buckner JC, Scheithauer BW, Kimmel DW, Schomberg PJ, Piepgras DG. Management of recurrent meningeal hemangiopericytoma. Cancer. 1998;82:1915–1920. [PubMed] [Google Scholar]
- 7.Jaaskelainen J, Louis DN, Paulus W. World Health Organization classification of tumours: pathology and genetics of tumours of the nervous system. In: Kleihues P, Cavenee WK, editors. Haemangiopericytoma. Lyon: 2004. pp. 190–192. [Google Scholar]
- 8.Mena H, Ribas JL, Pezeshkpour GH, Cowan DN, Parisi JE. Hemangiopericytoma of the central nervous system: a review of 94 cases. Hum Pathol. 1991;22:84–91. doi: 10.1016/0046-8177(91)90067-y. [DOI] [PubMed] [Google Scholar]
- 9.Fountas KN, Kapsalaki E, Kassam M, et al. Management of intracranial meningeal hemangiopericytomas: outcome and experience. Neurosurg Rev. 2006;29:145–153. doi: 10.1007/s10143-005-0001-9. [DOI] [PubMed] [Google Scholar]
- 10.Dufour H, Métellus P, Fuentes S, et al. Meningeal hemangiopericytoma: a retrospective study of 21 patients with special review of postoperative external radiotherapy. Neurosurgery. 2001;48:756–762. doi: 10.1097/00006123-200104000-00011. discussion 762-763. [DOI] [PubMed] [Google Scholar]
- 11.Dietzmann K, von Bossanyi P, Warich-Kirches M, et al. Immunohistochemical detection of vascular growth factors in angiomatous and atypical meningiomas, as well as hemangiopericytomas. Pathol Res Pract. 1997;193:503–510. doi: 10.1016/s0344-0338(97)80104-5. [DOI] [PubMed] [Google Scholar]
- 12.Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg M. Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Clin Ther. 2007;29:2289–2308. doi: 10.1016/j.clinthera.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Giles FJ, O'Dwyer M, Swords R. Class effects of tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Leukemia. 2009;23:1698–1707. doi: 10.1038/leu.2009.111. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H, O'Brien S, Talpaz M, et al. Outcome of patients with Philadelphia chromosome-positive chronic myelogenous leukemia post-imatinib mesylate failure. Cancer. 2007;109:1556–1560. doi: 10.1002/cncr.22569. [DOI] [PubMed] [Google Scholar]