Abstract
Background
Hypoxia of the renal medulla has been implicated in the development of renal injury, particularly acute renal failure, and its regulation in humans may therefore be relevant to certain renal disorders. Changes in oxygenation of the renal medulla can now be monitored noninvasively with blood oxygenation level–dependent (BOLD) magnetic resonance imaging (MRI). Using this method, water diuresis has been shown to improve medullary oxygenation in young persons. Urinary excretion of prostaglandin E2 (PGE2) likewise increases during water diuresis in younger but not in older people. We used BOLD MRI to measure the effects of aging and of inhibiting prostaglandin synthetase on the renal response to water diuresis in healthy human subjects.
Methods
Nine younger (25 to 31 years) and nine older (59 to 79 years) female volunteers were studied with BOLD MRI during antidiuresis in the postabsorptive state and during water diuresis. Simultaneously, urinary excretion of PGE2 was determined. PG synthetase was inhibited by administering ibuprofen.
Results
Renal medullary oxygenation, initially low, greatly improved during diuresis in younger subjects, whereas PGE2 excretion increased. In older women, however, water diuresis elicited no change in oxygenation of renal medulla or PGE2 excretion. Ibuprofen inhibited excretion of PGE2 and blocked the increase in medullary oxygenation normally produced by water diuresis in the young.
Conclusions
The increase in oxygenation of the renal medulla accompanying water diuresis depends on PGE2 synthesis. Attenuation of renal PGE2 synthesis in older people is probably responsible, at least in part, for the loss of the ability to improve medullary oxygenation that younger subjects possess. Inability to improve renal medullary oxygenation might predispose to hypoxic renal injury in older patients.
Keywords: radiology, hypoxia, renal injury, acute renal failure, medullary oxygenation, BOLD-MRI
The medulla of the mammalian kidney normally operates in a hypoxic environment maintained by the countercurrent arrangement of vessels and tubules necessary to conserve water [1]. Renal medullary hypoxia is relieved by loop diuretics that reduce the work and oxygen consumption of medullary tubules or by vasodilating agents that increase medullary blood flow [2]. Intrarenal oxygenation can be monitored noninvasively in humans by the technique of blood oxygenation level–dependent (BOLD) magnetic resonance imaging (MRI) [3], which depends on the principle that variations in the oxygen saturation of hemoglobin result in changes in local magnetic susceptibility and hence in the signal intensity of T2*–weighted magnetic resonance images. We have used this method to examine the way in which the response of the renal medulla to water diuresis is affected by normal aging and by inhibition of prostaglandin (PG) synthesis.
Methods
Study plan
To compare responses to water diuresis in younger and older subjects, we recruited nine healthy, young female volunteers 25 to 31 (mean 28) years of age and nine healthy older women 59 to 79 (mean 69) years of age. These studies were repeated during cyclooxygenase inhibition in six younger volunteers. In all subjects, the medical history and a preliminary physical examination were essentially negative. Blood pressure was below 150/90 mm Hg, and complete blood count and urinalysis were normal. To avoid contamination of the urine with PGs produced in the male reproductive tract, the study was limited to women. The study was approved by the Beth Israel Hospital Committee on Clinical Investigation, and all volunteers gave written informed consent prior to their participation in the study.
All subjects were asked to take nothing by mouth after 8:00 p.m. on the night before the study. The next morning, at 8:00 a.m., they reported to the Clinical Research Center, where they were weighed, vital signs were measured, a sample of blood was drawn for blood urea nitrogen (BUN) and serum creatinine, and a timed sample of urine was obtained by spontaneous voiding. They were then taken to the MRI suite, where baseline MRI images were obtained in the supine position. The subjects were taken out of the magnet and were asked to void again for a second urine collection. They then drank 20 ml of flavored water per kg of body weight within 15 minutes in order to induce a water diuresis. Urinary output was measured every 15 minutes. After it exceeded 5 ml per minute, the subject returned to the magnet, where a second set of BOLD MRI measurements was obtained. Immediately following this, a final sample of urine was obtained for measurement of osmolality, creatinine, and PGE2.
In the studies of cyclooxygenase inhibition, ibuprofen, 600 mg, was taken by mouth three times daily with meals for two days prior to the test and at 8 a.m. the morning of the study. These results were compared with a control study in each subject of water diuresis without ibuprofen, performed at least a week before or after ibuprofen was given.
Laboratory methods
Urinary osmolality was determined using freezing point depression with an Osmette osmometer (Precision Instruments, Sudbury, MA, USA). Urinary creatinine concentration was determined using a rate-dependent modification of the Jaffe reaction (SMAC II; Technicon Instruments, Tarrytown, NY, USA).
Urinary samples for determination of PGE2 were immediately aliquoted into containers acidified with 2 n HCl. Samples were then adjusted to pH 3 to 4. PGE2 was assayed on diluted urine samples using a standard double antibody radioimmunoassay with reagents obtained from New England Nuclear (DuPont, Boston, MA, USA). Interassay and intra-assay coefficients of variation are 20% and 5.1%, respectively. All samples were assayed in duplicate.
MRI methods
Magnetic resonance imaging takes advantage of the energy emitted as radio waves by water molecules, when their alignment in a strong magnetic field is changed. The BOLD MRI technique depends on the principle that hemoglobin (because of its iron content) changes its magnetic qualities depending on whether the hemoglobin is in the oxygenated or deoxygenated form. This change in the magnetic property of hemoglobin in turn influences the MRI signal from the neighboring water molecules in a predictable way. Because the ratio of oxyhemoglobin to deoxyhemoglobin is related to the pO2 of blood and because the pO2 of capillary blood is thought to be in equilibrium with the surrounding tissue, changes estimated by BOLD MRI can be interpreted as changes in tissue pO2 (Fig. 1). All measurements were performed on a 1.5T whole body scanner (Vision; Siemens Medical Systems, Erlangen, Germany). We used R2* (= 1/T2*) as the parameter to reflect relative oxygenation status [3]. We used a multiple gradient echo sequence (TR/TE/Flip angle = 60 ms/3–48 ms/40°) to acquire 16 T2*–weighted images within a single breath-hold of less than 15 seconds, which permits calculation of R2* maps [4]. For studies with and without ibuprofen, a modified sequence involving selective water excitation pulses was used. This increased the range of echo times slightly, 6 to 51 ms. We have shown that both of these sequences give equivalent results as far as R2* calculations are concerned [4]. The advantage of the selective water excitation is better delineation between renal medulla and intrarenal fat on R2* maps. A decrease in R2* implies an increase in tissue pO2 (Fig. 1).
Fig. 1. Correlation of blood oxygenation level-dependent (BOLD) MRI with pO2.
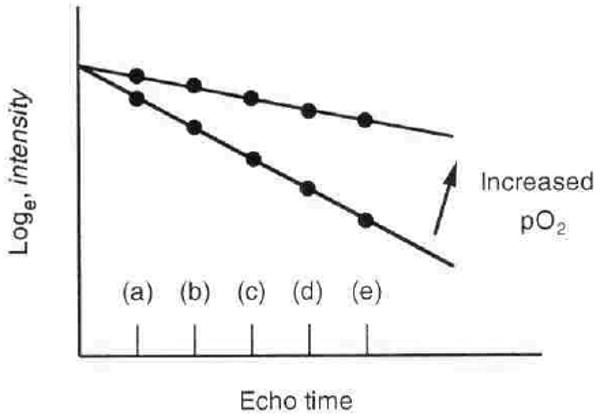
R2* = slope ∼ conc[deoxyHB] ∼ blood pO2 ∼ tissue pO2. The deoxygenation of hemoglobin changes its magnetic characteristics, leading to changes in a parameter of magnetic resonance called R2* (apparent spin-spin relaxation rate). R2* can be estimated from signal intensity measurements made at several different echo times (a through e). The slope of loge (intensity) vs. echo time determines R2* and is directly related to the amount of deoxygenated blood. A decrease in the slope implies an increase in the pO2 of blood. Because blood pO2 is thought to be in rapid equilibrium with tissue pO2, changes in BOLD signal intensity or R2* should reflect changes in the pO2 of the tissue.
Statistical methods
Results are expressed as mean ± sem. Results were analyzed for significance using Student's t-test, and the differences were considered not significant if P was more than 0.05.
Results
Effects of age on the response of the medulla to water diuresis
BOLD MRI measurements of medullary oxygenation (R2*) indicated that the renal medulla was appreciably less well oxygenated than the cortex in both younger and older subjects (Table 1 and Fig. 2). Water diuresis increased medullary oxygenation in the young (R2* = 17.9 ± 0.4 before water was drunk and 12.6 ± 0.5 during water diuresis, P < 0.01) as reported in earlier studies [3], whereas cortical oxygenation changed only slightly. By contrast, in older subjects, R2* in the renal medulla remained high after drinking water and was essentially unaltered by water diuresis. As noted earlier [5], the minimum urinary osmolality achieved during water diuresis was lower in younger than in older subjects (56 ± 3 vs. 86 ± 6 mOsm/kg, P < 0.01).
Table 1.
Effect of water diuresis in young and elderly subjects
| Antidiuresis | Diuresis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2* medulla |
R2* cortex |
PGE2 excretion pg/min |
V ml/min |
UOsm mOsm/kg |
CCr ml/min |
R2* medulla |
R2* cortex |
PGE2 excretion pg/min |
V ml/min |
UOsm mOsm/kg |
|
| Young (N = 9) |
17.9 ± 0.4 | 12.8 ± 0.4 | 228 ± 37 | 0.44 ± 0.07 | 810 ± 55 | 115 ± 0.5 | 12.6a ± 0.5 | 11.7c ± 0.3 | 571b ± 64 | 10.9a ± 0.6 | 56a ± 3 |
| Elderly (N = 9) |
18.2 ± 0.4 | 12.3 ± 0.4 | 208 ± 50 | 0.60 ± 0.08 | 664g ± 28 | 81af ± 6.3 | 17.1de ± 0.8 | 11.9d ± 0.3 | 122de ± 16 | 6.9ae ± 63 | 86ae ± 6 |
Different from antidiuresis (P < 0.01)
Different from antidiuresis (P < 0.02)
Different from antidiuresis (P < 0.05)
Not significantly different from antidiuresis (P > 0.05)
Different from young (P < 0.01)
Different from young (P < 0.02)
Different from young (P < 0.05)
Fig. 2. Comparison of changes in R2* (1/s) in response to waterload in 9 young and 9 elderly subjects.
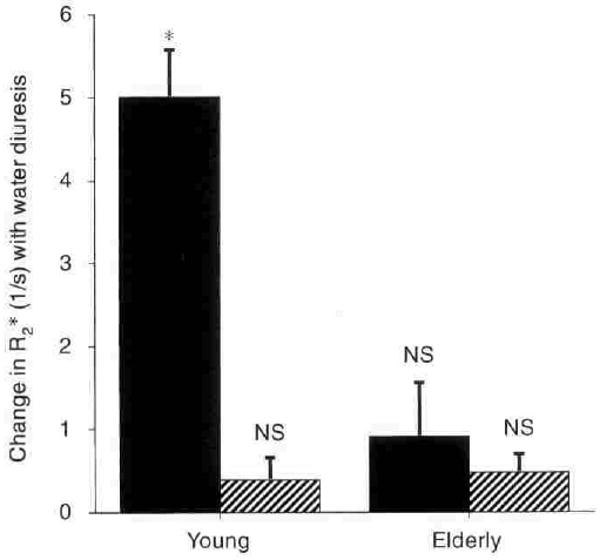
Symbols are: (■) medulla; (▨) cortex. Columns are mean ± sem. NS implies not significant. * implies P < 0.01.
Previous studies have shown that water diuresis is associated with an increase in the urinary excretion of PGE2 in younger subjects but not in older subjects [5, 6]. In these experiments as well, PGE2 excretion was more than doubled by water diuresis in younger subjects (228 ± 37 to 571 ± 64 pg/min), but did not change significantly in older subjects. Accordingly, additional experiments were planned to study the effects of inhibiting PGE2 synthesis on medullary oxygenation in younger individuals during water diuresis.
Effects of ibuprofen on the response of the medulla to water diuresis
In six younger women 25 to 31 years of age, the effects of water diuresis were studied again after taking 600 mg of ibuprofen, an inhibitor of PG synthesis, one hour before water loading on the day of the test and three times daily with meals for the previous two days (Table 2 and Fig. 3). As expected from the two-hour half-life of ibuprofen in circulating blood, baseline values for PGE2 excretion were unaffected during antidiuresis (measured 12 hr after the previous day's supper dose of ibuprofen), but urinary excretion of PGE2 was greatly inhibited during the water diuresis generated immediately following ibuprofen ingestion on the day of the test. Peak diuresis after water ingestion was slightly but not significantly diminished by ibuprofen, and the minimum urinary osmolality was slightly higher after the drug in five of the six subjects.
Table 2.
Effects of ibuprofen on the kidney during water diuresis in young subjects
| Antidiuresis | Diuresis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2* medulla |
R2* cortex |
PGE2 excretion pg/min |
V ml/min |
UOsm mOsm/kg |
CCr ml/min |
R2* medulla |
R2* cortex |
PGE2 excretion pg/min |
V ml/min |
UOsm mOsm/kg |
|
| No ibuprofen (6) | 17.9 ± 0.45 | 11.9 ± 0.4 | 237 ± 62 | 0.44 ± 0.12 | 839 ± 80 | 116 ± 16 | 13.2a ± 0.56 | 11.4 ± 0.35 | 478a ± 21 | 11.1a ± 0.9 | 55a ± 4 |
| Ibuprofen (6) | 18.45 ± 0.71 | 12.13 ± 0.34 | 213 ± 44 | 0.49 ± 0.14 | 846 ± 113 | 121 ± 16 | 17.6b ± 0.36 | 11.2 ± 0.28 | 89ab ± 11 | 9.1 ± 0.7 | 64 ± 6.4 |
Significantly different from antidiuresis, (P < 0.01)
Significantly different from no ibuprofen, (P < 0.01)
Fig. 3. Comparison of changes in R2* (1/s) in response to waterload in 6 young subjects with and without cyclooxygenase inhibition with ibuprofen.
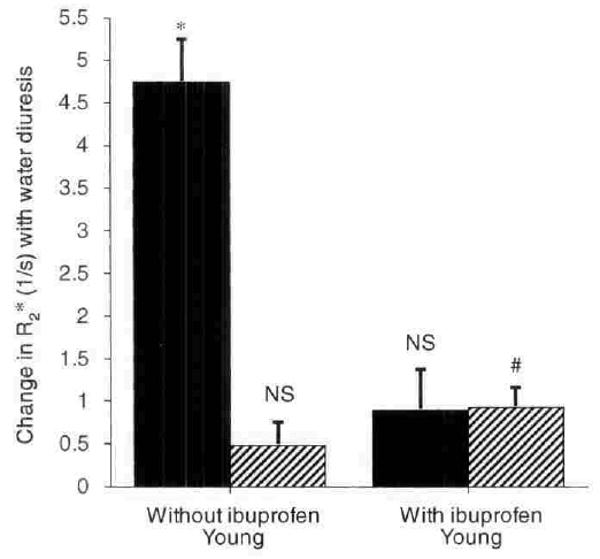
Symbols are: (■) medulla; (▨) cortex. Columns are mean ± sem. NS implies not significant. * implies P < 0.01; # implies P < 0.02.
The main finding in the studies with ibuprofen is that this drug suppressed the improvement in medullary oxygenation otherwise seen in younger subjects during water diuresis (Fig. 3). Water diuresis without ibuprofen produced a fall in medullary R2* from 17.9 ± 0.45 to 13.2 ± 0.56, whereas after ibuprofen, there was virtually no change in R2* of renal medulla (18.45 ± 0.71 to 17.6 ± 0.36).
Discussion
The marked and habitual hypoxia of the renal medulla, as compared with the renal cortex and with most other body tissues, has spurred speculation about its implications for human disease [1]. In particular, it has been postulated that anoxic injury to medullary structures initiates the phenomenon of acute renal failure [7]. If so, the intrinsic ability of the kidneys to improve medullary oxygenation after a variety of physiological and pathological stimuli might be correlated with susceptibility to acute renal injury.
Experiments in animals suggest that PGE2 is a paracrine factor that importantly influences medullary oxygenation. Produced by medullary collecting ducts and interstitial cells, PGE2 inhibits active transport by the medullary thick limb [8], inhibits Na,K-ATPase activity in renal cells [9, 10], and reduces their oxygen consumption [11] while enhancing medullary blood flow [12]. Receptors for PGE2 are clustered in high density in the vasa recta and the renal tubular cells lining the thick ascending limb within the outer medulla [13]. In intact rats, medullary pO2 is reduced by indomethacin, which blocks PG synthetase [14]. Pretreatment with indomethacin greatly increases susceptibility to experimental radiocontrast nephropathy and predisposes to acute renal failure in humans [15]. On the other hand, when urinary excretion of PGE2 is increased in rats by dietary manipulation, hypoxic damage to their perfused kidneys is substantially reduced [16]. Therefore, there are strong reasons to believe that renal PGs might influence medullary oxygenation in human kidneys.
These experiments provide direct evidence in support of this hypothesis. Inhibition of PG synthesis by ibuprofen completely abolished the increase in medullary pO2 that normally accompanies water diuresis in healthy, younger subjects. Urinary excretion of PGE2, which is thought to be derived exclusively from PGs synthesized in the renal medulla [17], fell to low levels during water diuresis after ibuprofen was given to younger subjects, whereas without ibuprofen, urinary PGE2 excretion was increased by water diuresis. Of note, the dose of ibuprofen that was given did not substantially impair the excretion of a water load or significantly alter the minimum urinary osmolality achieved after water was imbibed. The marked effect of ibuprofen on medullary pO2 cannot therefore be ascribed to an inhibition of diuresis per se, but probably reflects the action of locally synthesized PGs and their inhibition on medullary blood flow during water diuresis.
In the light of these results, the difference we noted between younger and older subjects in the response of renal medullary pO2 to water diuresis is not unexpected. Although urinary excretion of PGE2 (and, presumably, renal medullary synthesis of PGE2) is stimulated by water diuresis in the young [5, 6], this response is known to be markedly reduced with age [5]. It seems reasonable to assume that this is responsible, at least in part, for the defect in medullary oxygenation seen with BOLD MRI during diuresis in older people. The action of other local endogenous vasodilators in the renal medulla, such as nitric oxide [18] or dopamine [5], may also become attenuated with age. These experiments suggest that a feature of normal aging is the loss of the ability to improve renal medullary pO2 rapidly during water diuresis. An analogous inability to improve medullary oxygenation in response to physiological or pathological stimuli might help to explain the increased susceptibility of older people to hypoxic renal damage and acute renal failure following circulatory, septic, and toxic insults [19–22]. The physiological basis of this hypothesis can now be investigated noninvasively in human subjects using BOLD MRI.
Acknowledgments
This work was supported in part by grants from the American Heart Association and the National Institutes of Health (DK 53221) to P.V.P. and by the Kidney Foundation of Massachusetts and Rhode Island and the National Institutes of Health (DK 18078) to F.H.E. The authors thank Dr. Wei Li and Ms. Katherine Spokes for their technical support, and the BIDMC NIH General Clinical Research Center (M01-RRO-1032), which assisted in carrying out these experiments.
References
- 1.Brezis M, Rosen S. Hypoxia of the renal medulla: Its implications for disease. N Engl J Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 2.Brezis M, Agmon Y, Epstein FH. Determinants of intrarenal oxygenation. I. Effects diuretics. Am J Physiol. 1994;267:F1059–F1062. doi: 10.1152/ajprenal.1994.267.6.F1059. [DOI] [PubMed] [Google Scholar]
- 3.Prasad P, Edelman R, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94:3271–3275. doi: 10.1161/01.cir.94.12.3271. [DOI] [PubMed] [Google Scholar]
- 4.Prasad P, Chen Q, Goldfarb J, Epstein F, Edelman R. Breath-hold R2* mapping with a multiple gradient recalled echo sequence: Application to the evaluation of intra-renal oxygenation. J Magn Reson Imaging. 1997;7:1163–1165. doi: 10.1002/jmri.1880070633. [DOI] [PubMed] [Google Scholar]
- 5.Kuhlik A, Epstein FH, Elahi D, Clark B. Urinary prostaglandin E2 and dopamine responses to water loading in young and elderly humans. Geriatr Nephrol Urol. 1995;5:79–83. [Google Scholar]
- 6.Walker RM, Brown RS, Stoff JS. Role of renal prostaglandins during antidiuresis and water diuresis in man. Kidney Int. 1982;21:365–370. doi: 10.1038/ki.1982.31. [DOI] [PubMed] [Google Scholar]
- 7.Brezis M, Rosen S, Epstein FH. The pathophysiological implications of medullary hypoxia. Am J Kidney Dis. 1989;13:253–258. doi: 10.1016/s0272-6386(89)80062-9. [DOI] [PubMed] [Google Scholar]
- 8.Stokes JB. Effect of prostaglandin E2 on chloride transport across the rabbit thick ascending limb of Henle: Selective inhibitions of the medullary portion. J Clin Invest. 1979;64:495–502. doi: 10.1172/JCI109487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jabs K, Zeidel ML, Silva P. Prostaglandin E2 inhibits Na+-K+-ATPase activity in the inner medullary collecting duct. Am J Physiol. 1989;257:F424–F430. doi: 10.1152/ajprenal.1989.257.3.F424. [DOI] [PubMed] [Google Scholar]
- 10.Cohen-Luria R, Rimon G, Moran A. PGE2 inhibits Na-K-ATPase activity and ouabain binding in MDCK cells. Am J Physiol. 1993;264:F61–F65. doi: 10.1152/ajprenal.1993.264.1.F61. [DOI] [PubMed] [Google Scholar]
- 11.Lear S, Silva P, Kelley VE, Epstein FH. Prostaglandin E2 inhibits oxygen consumption in rabbit medullary thick ascending limb. Am J Physiol. 1990;258:F1372–F1378. doi: 10.1152/ajprenal.1990.258.5.F1372. [DOI] [PubMed] [Google Scholar]
- 12.Chang LC, Splawinski JA, Oates JA, Nies AS. Enhanced renal prostaglandin production in the dog. II. Effects on intrarenal hemodynamics. Circ Res. 1975;36:204–207. doi: 10.1161/01.res.36.1.204. [DOI] [PubMed] [Google Scholar]
- 13.Eriksen EF, Richelsen B, Gesser BP, Jacobsen NO, Stengaard-Pedersen K. Prostaglandin-E2 receptors in the rat kidney: Biochemical characterization and localization. Kidney Int. 1987;32:181–186. doi: 10.1038/ki.1987.190. [DOI] [PubMed] [Google Scholar]
- 14.Heyman SN, Brezis M, Reubinoff CA, Greenfeld Z, Lechene C, Epstein FH, Rosen S. Acute renal failure with selective medullary injury in the rat. J Clin Invest. 1988;82:401–412. doi: 10.1172/JCI113612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantley L, Spokes K, Clark B, McMahon E, Carter J, Epstein FH. Role of endothelin and prostaglandins in radiocontrast-induced renal artery constriction. Kidney Int. 1993;44:1217–1223. doi: 10.1038/ki.1993.371. [DOI] [PubMed] [Google Scholar]
- 16.Silva P, Rosen S, Spokes K, Taylor M, Epstein FH. Influence of endogenous prostaglandins on mTAL injury. J Am Soc Nephrol. 1990;1:808–814. doi: 10.1681/ASN.V15808. [DOI] [PubMed] [Google Scholar]
- 17.Frolich JC, Wilson TW, Sweetman BJ, Smigel M, Nies AS, Carr K, Watson JT, Oates JA. Urinary prostaglandins: Identification origin. J Clin Invest. 1975;55:763–770. doi: 10.1172/JCI107987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brezis M, Heyman SN, Dinour D, Epstein FH, Rosen S. Role of nitric oxide in renal medullary oxygenation: Studies in isolated and intact rat kidneys. J Clin Invest. 1991;88:390–395. doi: 10.1172/JCI115316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar R, Hill CM, McGeown MG. Acute renal failure in the elderly. Lancet. 1973;1:90–91. doi: 10.1016/s0140-6736(73)90480-7. [DOI] [PubMed] [Google Scholar]
- 20.Lunding M, Steiness I, Thaysen J. Acute renal failure due to tubular necrosis: Immediate prognosis and complications. Acta Med Scand. 1964;176:103–119. doi: 10.1111/j.0954-6820.1964.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 21.Byrd L, Sherman RL. Radiocontrast-induced acute renal failure: A clinical and pathophysiologic review. Medicine. 1979;58:270–279. doi: 10.1097/00005792-197905000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Henrich WL. Nephrotoxicity of nonsteroidal anti-inflammatory agents. Am J Kidney Dis. 1983;2:478–484. doi: 10.1016/s0272-6386(83)80083-3. [DOI] [PubMed] [Google Scholar]


