Abstract
Postprandial triglyceridemia is an emerging risk factor for cardiovascular disease. However, most of the genes that influence postprandial triglyceridemia are not known. We evaluated whether a common nonsynonymous SNP rs1260326/P446L in the glucokinase regulatory protein (GCKR) gene influenced variation in the postprandial lipid response after a high-fat challenge in seven hundred and seventy participants in the Amish HAPI Heart Study who underwent an oral high-fat challenge and had blood samples taken in the fasting state and during the postprandial phase at 1, 2, 3, 4, and 6 hours. We found that the minor T allele at rs1260326 was associated with significantly higher fasting TG levels after adjusting for age, sex, and family structure (Pa = 0.06 for additive model, and Pr=0.0003 for recessive model). During the fat challenge, the T allele was associated with significantly higher maximum TG level (Pa = 0.006), incremental maximum TG level (Pa = 0.006), TG area under the curve (Pa = 0.02) and incremental TG area under the curve (Pa = 0.03). Our data indicate that the rs1260326 T allele of GCKR is associated with both higher fasting levels of TG as well as the postprandial TG response, which may result in higher atherogenic risk.
Keywords: GCKR, polymorphism, postprandial, lipid
Introduction
Fasting dyslipidemia, including high LDL cholesterol and low HDL cholesterol, is a major risk factor for cardiovascular disease (CVD). Of equal or potentially greater importance, however, is the impact of postprandial dyslipidemia on cardiovascular health, given the abundance of food and frequent eating patterns that result in increased time spent in the postprandial state relative to the fasting state. Several studies have demonstrated that a heightened postprandial lipid response or nonfasting triglyceride (TG) level is associated with coronary artery disease (Groot PH et al. 1991; Kugiyama K et al. 1999; Stampfer MJ et al. 1996; Bansal S et al. 2007; Eberly LE et al. 2003). There is substantial inter-individual variability in postprandial lipid response and several studies have reported associations between polymorphisms at candidate genes and postprandial lipid response, as well as interactions with dietary factors (Ordovas JM and Corella D 2004).
A common single nucleotide polymorphism (SNP) (rs780094) in the gene encoding the glucokinase regulatory protein (GCKR), located on chromosome 2, was recently associated through a genome-wide association study with variation in fasting triglyceride levels in a Scandinavian population, with this SNP explaining approximately 1% of its residual variance (Saxena R et al. 2007). This association has since been consistently replicated in other large population samples (Kathiresan S et al. 2008; Willer CJ et al. 2008; Sparso T et al. 2008; Scott LJ et al. 2007). Intriguingly, the allele associated with elevated TG levels is also associated with lower fasting blood glucose, less insulin resistance, and lower risk of type 2 diabetes (Saxena R et al. 2007). Rs780094 is located in a large block spanning at least 500 kb. A common nonsynonymous GCKR mutation rs1260326 (P446L) is in linkage disequilibrium with rs780094 (r2 = 0.93 in Hapmap CEU sample) and was found to be associated with TG and the metabolic traits in the same way (Willer CJ et al. 2008; Vaxillaire M et al. 2008; Koster B et al. 2005). It was also the strongest association signal in the region by fine mapping approaches in a recent study (Orho-Melander M et al. 2008).
Glucokinase (GK), the first glycolytic enzyme, catalyzes the phosphorylation of glucose, the first step in glycogen synthesis in the liver and glucose metabolism and insulin secretion in the beta-cell. GCKR plays a major regulatory role in the post-transcriptional regulation of GK in the liver. It is localized in the nucleus and functions to sequester GK thus inhibiting its degradation (Farrelly D et al. 1999; Slosberg ED et al. 2001). GCKR knockout mice have decreased GK activity and elevated glucose levels (Farrelly D et al. 1999). Adenoviral-mediated over-expression of GCKR in murine liver increases GK activity and lowered fasting blood glucose (Slosberg ED et al. 2001), and over-expression of liver GK in rats and mice leads to lowered blood glucose as well as increased triglyceride levels (O'Doherty RM et al. 1999). The role of GK and GCKR in lipid metabolism is less well established. Long-term over-expression of GK can lead to TG-mediated insulin resistance and hyperglycemia in mice (Ferre T et al. 2003).
The potential impact of sequence variation in GCKR on postprandial lipid response is unknown despite the significance of this phenotype on CVD risk. The goal of the current study was to evaluate the impact of the common nonsynonymous SNP, rs1260326 in GCKR on both fasting TG and TG levels after a high-fat challenge.
Methods
Subjects/Phenotyping
Study subjects were Amish men and women, enrolled between 2003 and 2006 in the Heredity and Phenotype Intervention (HAPI) Heart Study, which was designed to identify genes that interact with the environment to influence CVD risk (Mitchell BD et al. 2008). Subjects were generally healthy and were identified through their participation in one of our previous studies as well as by word of mouth, advertisements, Amish-wide mailings, and referrals from local physicians. Participating subjects were aged 20 years and older. Those with severe hypertension (BP > 180/105 mm Hg), malignancy, or kidney, liver or thyroid disease were not eligible for the study. All subjects discontinued medications (including lipid-lowering medications (n = 7 subjects)), vitamins and nutritional supplements for seven days prior to and throughout the study.
Of the 868 total subjects enrolled in the HAPI Heart Study, 809 completed a high fat challenge test, during which lipid levels were measured following oral administration of a high fat milk shake. Subjects were instructed to fast for 12 hours prior to their appointment, to abstain from excessive physical activity on the morning of the test. Among them, 770 were successfully genotyped for SNP rs1260326. Genotyping was performed using a Taqman® assay.
The research protocol was approved by the Institutional Review Board of the University of Maryland School of Medicine. All subjects signed informed consent, which included permission to perform genotyping.
Phenotype Measurement
The high fat challenge, prepared in the form of a whipping cream milk shake, was standardized to consist of 782 calories per m2 of body surface with 77.6% of calories from fat, 19.2% from carbohydrate, and 3.1% from protein. Following ingestion, blood was drawn at 1, 2, 3, 4, and 6 hours to assess the TG excursion. The subject rested and remained otherwise fasting during the 6 hours post-fat challenge.
Fasting and post-challenge TG and fasting total cholesterol and HDL-C levels were measured by Quest Diagnostics (Horsham, PA). Fasting TG levels were < 400 mg/dl in all subjects, and LDL-C levels were calculated by the Friedewald equation (Friedewald WT et al. 1972). TG total area under the curve (TAUCTG) was calculated by the trapezoid method, and incremental area under the curve (iAUCTG) was calculated as TAUCTG – 6 × fasting TG. Cholesterol in all lipoprotein subfractions other than chylomicron were measured at zero and four hours by Vertical Auto Profile (VAP) technology (Atherotech, Birmingham, AL) (Kulkarni KR 2006).
Height and weight were measured using a stadiometer and calibrated scale with shoes removed and in light clothing, and body mass index (BMI) (kg/m2) was computed. Systolic blood pressure (SBP) (1st phase) and diastolic blood pressure (DBP) (5th phase) were obtained in triplicate using a standard sphygmomanometer with the subject sitting for at least 5 minutes. Glucose concentrations were assayed with a YSI glucose analyzer (YSI Life Sciences, Yellow Springs, OH) using the glucose oxidase method (interassay coefficient of variation = 1.52%). Insulin was measured by radioimmunoassay (coefficient of variation = 4.42%).
Statistical Methods
The Amish men and women participating in the study are all related to one another and the relatedness can be connected back 14 generations. In the current study, there are 256 parent-offspring pairs, 512 sibling pairs, 171 first cousin pairs, and 11 grandparent-grandchild pairs. Genotypes were checked for Mendelian consistency using the PedCheck software program (O'Connell JR and Weeks DE 1998) in the extended Amish pedigree. The observed distribution of genotypes was tested for fit to Hardy-Weinberg expectations using the χ2 test. Association analyses of quantitative traits were performed using the measured genotype approach that models variation in the trait of interest as a function of measured environmental covariates, measured genotype, and a polygenic component to account for phenotypic correlation due to relatedness. A t-test was used to assess significance of the measured genotype beta coefficient. We included sex and sex-specific age and age2 and in some models body mass index (BMI) as covariates, and coded the SNPs using additive, dominant and recessive models. The polygenic component was modeled using the relationship matrix derived from the complete 14-generation pedigree structure to properly control for the relatedness of all subjects in the study. These analyses were carried out using software developed in our group (O'Connell JR, manuscript in preparation). Quantitative traits that were not normally distributed (fasting TG, glucose, and insulin) were transformed by their natural logarithm. P-values for the additive and recessive models are reported unless specified otherwise.
Results
The mean ± SD age of the 770 HAPI Heart subjects was 43.4 ± 14.0 years and 46% were women. Mean ± SD body mass index (BMI) was 25.5 ± 3.2 in men and in 27.6 ± 5.3 women. Baseline and postprandial lipid levels are shown in Table 1. The majority of subjects had maximal TG levels at 3 to 4 hours after the high-fat challenge. As expected, HDL-C and LDL-C levels did not change appreciably during the high fat challenge.
Table 1.
Lipid levels at baseline and after high fat challenge in total subjects (mean, SD)
| Baseline | Hour 1 | Hour 2 | Hour 3 | Hour 4 | Hour 6 | |
|---|---|---|---|---|---|---|
| Total cholesterol, mg/dL | 208 (46) | 215 (48) | 213 (47) | 213 (46) | 213 (46) | 215 (47) |
| HDL-C, mg/dL | 56 (14) | 57 (15) | 55 (15) | 54 (15) | 53 (15) | 53 (15) |
| LDL-C, mg/dL | 138 (42) | 140 (44) | 131 (43) | 126 (43) | 125 (43) | 131 (43) |
| TG*, mg/dL | 55 (41, 78) | 73 (55, 104) | 118 (85, 163) | 147 (106, 211) | 151 (107, 225) | 130 (87, 206) |
Median (interquantile range)
The frequency of the rs1260326 T allele was 0.294, and the distribution of genotypes was slightly inconsistent with Hardy-Weinberg-equilibrium (p = 0.02), likely due to relatedness among the study group. Characteristics of the study population according to rs1260326 genotype are summarized in Table 2. Body mass index, blood pressure, HDL-C and LDL-C did not differ significantly among the rs1260326 genotypes, although there was a trend toward lower fasting glucose among those with the T allele (Pa = 0.06).
Table 2.
Baseline clinical characteristics of the study population according to rs1260326 genotype
| CC |
CT |
TT |
P | |
|---|---|---|---|---|
| N | 398 | 292 | 80 | |
| Age, yr | 43.0 (14.1) | 43.7 (14.0) | 44.5 (13.5) | 0.36 |
| Female, % | 45.7% | 49.0% | 33.8% | 0.89 |
| BMI, kg/m2 | 26.3 (4.4) | 26.9 (4.7) | 26.0 (4.1) | 0.84 |
| Systolic blood pressure, mmHg | 121.0 (13.8) | 119.9 (14.3) | 121.8 (14.8) | 0.86 |
| Diastolic blood pressure, mmHg | 76.4 (8.2) | 76.0 (9.3) | 78.7 (9.0) | 0.45 |
| Total cholesterol, mg/dL | 209 (47) | 207 (46) | 203 (43) | 0.81 |
| HDL-C, mg/dL | 56 (15) | 56 (14) | 53 (13) | 0.73 |
| LDL-C, mg/dL | 140 (43) | 137 (42) | 134 (40) | 0.71 |
| Fasting glucose, mg/dL | 87 (10) | 86 (7) | 85 (6) | 0.06 |
| Fasting insulin, mU/dL | 9.1 (4.3) | 8.7 (3.6) | 8.9 (6.1) | 0.84 |
| Current smoker % | 10.7% | 9.0% | 12.7% | 0.73 |
Mean (SD) or frequency
Adjusted for age (except for age), sex, and family structure
Current smokers include use of cigar, pipe, and cigarettes
Fasting glucose and fasting insulin were natural logarithm transformed before analysis BMI, body mass index; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol
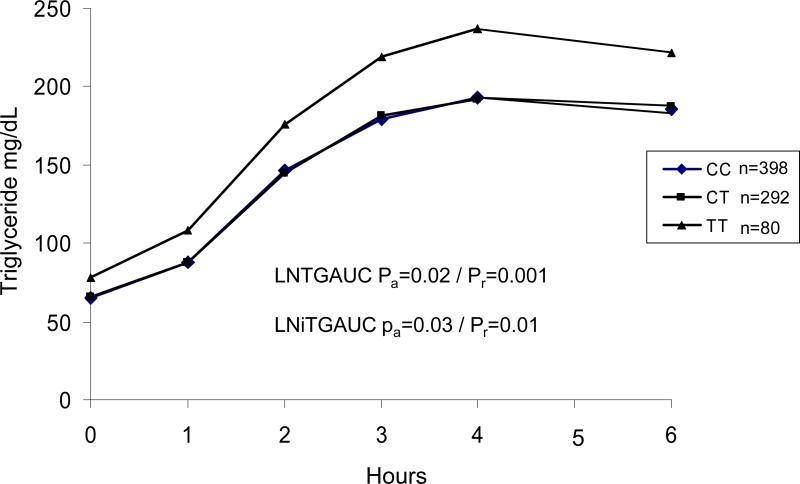
Rs1260326 was significantly associated with fasting TG after adjusting for age, sex, BMI and family structure (Pa = 0.06 for additive model, and Pr = 0.0003 for recessive model) (Table 3). This SNP explained 0.6% of residual variance in age- and sex-adjusted fasting TG levels. At each time point after fat meal, rs1260326 genotype was significantly associated with TG levels (Table 3 and Figure 1), with TT homozygotes consistently having higher TG levels. The T allele at rs1260326 was associated with higher maximum TG level (MAXTG) (Pa = 0.006) and TG area under the curve (TGAUC)(Pa = 0.02). Rs1260326 genotype was also significantly associated with incremental maximum TG (iMAXTG) and incremental TG area under the curve (iTGAUC)( Pa = 0.006 and Pa = 0.03, respectively). However, after adjusting for fasting TG, the association between genotype and post-prandial TG levels was attenuated, achieving nominal significance (P = 0.03- 0.05) only at 1 hour after the fat challenge and for MAXTG and iMAXTG.
Table 3.
Triglyceride levels at baseline and after the high fat challenge by rs1260326 genotype
| CC |
CT |
TT |
Pa | Pr | |
|---|---|---|---|---|---|
| N | 398 | 292 | 80 | ||
| Body suface area*, m2 | 1.85 (0.18) (1.30-2.36) | 1.86 (0.18) (1.34-2.29) | 1.86 (0.17) (1.49-2.27) | ||
| Calories*, cal | 1446 (142) (1020-1843) | 1455 (139) (1044-1791) | 1458 (0.17) (1169-1773) | ||
| Fasting TG, mg/dL | 55 (40, 76) | 54 (41, 77) | 66 (48, 111) | 0.06 | 0.0003 |
| Hour 1 TG, mg/dL | 71 (54, 103) | 72 (56, 98) | 90 (66,130) | 0.01 | <0.0001 |
| Hour 2 TG, mg/dL | 115 (84,164) | 114 (84, 157) | 142 (101, 212) | 0.05 | 0.0003 |
| Hour 3 TG, mg/dL | 143 (102, 209) | 147 (106, 204) | 190 (116, 268) | 0.04 | 0.001 |
| Hour 4 TG, mg/dL | 148 (106, 220) | 151 (105, 219) | 186 (127, 288) | 0.05 | 0.0002 |
| Hour 6 TG, mg/dL | 125 (88, 198) | 130 (82, 206) | 172 (109, 252 | 0.13 | 0.003 |
| MAXTG, mg/dL | 167 (123, 250) | 181 (120, 249) | 207 (149, 320) | 0.006 | <0.0001 |
| iMAXTG, mg/dL | 112 (77, 174) | 117 (78, 165) | 139 (97, 224) | 0.006 | 0.0007 |
Mean (SD) (range), body surface area = √(heightcm*weightkg)/3,600; calories are energy intake from the milk shake.
TGs are median (interquantile range).
TG levels were natural logarithm transformed before statistical analysis.
P values were adjusted for age, age2, sex, BMI and family structure.
Pa and Pr were from genetic additive and recessive models, respectively
Figure 1.
Line plots of postprandial plasma triglyceride in HAPI subjects. TGAUC, triglyceride area under curve; iTGAUC, incremental triglyceride area under curve. P values were adjusted for age, age2, sex, BMI, and family structure. System International conversion factor is 0.01129 mmol/liter for triglyceride.
The lipoprotein subfraction cholesterol levels were measured at zero and four hours after the high fat challenge. The T allele at rs1260326 was associated with significantly higher levels of fasting and postprandial response in total VLDL-C (Pa = 0.04 and Pa = 0.02 respectively), from the liver-derived triglyceride-rich lipoprotein particles, as well as with buoyant VLDL-C (Pa = 0.02 and Pa = 0.01 respectively) and small VLDL particle (VLDL3) cholesterol (Pr = 0.001 and Pa = 0.05 respectively) (Table 4). Similarly, the T allele was associated with a significantly higher postprandial response for remnant lipoprotein (Pr = 0.04), and IDL1 (Pa = 0.03) cholesterol particles. In contrast, the T allele was associated with significantly lower LDL2 (relatively buoyant pattern “A” type particles) cholesterol at four hours after the high fat challenge (Pr = 0.03).
Table 4.
Lipid subfraction levels at baseline and after the high fat challenge by rs1260326 genotype
| Fasting |
4hrs after high fat challenge |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CC |
CT |
TT |
Pa | Pr | CC |
CT |
TT |
Pa | Pr | |
| N | 350 | 264 | 77 | 350 | 264 | 77 | ||||
| Total VLDL-C* | 2.80 (0.27) | 2.79 (0.26) | 2.90 (0.27) | 0.04 | 0.0001 | 2.96 (0.27) | 2.97 (0.26) | 3.07 (0.28) | 0.02 | 0.001 |
| 1&2-buoyant VLDL-C** | 2.74 (0.38) | 2.74 (0.37) | 2.90 (0.43) | 0.02 | 0.0001 | 3.06 (0.47) | 3.08 (0.49) | 3.26 (0.55) | 0.01 | 0.0003 |
| 3-dense VLDL-C** | 3.03 (0.47) | 3.01 (0.45) | 3.17 (0.45) | 0.11 | 0.001 | 3.22 (0.41) | 3.24 (0.38) | 3.35 (0.40) | 0.05 | 0.009 |
| Remnant lipoprotein-C* | 2.73 (0.66) | 2.72 (0.65) | 2.95 (0.56) | 0.07 | 0.002 | 2.83 (0.56) | 2.85 (0.51) | 2.96 (0.60) | 0.08 | 0.04 |
| IDL-C* | 2.56 (0.61) | 2.54 (0.60) | 2.70 (0.60) | 0.18 | 0.02 | 2.58 (0.53) | 2.59 (0.48) | 2.69 (0.56) | 0.11 | 0.05 |
| IDL1-C* | 1.47 (0.51) | 1.47 (0.49) | 1.63 (0.48) | 0.10 | 0.002 | 1.56 (0.37) | 1.58 (0.37) | 1.68 (0.35) | 0.03 | 0.004 |
| IDL2-C | 8.39 (6.91) | 8.14 (6.52) | 9.39 (6.26) | 0.33 | 0.08 | 7.94 (6.31) | 7.78 (5.66) | 8.82 (5.79) | 0.27 | 0.10 |
| HDL2-C | 15.1 (6.3) | 15.0 (6.3) | 14.0 (5.4) | 0.32 | 0.11 | 15.9 (6.6) | 15.8 (6.5) | 14.9 (6.1) | 0.50 | 0.14 |
| HDL3-C | 42 (7) | 41 (7) | 40 (7) | 0.36 | 0.12 | 39 (7) | 39 (7) | 38 (6) | 0.70 | 0.23 |
| Total non-HDL-C | 158 (43) | 156 (44) | 157 (40) | 0.94 | 0.75 | 160 (43) | 157 (42) | 157 (40) | 0.93 | 0.84 |
| LDL1-C* | 2.96 (0.58) | 2.96 (0.55) | 3.02 (0.48) | 0.46 | 0.37 | 3.02 (0.52) | 3.04 (0.48) | 3.04 (0.54) | 0.56 | 0.84 |
| LDL2-C | 48 (25) | 46 (23) | 41 (22) | 0.27 | 0.05 | 48 (24) | 47 (22) | 41 (20) | 0.22 | 0.03 |
| LDL3-C | 49 (22) | 48 (23) | 50 (22) | 0.91 | 0.68 | 45 (21) | 44 (23) | 47 (21) | 0.97 | 0.49 |
| LDL4-C* | 1.18 (1.20) | 1.32 (1.12) | 1.38 (1.06) | 0.23 | 0.40 | 1.28 (1.12) | 1.39 (1.08) | 1.47 (1.01) | 0.20 | 0.41 |
Data are in mean (SD) mg/dL. P values were adjusted for age, age2, sex, BMI and family structure
Pa and Pr were from genetic additive and recessive model respectively
P values were based on natural logarithm transformed lipid values
based on square-root transformed lipid values
VLDL-C, very low density lipoprotein cholesterol; IDL-C, intermediate density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol
Discussion
In this short-term intervention study, we replicated the previously reported association between the T allele of rs1260326 in GCKR and higher fasting TG levels. We estimate that the variance in fasting TG levels accounted by rs1260326 is approximately 0.6%, an effect size comparable to the 0.2% estimated in a French population (Vaxillaire M et al. 2008) and 0.1%-1.2% explained by rs780094 across other studies (Orho-Melander M et al. 2008). Similar to previous studies that have reported the minor T allele to be associated with lower fasting glucose levels, we also find the same trends in our Amish population, although this association did not reach statistical significance. In our cohort, only four subjects had diabetes. Adjusting the data for the diabetes status or removing these individuals from analysis did not appreciably change the results.
In addition to higher fasting TG, we demonstrated that subjects with the rs1260326 T allele have higher absolute plasma postprandial TG and incremental TG concentrations, as well as postprandial VLDL-C levels (the triglyceride rich particle of liver origin). The mechanism through which GCKR influences variation in fast and postprandial lipid response (as indicated by higher iMAXTG and iTGAUC) remains to be elucidated. Possibly, this effect could be attributed to variability in VLDL production in liver. Indeed, GCKR expression is highest in human liver. Elevated TG levels may be secondary to increased glucose metabolism caused by overexpression or increased activity of GCKR, which in turn would be expected to increase GK activity. In the liver, increased glycolytic flux as a consequence of increased GK activity would be expected to increase levels of glycerol-3-phosphate and malonyl CoA. Malonyl CoA functions as the physiological inhibitor of carnitine-palmitoyl transferase I, the rate limiting enzyme for beta-oxidation, as well as an intermediate for de novo lipogenesis. Thus, increases in levels of malonyl CoA would inhibit fatty acid oxidation and drive fatty acyl-CoA into TG and VLDL synthesis (McGarry JD and Foster DW 1980; Ruderman NB et al. 2003; Karper F 1999). Animal studies of GK overexpression, which results in increased circulating TG, support this potential mechanism (O'Doherty RM et al. 1999).
The increase in plasma triglyceride concentration after a fat meal is predominantly due to triglyceride contained in apoB-48-containing lipoproteins (chylomicrons), and to a lesser extent to triglyceride contained in apoB-100-containging lipoprotein (VLDL) (Cohn JS et al. 1993). Our study did not have direct measurement of postprandial chylomicron-TG concentration, the TG-rich particle of intestinal origin. We thus do not know whether GCKR genetic variation affects absorption or intestinal trafficking of TG-rich chylomicrons.
In extrahepatic tissue, the lower serum insulin levels may result in increased lipolysis in adipose tissue, which may also contribute to higher fasting (and also perhaps postprandial) TG levels. Chronic elevation in circulating lipids could have further deleterious consequences. For instance, chronic exposure of islet beta-cells to increased levels of free fatty acids may result in “lipotoxicity” and resulting deterioration of function (Zhou YP and Grill V 1999; Carpentier AC 2008). Furthermore, chronic increases in hepatic lipogenesis and circulating lipids may lead to insulin resistance in muscle (Petersen KF and Shulman GI 2002).
Postprandial TG was found to be an independent CVD risk factor in large prospective studies (Stampfer MJ et al. 1996; Bansal S et al. 2007; Eberly LE et al. 2003; Nordestgaard BG et al. 2007) and may be superior to fasting levels for predicting CVD risk due to the fact that postprandial TG levels better indicate the levels of atherogenic remnant lipoproteins. Remnant lipoproteins, the smaller triglyceride-rich lipoprotein derived from VLDL and chylomicrons, can penetrate the arterial intima and are preferentially trapped within the arterial wall (Nordestgaard BG et al. 1995; Rutledge JC et al. 2000). The GCKR rs1260326 T allele was associated not only with greater elevation in postprandial total TG but also postprandial dense VLDL and remnant lipoprotein particle concentrations as measured by their respective cholesterol levels. Thus, those with the GCKR minor allele may be exposed to higher CVD risk and may benefit more from diets lower in fat.
The association between variants in GCKR and TG was initially identified through an agnostic GWAS approach (Saxena R et al. 2007). The TG-associated SNPs are located in a large haplotypes block spanning at least 500 kb, thus variants in GCKR or other nearby gene(s) may be causative, though the recent fine mapping study of a 117 kb region strongly supports the role of GCKR (Orho-Melander M et al. 2008). In the Amish, rs780094, the SNP initially reported to be associated with TG levels, is in strong LD with the nonsynonymous SNP rs1260326 (r2 = 0.96). Whether rs1260326/P446L is the functional SNP and responsible for the observed associations cannot be determined on statistical grounds because of the high LD of the associated SNP with other SNPs in this region, and available functional evidence (Orho-Melander M et al. 2008; Veiga-da-Cunha M et al. 2003) is equivocal. A recent study found that the carriers of both the variant allele for the GCKR rs780094 (CT or TT) and the variant alleles at one of the two APOA5 SNPs (-113T/C or 56C/G) had greater postprandial TG response than did subjects in other genotypes (Perez-Martinez P et al. 2009). This result is in line with ours, given GCKR rs780094 and rs1260326 were found to be in high LD in both Amish and other Caucasians.
In conclusion, our data suggest that the rs1260326 polymorphism affects levels of both fasting and postprandial TGs, especially liver-derived atherogenic TG-rich particles, which would be expected to increase CVD risk. The paradoxical effect of this polymorphism to increase fasting and postprandial TG but decrease fasting glucose and risk of diabetes on overall health will require additional study in prospective populations with CVD and other outcome data. Identification of the functional variant and further understanding of the mechanisms underlying these associations may provide new insights into prevention and treatment of hypertriglyceridemia and CVD.
Acknowledgements
This work supported by NIH research grants U01 HL72515, the University of Maryland General Clinical Research Center, grant M01 RR 16500, the Clinical Nutrition Research Unit of Maryland (P30 DK072488), the Baltimore Diabetes Research and Training Center grant (P60 DK079637), and the Baltimore Veterans Administration Medical Center Geriatrics Research and Education Clinical Center. We thank our Amish research volunteers for their long-standing partnership in research, and the research staff at the Amish Research Clinic for their hard work and dedication.
References
- Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- Carpentier AC. Postprandial fatty acid metabolism in the development of lipotoxicity and type 2 diabetes. Diabetes Metab. 2008;34(2):97–107. doi: 10.1016/j.diabet.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Cohn JS, Johnson EJ, Millar JS, Cohn SD, Milne RW, Marcel YL, Russell RM, Schaefer EJ. Contribution of apoB-48 and apoB-100 triglyceride-rich lipoproteins (TRL) to postprandial increases in the plasma concentration of TRL triglycerides and retinyl esters. J Lipid Res. 1993;34:2033–2040. [PubMed] [Google Scholar]
- Eberly LE, Stamler J, Neaton JD, Multiple Risk Factor Intervention Trial Research Group Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med. 2003;163(9):1077–1083. doi: 10.1001/archinte.163.9.1077. [DOI] [PubMed] [Google Scholar]
- Farrelly D, Brown KS, Tieman A, Ren J, Lira SA, Hagan D, Gregg R, Mookhtiar KA, Hariharan N. Mice mutant for glucokinase regulatory protein exhibit decreased liver glucokinase: A sequestration mechanism in metabolic regulation. Proc Natl Acad Sci U S A. 1999;96(25):14511–145161. doi: 10.1073/pnas.96.25.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre T, Riu E, Franckhauser S, Agudo J, Bosch F. Long-term overexpression of glucokinase in the liver of transgenic mice leads to insulin resistance. Diabetologia. 2003;46(12):1662–1668. doi: 10.1007/s00125-003-1244-z. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- Groot PH, van Stiphout WA, Krauss XH, Jansen H, van Tol A, van Ramshorst E, Chin-On S, Hofman A, Cresswell SR, Havekes L. Postprandial lipoprotein metabolism in normolipidemic men with and without coronary artery disease. Arterioscler Thromb. 1991;11(3):653–66227. doi: 10.1161/01.atv.11.3.653. [DOI] [PubMed] [Google Scholar]
- Karpe F. Postprandial lipoprotein metabolism and atherosclerosis. J Intern Med. 1999;246(4):341–355. doi: 10.1046/j.1365-2796.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, Cooper GM, Roos C, Voight BF, Havulinna AS, Wahlstrand B, Hedner T, Corella D, Tai ES, Ordovas JM, Berglund G, Vartiainen E, Jousilahti P, Hedblad B, Taskinen MR, Newton-Cheh C, Salomaa V, Peltonen L, Groop L, Altshuler DM, Orho-Melander M. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40(2):189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster B, Fenger M, Poulsen P, Vaag A, Bentzen J. Novel polymorphisms in the GCKR gene and their influence on glucose and insulin levels in a danish twin population. Diabet Med. 2005;22(12):1677–1682. doi: 10.1111/j.1464-5491.2005.01700.x. [DOI] [PubMed] [Google Scholar]
- Kugiyama K, Doi H, Takazoe K, Kawano H, Soejima H, Mizuno Y, Tsunoda R, Sakamoto T, Nakano T, Nakajima K, Ogawa H, Sugiyama S, Yoshimura M, Yasue H. Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation. 1999;99(22):2858–2860. doi: 10.1161/01.cir.99.22.2858. [DOI] [PubMed] [Google Scholar]
- Kulkarni KR. Cholesterol profile measurement by vertical auto profile method. Clin Lab Med. 2006;26(4):787–8023. doi: 10.1016/j.cll.2006.07.004. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Mitchell BD, McArdle PF, Shen H, Rampersaud E, Pollin TI, Bielak LF, Jaquish C, Douglas JA, Roy-Gagnon MH, Sack P, Naglieri R, Hines S, Horenstein RB, Chang YP, Post W, Ryan KA, Brereton NH, Pakyz RE, Sorkin J, Damcott CM, O'Connell JR, Mangano C, Corretti M, Vogel R, Herzog W, Weir MR, Peyser PA, Shuldiner AR. The genetic response to short-term interventions affecting cardiovascular function: Rationale and design of the heredity and phenotype intervention (HAPI) heart study. Am Heart J. 2008;155(5):823–828. doi: 10.1016/j.ahj.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Non-fasting triglycerides and risk of for myocardial infarction and death among women and men. Ugeskr Laeger. 2007;169(45):3865–3868. [PubMed] [Google Scholar]
- Nordestgaard BG, Wootton R, Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol. 1995;15(4):534–542. doi: 10.1161/01.atv.15.4.534. [DOI] [PubMed] [Google Scholar]
- O'Connell JR, Weeks DE. PedCheck: A program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63(1):259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty RM, Lehman DL, Telemaque-Potts S, Newgard CB. Metabolic impact of glucokinase overexpression in liver: Lowering of blood glucose in fed rats is accompanied by hyperlipidemia. Diabetes. 1999;48(10):2022–2027. doi: 10.2337/diabetes.48.10.2022. [DOI] [PubMed] [Google Scholar]
- Ordovas JM, Corella D. Nutritional genomics. Annu Rev Genomics Hum Genet. 2004;5:71–118. doi: 10.1146/annurev.genom.5.061903.180008. [DOI] [PubMed] [Google Scholar]
- Orho-Melander M, Melander O, Guiducci C, Perez-Martinez P, Corella D, Roos C, Tewhey R, Rieder MJ, Hall J, Abecasis G, Tai ES, Welch C, Arnett DK, Lyssenko V, Lindholm E, Saxena R, de Bakker PI, Burtt N, Voight BF, Hirschhorn JN, Tucker KL, Hedner T, Tuomi T, Isomaa B, Eriksson KF, Taskinen MR, Wahlstrand B, Hughes TE, Parnell LD, Lai CQ, Berglund G, Peltonen L, Vartiainen E, Jousilahti P, Havulinna AS, Salomaa V, Nilsson P, Groop L, Altshuler D, Ordovas JM, Kathiresan S. A common missense variant in the glucokinase regulatory protein gene (GCKR) is associated with increased plasma triglyceride and C-reactive protein but lower fasting glucose concentrations. Diabetes. 2008 doi: 10.2337/db08-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martinez P, Corella D, Shen J, Arnett DK, Yiannakouris N, Tai ES, Orho-Melander M, Tucker KL, Tsai M, Straka RJ, Province M, Kai CS, Perez-Jimenez F, Lai CQ, Lopez-Miranda J, Guillen M, Parnell LD, Borecki I, Kathiresan S, Ordovas JM. Association between glucokinase regulatory protein (GCKR) and apolipoprotein A5 (APOA5) gene polymorphisms and triacylglycerol concentrations in fasting, postprandial, and fenofibrate-treated states. Am J Clin Nutr. 2009;89(1):391–9. doi: 10.3945/ajcn.2008.26363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol. 2002;90(5A):11G–18G. doi: 10.1016/s0002-9149(02)02554-7. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Saha AK, Kraegen EW. Minireview: Malonyl CoA, AMP-activated protein kinase, and adiposity. Endocrinology. 2003;144(12):5166–5171. doi: 10.1210/en.2003-0849. [DOI] [PubMed] [Google Scholar]
- Rutledge JC, Mullick AE, Gardner G, Goldberg IJ. Direct visualization of lipid deposition and reverse lipid transport in a perfused artery : Roles of VLDL and HDL. Circ Res. 2000;86(7):768–773. doi: 10.1161/01.res.86.7.768. [DOI] [PubMed] [Google Scholar]
- Diabetes Genetics Initiative of Broad Institute of Harvard and MIT. Lund University. Novartis Institutes of BioMedical Research. Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316(5829):1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in finns detects multiple susceptibility variants. Science. 2007;316(5829):1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slosberg ED, Desai UJ, Fanelli B, St Denny I, Connelly S, Kaleko M, Boettcher BR, Caplan SL. Treatment of type 2 diabetes by adenoviral-mediated overexpression of the glucokinase regulatory protein. Diabetes. 2001;50(8):1813–1820. doi: 10.2337/diabetes.50.8.1813. [DOI] [PubMed] [Google Scholar]
- Sparso T, Andersen G, Nielsen T, Burgdorf KS, Gjesing AP, Nielsen AL, Albrechtsen A, Rasmussen SS, Jorgensen T, Borch-Johnsen K, Sandbaek A, Lauritzen T, Madsbad S, Hansen T, Pedersen O. The GCKR rs780094 polymorphism is associated with elevated fasting serum triacylglycerol, reduced fasting and OGTT-related insulinaemia, and reduced risk of type 2 diabetes. Diabetologia. 2008;51(1):70–75. doi: 10.1007/s00125-007-0865-z. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276(11):882–888. [PubMed] [Google Scholar]
- Vaxillaire M, Cavalcanti-Proenca C, Dechaume A, Tichet J, Marre M, Balkau B, Froguel P, DESIR Study Group The common P446L polymorphism in GCKR inversely modulates fasting glucose and triglyceride levels and reduces type 2 diabetes risk in the DESIR prospective general french population. Diabetes. 2008;57(8):2253–2257. doi: 10.2337/db07-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-da-Cunha M, Delplanque J, Gillain A, Bonthron DT, Boutin P, Van Schaftingen E, Froguel P. Mutations in the glucokinase regulatory protein gene in 2p23 in obese french caucasians. Diabetologia. 2003;46(5):704–711. doi: 10.1007/s00125-003-1083-y. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, Strait J, Duren WL, Maschio A, Busonero F, Mulas A, Albai G, Swift AJ, Morken MA, Narisu N, Bennett D, Parish S, Shen H, Galan P, Meneton P, Hercberg S, Zelenika D, Chen WM, Li Y, Scott LJ, Scheet PA, Sundvall J, Watanabe RM, Nagaraja R, Ebrahim S, Lawlor DA, Ben-Shlomo Y, Davey-Smith G, Shuldiner AR, Collins R, Bergman RN, Uda M, Tuomilehto J, Cao A, Collins FS, Lakatta E, Lathrop GM, Boehnke M, Schlessinger D, Mohlke KL, Abecasis GR. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40(2):161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YP, Grill V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of langerhans. J Clin Endocrinol Metab. 1995;80(5):1584–1590. doi: 10.1210/jcem.80.5.7745004. [DOI] [PubMed] [Google Scholar]