Abstract
This study seeks to measure the treatment effect of glucose–insulin–potassium (GIK) infusion on mortality in critically ill patients. A systematic review of randomized controlled trials is conducted, comparing GIK treatment with standard care or placebo in critically ill adult patients. The primary outcome variable is mortality. Two authors independently extract data and assess study quality. The primary analysis is based on the random effects model to produce pooled odds ratios (ORs) with 95% confidence intervals (CIs). The search yields 1720 potential publications; 23 studies are included in the final analysis, providing a sample of 22 525 patients. The combined results demonstrate no heterogeneity (P = .57, I2 = 0%) and no effect on mortality (OR = 1.02; 95% CI, 0.93–1.11) with GIK treatment. No experimental studies of shock or sepsis populations are identified. This meta-analysis finds that there is no mortality benefit to GIK infusion in critically ill patients; however, study populations are limited to acute myocardial infarction and cardiovascular surgery patients. No studies are identified using GIK in patients with septic shock or other forms of circulatory shock, providing an absence of evidence regarding the effect of GIK as a therapy in patients with shock.
Keywords: Clinical research, critical care, emergency medicine, clinical trials, anesthesiology
Infusion of a glucose–insulin–potassium (GIK) solution was first proposed in the 1960s as treatment for acute myocardial infarction (AMI) with the intention of providing electrical stability to the myocardium.1 The proposed mechanisms of action of GIK therapy in cardiovascular disease include suppression of circulating levels and myocardial uptake of free fatty acids, which are toxic to ischemic myocardium; improvement in the efficiency of myocardial energy production through the provision of exogenous glucose; and stabilization of intracellular potassium, which may be depleted during times of myocardial ischemia.2,3 Since the sentinel report, numerous randomized controlled trials have been performed using GIK. Pooled data have suggested morbidity and mortality benefits associated with GIK therapy in cardiothoracic surgery4 and AMI3 populations. The largest GIK study performed in AMI patients to date failed to find similar benefits.2 Thus, there remain significant conflicting evidence and debate regarding the utility of GIK infusion in various patient populations.
Extensive preclinical rationales support the use of GIK as an inotropic and metabolic therapy in several critical illnesses. The heart develops insulin resistance in shock and sepsis, and insulin-euglycemia produces a potent inotropic effect on the left ventricle.5,6 Sepsis can lead to excessive phosphorylation of the pyruvate dehydrogenase complex, thus impairing net carbohydrate oxidation in skeletal and heart muscle.7–9 Deficits in carbohydrate oxidation can be expected to be worsened by the effect of diabetes mellitus, a common comorbidity in humans.10 Insulin reactivates the pyruvate dehydrogenase complex, via activation of pyruvate dehydrogenase phosphatase, to permit maximal glucose and lactate oxidation by heart muscle.11 Additionally, it can be hypothesized that via its action on the tyrosine kinase pathway, insulin can reduce intracellular signals that lead to the production of inflammatory molecules that possess negative inotropic properties, including tumor necrosis factor.12
Recognizing that numerous clinical trials of GIK infusion in various critically ill populations have been conducted over the past 4 decades, we hypothesized that their results could be aggregated to produce more definitive conclusions about the treatment effect of GIK infusion in the critically ill. Therefore, we conducted a systematic review and meta-analysis to examine the available evidence investigating GIK treatment of critically ill patients; our goal was to determine the treatment effect of such a strategy on mortality and to determine whether certain predefined subpopulations (patients with septic shock or other forms of circulatory shock) are afforded survival benefit with GIK treatment.
METHODS
Search Strategy for Identification of Studies
We followed a written protocol that was designed in accordance with recommended guidelines and finalized prior to beginning the study.13 We searched MEDLINE (1965 to April 2008), EMBASE (1974 to April 2008), and CINHAL (1982 to April 2008) using the following search terms: (critical care or intensive care unit or CABG or coronary artery bypass or cardiac surgery or heart surgery or myocardial infarction or stroke or sepsis or shock) and (GIK or glucose–insulin–potassium or glucose-insulin or glucose or insulin or hyperinsulinemia-euglycemia) and clinical trial. The search was updated in October 2008. To identify potential unpublished data, we contacted 2 experts in the field of critical care, searched abstracts from the Society of Critical Care Medicine and American College of Chest Physicians annual meetings from 1997 to 2007, reviewed published practice guidelines for critical care from 2000 to 2007, and searched Web sites containing details for clinical trial registration. Additionally, reference lists of the articles chosen for inclusion and the reference lists of previous reviews3,4,14,15 were screened to identify further studies for inclusion.
Inclusion Criteria
We considered studies eligible for review, regardless of language or publication type, if they were randomized controlled trials of human adults (age >17 years) with critical illness (defined as either patients enrolled in an intensive care unit [ICU] or operating room or a control group with a mortality rate of greater than or equal to 15%) that had (1) a clearly defined control group in which patients received standard of care therapy or placebo and (2) an intervention group in which patients received a continuous infusion of insulin, glucose, and potassium. Reviews, correspondence, editorials, and nonhuman studies were excluded; however, their reference lists were screened, if relevant, to identify further studies for inclusion. We attempted to contact corresponding authors for clarification of missing or incomplete data.
Study Selection and Data Abstraction
Two reviewers (MAP and AEJ) independently screened the titles and abstracts of identified studies for potential eligibility. After the relevance search, the 2 reviewers compared their exclusion logs, and the κ statistic was calculated to assess interobserver agreement. Cases of disagreement were resolved by conference between the reviewers. If agreement could not be reached, the full manuscript was obtained for review.
The full manuscript of each study that passed the relevance screen was reviewed by 2 of the investigators (MAP and MSR). Study data were abstracted independently by each reviewer using a standardized data collection form. In cases of disagreement in abstracted data, a third reviewer abstracted the data and consensus was reached by conference between the 3 reviewers.
Assessment of Quality
Quality was assessed using the Cochrane Collaboration’s tool for assessing the risk of bias evaluating 4 domains13:
Sequence generation: grade A = sequence was adequately generated, grade B = sequence was inadequately generated, grade C = unknown.
Concealment allocation: grade A = performed concealment allocation, grade B = did not use concealment allocation, grade C = unknown.
Blinding: grade A = investigators blinded to allocation, grade B = investigators not blinded to allocation, grade C = unknown.
Selective outcome reporting: grade A = free of suggestion of selective outcome reporting, grade B = suggestion of selective outcome reporting, grade C = unknown.
Main Outcome Measure
We defined mortality at the end of the time frame reported by the authors to be the primary dependent variable for analysis. In the event authors reported mortality at more than one time point, we used inhospital mortality preferentially.
Statistical Analysis
All data were entered into StatsDirect version 2.7.2 (StatsDirect, Cheshire, UK). Heterogeneity was tested using a chi-square test with a P value <.10 to indicate significant heterogeneity between the trials. In addition, I2 was calculated to provide an estimate of the variability across studies beyond that attributable to chance.16 The results of studies were pooled using a random effects model.17 The individual and pooled statistics were calculated as odds ratios (ORs) with 95% confidence intervals (CIs). The validity of using funnel plots and formal statistical methods to detect the presence of publication bias has recently been called into question; therefore, a funnel plot was constructed for visual inspection but we do not attempt to explain any asymmetry.18,19 Additionally, we collected data on the adverse events of hypoglycemia and hyperkalemia, as defined by the authors of the respective publications, in the control and GIK arms. We report them as a proportion of the total enrolled populations among the publications that reported adverse events and according to the respective study group.
Subgroup and Sensitivity Analysis
Given the large amount of clinical heterogeneity attributable to our definition of critically ill patients and the wide range of year of publication and practice style, we conducted the following a priori defined subgroup analyses: (1) high-quality studies (studies rated grade A for at least 3 of 4 quality domains previously discussed; (2) studies of patients with septic shock (using standard criteria); and (3) studies of patients with other forms of circulatory shock (as defined by each study). Finally, a sensitivity analysis was performed to examine the use of a fixed effect versus random effects model.
We performed a post hoc (not described in our original protocol) analysis of patients with acute myocardial infarction (AMI) only and those undergoing cardiovascular surgery (CVS) only. We chose to perform these subgroup analyses after data collection given that these were the only 2 patient populations represented in the included studies.
RESULTS
Search and Selection
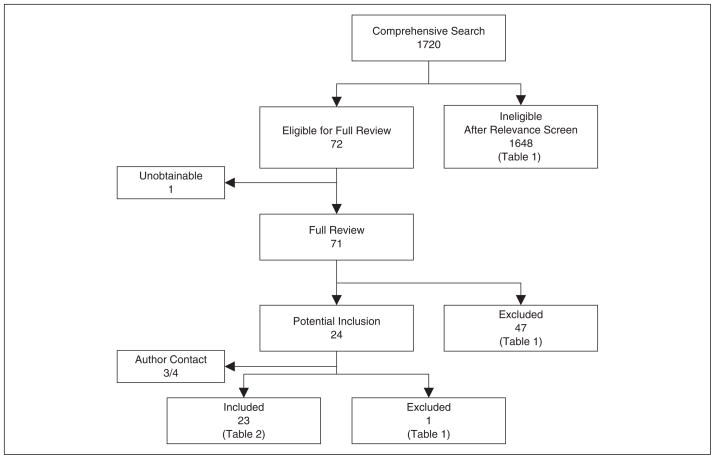
The comprehensive search yielded a total of 1720 relevant publications; details of the search and study selection are provided in Figure 1 and Table I.
Figure 1.
Search, inclusion, and exclusion flow diagram.
Table I.
Primary Reason for Study Exclusion
| After relevance screen | |
| Review/letter/editorial | 384 |
| Nonrandomized/nontreatment study | 20 |
| Wrong focus or population | 1244 |
| After full manuscript review | |
| Review article | 1 |
| Duplicate publication | 4 |
| Not critically ill | 16 |
| Wrong outcome | 18 |
| Wrong treatment | 5 |
| Wrong focus or population | 3 |
| Manuscript unobtainable for review | 1 |
| After attempted author contact | |
| Failed author contact | 1 |
| Total | 1697 |
Inclusion
After the relevance search (blinded interobserver agreement of 98% with 30 disagreements among 1720 articles, κ = 0.79), a complete manuscript review was performed on 71 articles; 1 article was unobtainable for review despite multiple requests to the National Libraries of Medicine, Online Computer Library Center, Pramukh Swami Medical College Library (India), PSG Institute of Medical Sciences and Research (India), Regional Medical Research Centre for Tribals Central Library (India), KG Medical University (India), and e-mail requests to the editor of the journal and all of the primary and secondary authors.
A total of 23 studies met our predefined criteria and were included in the final analysis, providing a total sample of 22 525 patients.2,20–41 Three full-length articles were translated to English from French,23 Turkish,31 and Chinese.40 All 3 met our criteria and were included in the final analysis.
Study Descriptions
The incidence of mortality in the studies ranged from 0 to 36% in the control group and 0 to 16% in the study group. Nine studies took place in an ICU, 13 studies took place in an operating room, and in 1 study the location was unclear but the predefined control group mortality rate allowed inclusion of the study. Pertinent details of the included studies are provided in Table II.
Table II.
Summary of Included Studies
| Author | Year | Indication | Study Setting | GIK Formulation, U/kg/h insulin | Infusion Duration, h | Mortality Timing | Treatment, N | Control, N | Treatment Mortality, n | Control Mortality, n | Sequence Generationa | Allocation Concealmentb | Blindingc | Selective Outcome Reportingd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pentecost | 1968 | AMI | ICU | Not weight-based | 48 | In-hospital | 100 | 100 | 15 | 16 | C | C | A | B |
| Thys | 1974 | AMI | ICU | Not weight-based | 168 | In-hospital | 85 | 89 | 14 | 10 | C | C | C | C |
| Rackley | 1976 | AMI | ICU | 0.075 | 48 | In-hospital | 8 | 8 | 1 | 2 | C | C | C | C |
| Stanley | 1978 | AMI Unclear | 0.075 | 48 | In-hospital | 55 | 55 | 4 | 9 | C | C | A | C | |
| Mantle | 1981 | AMI | ICU | 0.075 | 48 | In-hospital | 41 | 44 | 2 | 4 | C | C | C | B |
| Rogers | 1982 | AMI | ICU | 0.075 | 48 | In-hospital | 96 | 94 | 4 | 9 | C | C | C | B |
| Oldfield | 1986 | MVR | OR | Not weight-based | 12 | In-hospital | 20 | 23 | 0 | 2 | C | C | C | C |
| Coleman | 1989 | CABG | OR | 0.08 | 48 | 60-d | 11 | 11 | 1 | 4 | C | C | A | B |
| Lazar | 1997 | CABG | OR | 0.05 | 12+ | In-hospital | 16 | 15 | 0 | 0 | B | C | C | B |
| Lazar | 2000 | CABG | OR | Not weight-based | 12+ | In-hospital | 20 | 20 | 0 | 0 | B | C | C | A |
| Turkoz | 2000 | CABG | OR | 0.14 | Othere | In-hospital | 15 | 16 | 1 | 0 | C | C | C | B |
| Lindholm | 2001 | CABG±AVR | OR | Not weight-based | Otherg | In-hospital | 16 | 14 | 0 | 1 | C | C | C | C |
| Smith | 2002 | CABG | OR | 0.05 | 10 | 30-d | 22 | 22 | 0 | 0 | A | C | A | B |
| Lell | 2002 | CABG | OR | 0.075 | 12 | In-hospital | 21 | 20 | 0 | 0 | C | A | A | B |
| Lazar | 2003 | CABG | OR | Not weight-based | 12 | 30-d | 72 | 69 | 0 | 0 | C | C | A | A |
| Pache | 2004 | AMI | ICU | 0.072 | 24 | 30-d | 155 | 157 | 7 | 5 | A | C | A | A |
| Krljanac | 2005 | AMI | ICU | 0.05 | 24 | 1-y | 78 | 40 | 3 | 4 | C | C | A | C |
| Koskenkari | 2005 | CABG+AVR | OR | 1 | 6+ | In-hospital | 20 | 20 | 0 | 1 | C | C | C | B |
| Mehta | 2005 | AMI | ICU | 0.075 | 24 | 30-d | 10 088 | 10 107 | 1004 | 976 | A | A | A | A |
| Ranasinghe | 2006 | CABG | OR | 0.0525 | 6+ | In-hospital | 157 | 160 | 3 | 3 | A | A | A | A |
| Quinn | 2006 | CABG | OR | 0.0525 | 6+ | In-hospital | 138 | 142 | 3 | 2 | A | A | A | C |
| Li | 2006 | AMI | ICU | 0.075 | 24 | In-hospital | 9 | 12 | 0 | 0 | C | C | C | C |
| Zuurbier | 2008 | CABG | OR | 0.1 | 24+ | In-hospital | 23 | 21 | 0 | 0 | A | A | C | C |
AMI, acute myocardial infarction; AVR, aortic valve replacement; CABG, coronary artery bypass grafting; GIK, glucose–insulin–potassium; ICU, intensive care unit; MVR, mitral valve replacement; N, number of subjects in each group; n, number of subjects with primary outcome in each group.
Sequence generation: grade A = adequate, grade B = inadequate, grade C = unknown.
Allocation concealment: grade A = performed, grade B = not performed, grade C = unknown.
Blinding: grade A = blinded, grade B = not blinded, grade C = unknown.
Selective outcome reporting: grade A = not suggested, grade B = suggested, grade C = unknown.
GIK administered from induction of anesthesia until the beginning of cardiopulmonary bypass (CPB).
GIK administered from 15 minutes before rewarming until 30 minutes after CPB discontinued.
Quality Assessment
Two authors (MAP, MSR) independently graded the publications on the measures of sequence generation, concealment of allocation, blinding, and selective outcome reporting. Four studies were graded A in 3 of 4 criteria,2,35,38,39 meeting our definition of high quality.
Analyses
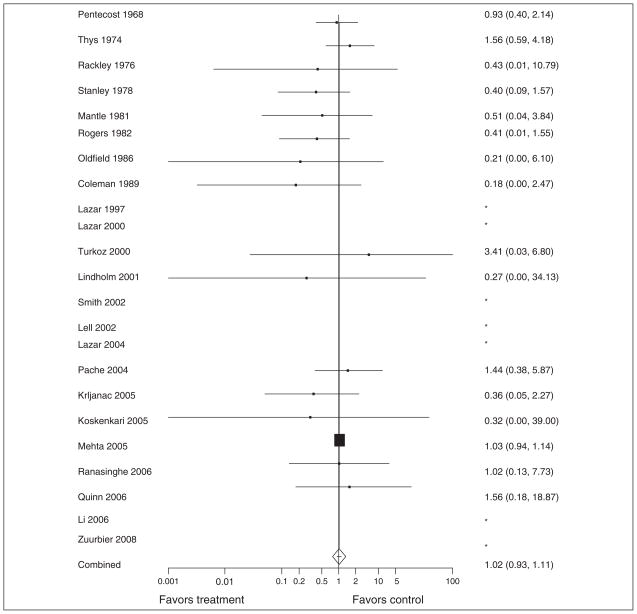
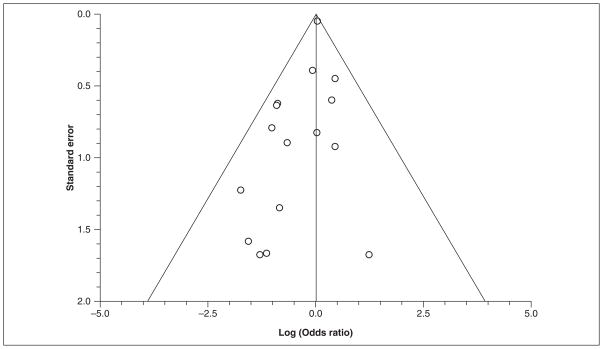
When all 23 studies were considered, there was no evidence of statistical heterogeneity (P = .57; I2 = 0%); however, there was clinical heterogeneity among the studies. The summary OR demonstrates no significant reduction in mortality (OR = 1.02; 95% CI, 0.93–1.11) among subjects receiving GIK (Figure 2). To assess for potential publication bias, a funnel plot of all included studies is provided in Figure 3.
Figure 2.
Forest plot of all included studies. Odds ratios with 95% confidence intervals are shown on the right and the corresponding study is shown on the left. *Studies that were excluded from the summary odds ratio calculation because there were no occurrences of the primary outcome in the study.
Figure 3.
Funnel plot of included studies. Individual included studies are represented by dots and are plotted by the standard error (SE) on the y-axis and the log odds ratio on the x-axis. This plot is provided for visual inspection for detection of publication bias.
Subgroup analysis of high-quality studies (defined above and shown in Table 2), studies with only AMI patients (10 studies, Table 2), and studies with only CVS patients (13 studies, Table 2) similarly showed no mortality reduction among subjects who received GIK (OR = 1.04; 95% CI, 0.95–1.14 for high-quality studies; OR = 0.98; 95% CI, 0.81–1.19 for AMI studies; and OR = 0.70; 95% CI, 0.29–1.72 for CVS studies). None of the above point estimates were significantly different when using a fixed effect model.
Of the 22 525 patients included in the final analysis, 20 195 (89.7%) were enrolled in a single, multi-center study of GIK in AMI patients.2 We performed a post hoc analysis to determine whether this study was dominating the model because of its size. When this study was excluded and the analysis repeated, there was no significant difference in the treatment effect (OR = 0.78; 95% CI, 0.54–1.11).
Table III provides a summary of all of the studies involving GIK administration to patients with septic shock.42–47 None of the studies with septic shock populations were randomized trials; thus, none were appropriate for inclusion in our meta-analysis. Additionally, our comprehensive literature search did not identify any randomized trials of GIK treatment in other circulatory shock populations, and no subpopulations of circulatory shock patients were reported in any of the studies meeting our inclusion criteria.
Table III.
Summary of Studies in Sepsis Populations
| Study | N | GIK Formulation | Duration of Infusion | Mortality | % Change MAP Post-GIK | % Change Cardiac Function Post-GIK | Adverse Events |
|---|---|---|---|---|---|---|---|
| Clowes 1974 | 10 | 1 g/kg glucose; 1.5 U/kg insulin; 10 mEq KCL | 10 min | 40% | 30% increase | 187% increase (CO) | None reported |
| Weisul 1975 | 6 | 1 g/kg glucose; 1.5 U/kg insulin; 15 mEq KCL | 10 min | 67% | 30% increase | 244% increase (CI) | None reported |
| Vincent 1981 | 6 | 75 g glucose; 100 U insulin; 15 mEq KCL | 40 min | NR | NR | 27% increase (CO) | None reported |
| Bronsveld 1985 | 15 | 1 g/kg 50% glucose; 1.5 U/kg insulin; 10 mmol KCL | 20 min | 67% | 5% increase | 12.5% increase (CI) | None reported |
| Mauritz 1986 | 14 | 1 g/kg 70% glucose; 1.5 U/kg insulin; 10 mmol KCL | 15 min | 86% | 61% increase | 50% increase (CI) | None reported |
| Hamdulay 2006 | 2 | 30% glucose; 50 U insulin; 80 mmol KCL | 18–72 h | 100% | 9% increase | 88% increase (CO) | None reported |
CI, cardiac index; CO, cardiac output; GIK, glucose–insulin–potassium; KCL, potassium chloride; MAP, mean arterial pressure; N, number of patients in study; NR, not reported.
Six studies2,29,36,37,39,40 explicitly reported the number of occurrences of hypoglycemia, and among these studies the incidence of hypoglycemia in GIK patients was 0.5% (54/10 324) and in controls was 0.1% (11/10 309). Four studies2,31,36,40 explicitly reported the number of occurrences of hyperkalemia, and among these studies the incidence of hyperkalemia was 4.3% (434/10 166) in GIK patients and 1.6% (161/10 147) in controls.
DISCUSSION
This meta-analysis evaluates the treatment effect of GIK therapy in critically ill patients. Using pooled data from 23 studies that randomized a total of 22 525 subjects, we found no significant effect on mortality (OR = 1.02; 95% CI = 0.93–1.11). We identified no studies that included septic shock or other forms of circulatory shock patients in an experimental design. Of the studies reporting adverse events, hyperkalemia occurred more commonly than hypoglycemia; however, both had low cumulative incidence.
This systematic review used both a comprehensive search and standard methods for summarizing the treatment effect of GIK therapy in critically ill patients. The subgroup analyses we present included the high-quality studies, studies with AMI populations only, and studies with CVS populations only. None of these analyses demonstrated significant mortality benefits associated with administration of GIK. Our other subgroup analyses were to examine only populations of septic shock and other forms of circulatory shock; however, we were unable to identify any experimental studies examining these groups of patients. This was surprising to us given the amount of basic science research that supports the potential physiological benefits of GIK therapy for septic shock.48–52 Because we specifically sought out studies of septic shock and were unable to include any in the analysis, this study adds to previously published meta-analyses of GIK by allowing a conclusion that the current literature provides an absence of evidence regarding the effect of GIK as a therapy for septic shock.
It is important to note the clinical heterogeneity among the studies included in this analysis. All of the included studies were either in patients with the diagnosis of AMI (10 studies) or in CVS patients (13 studies). Thus, our results are limited to those populations and should not be extrapolated to heterogeneous groups of critically ill patients or those with other diagnoses such as acute respiratory distress syndrome or sepsis. Furthermore, the studies in this analysis span a time frame of 40 years. Many new therapeutic options, particularly for myocardial infraction and cardiothoracic surgery, have changed during this time. A limitation of this meta-analysis is the possibility that co-interventions (eg, thrombolytic or percutaneous coronary angioplasty) may have influenced the results of included studies; there was no practical way to capture or control for such interventions in this meta-analysis.
One previous meta-analysis, by Pittas et al, examined the effect of insulin therapy for critically ill hospitalized patients.15 Pittas et al found that insulin therapy initiated in the hospital has beneficial effects on short-term mortality in critically ill patients. There are several plausible explanations for the different findings between the Pittas study and the present analysis. First, the Pittas analysis included studies in which patients were treated with any type or form of insulin therapy, whereas we limited our inclusion to only studies that administered a GIK solution. Second, the Pittas study used a broad definition of critical illness by including all patients with AMI and stroke and those in the ICU or operating room. This broad inclusion may have resulted in misclassification of critical illness and affected the results. The definition of critical illness used in the present analysis was more conservative, most likely resulting in a more homogenous sample. Finally, the sample of patients in this meta-analysis is 5 times larger (22 525 vs 4733) than the sample of subjects that received GIK in the Pittas study, resulting in a much higher power to detect the true treatment effect.
As previously mentioned, 20 195 of the 22 525 (89.7%) subjects included in this meta-analysis were from a single publication (Mehta et al2). In an attempt to control for the potential for this study to dominate the analysis, we performed a subgroup analysis where we excluded this report from the analysis and re-performed the statistical model. The resulting odds ratio was 0.78 (95% CI, 0.54–1.11), and although this was not statistically significant, there is clearly a trend toward improvement in mortality with the odds ratio well below 1 and the upper bounds of the confidence interval just above 1. This suggests that this large study had an important influence on our overall results; however, we are reassured by the finding that the statistical significance of the subgroup analysis (nonsignificant mortality reduction) was the same as our overall findings.
Any meta-analysis is prone to bias given its retrospective approach; however, we used a number of important steps in an attempt to minimize the impact of bias. First, we followed a protocol that was written prior to starting the search and analysis. Second, we used independent observers to both abstract data and grade study quality. Third, our meta-analysis addressed all of the suggested necessary components of the AMSTAR methodological quality measurement tool for systematic reviews.53
CONCLUSION
Data synthesized from 23 randomized controlled trials demonstrated no treatment effect of GIK therapy in critically ill patients; however, study populations included were limited to acute myocardial infarction and cardiovascular surgery patients. No studies were identified that used GIK in patients with septic shock or other forms of circulatory shock, providing an absence of evidence regarding GIK as a treatment for shock. Adverse events related to GIK infusion were uncommon. Future studies should explore the potential benefits of GIK in sepsis, shock, or other heterogeneous critically ill populations in experimental research designs.
Acknowledgments
The authors thank the following corresponding authors: Shamir Mehta, Adnan Kastrati, and C. J. Zuurbier. In addition, we thank Ming Qi, MD, for help with article translation and both Bridget Loven and Leonora Kaufmann for help with the literature search and article retrieval.
Financial disclosure: Alan E. Jones has received research support from Critical Biologics and Hutchinson Technology, and Stephen Trzeciak has received research support from Novo Nordisk, Biosite, and Eli Lilly.
References
- 1.Fishleder BL, Frieldand C, De MA, et al. Effects of an intravenous infusion of a potassium-glucose-insulin solution on the electrocardiographic signs of myocardial infarction: a preliminary clinical report. Am J Cardiol. 1962;9:166–181. doi: 10.1016/0002-9149(62)90035-8. [DOI] [PubMed] [Google Scholar]
- 2.Mehta SR, Yusuf S, Diaz R, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437–446. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- 3.Fath-Ordoubadi F, Beatt KJ. Glucose-insulin-potassium therapy for treatment of acute myocardial infarction: an overview of randomized placebo-controlled trials. Circulation. 1997;96:1152–1156. doi: 10.1161/01.cir.96.4.1152. [DOI] [PubMed] [Google Scholar]
- 4.Bothe W, Olschewski M, Beyersdorf F, et al. Glucose-insulin-potassium in cardiac surgery: a meta-analysis. Ann Thorac Surg. 2004;78:1650–1657. doi: 10.1016/j.athoracsur.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Law WR, McLane MP, Raymond RM. Effect of insulin on myocardial contractility during canine endotoxin shock. Cardiovasc Res. 1988;22:777–785. doi: 10.1093/cvr/22.11.777. [DOI] [PubMed] [Google Scholar]
- 6.Raymond RM, McLane MP, Law WR, et al. Myocardial insulin resistance during acute endotoxin shock in dogs. Diabetes. 1988;37:1684–1688. doi: 10.2337/diab.37.12.1684. [DOI] [PubMed] [Google Scholar]
- 7.Vary TC, Hazen S, Vary TC, et al. Sepsis alters pyruvate dehydrogenase kinase activity in skeletal muscle. Mol Cell Biochem. 1999;198:113–118. doi: 10.1023/a:1006993910781. [DOI] [PubMed] [Google Scholar]
- 8.Vary TC. Sepsis-induced alterations in pyruvate dehydrogenase complex activity in rat skeletal muscle: effects on plasma lactate [abstract] Shock. 1996;6:89–94. doi: 10.1097/00024382-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Vary TC, Siegel JH, Nakatani T, et al. Effect of sepsis on activity of pyruvate dehydrogenase complex in skeletal muscle and liver. Am J Physiol. 1986;250:E634–E640. doi: 10.1152/ajpendo.1986.250.6.E634. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi K, Neely JR. Effects of increased cardiac work on pyruvate dehydrogenase activity in hearts from diabetic animals. J Mol Cell Cardiol. 1983;15:347–357. doi: 10.1016/0022-2828(83)90319-x. [DOI] [PubMed] [Google Scholar]
- 11.Hutson NJ, Kerbey AL, Randle PJ, et al. Regulation of pyruvate dehydrogenase by insulin action. Prog Clin Biol Res. 1979;31:707–719. [PubMed] [Google Scholar]
- 12.Kumar A, Thota V, Dee L, et al. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 4.2.6. Chichester, UK: Wiley; 2006. [Google Scholar]
- 14.Schipke JD, Friebe R, Gams E. Forty years of glucose-insulin-potassium (GIK) in cardiac surgery: a review of randomized, controlled trials. Eur J Cardiothorac Surg. 2006;29:479–485. doi: 10.1016/j.ejcts.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2004;164:2005–2011. doi: 10.1001/archinte.164.18.2005. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter JE, Schmidt FL. Fixed effects vs random effects meta-analysis models: implications for cumulative research knowledge. International Journal of Selection and Assessment. 2000;8:275–292. [Google Scholar]
- 18.Terrin N, Schmid CH, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J Clin Epidemiol. 2005;58:894–901. doi: 10.1016/j.jclinepi.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Tang JL, Liu JL. Misleading funnel plot for detection of bias in meta-analysis. J Clin Epidemiol. 2000;53:477–484. doi: 10.1016/s0895-4356(99)00204-8. [DOI] [PubMed] [Google Scholar]
- 20.Pentecost BL, Mayne NM, Lamb P. Controlled trial of intravenous glucose, potassium, and insulin in acute myocardial infarction. Lancet. 1968;1:946–948. doi: 10.1016/s0140-6736(68)90903-3. [DOI] [PubMed] [Google Scholar]
- 21.Rackley CE, Rogers WJ, McDaniel HG, et al. Randomized study of glucose insulin potassium in patients with acute myocardial infarction [abstract] Clin Res. 1976;24:421A. [Google Scholar]
- 22.Stanley AWH, Jr, Prather JW. Glucose-insulin-potassium, patient mortality and the acute myocardial infarction: results from a prospective randomized study [abstract] Circulation. 1978;58(Supp 4 II):II–61. [Google Scholar]
- 23.Thys JP. The value of the polarizing treatment in myocardial infarction. Acta Cardiol. 1974;29:19–29. [PubMed] [Google Scholar]
- 24.Mantle JA, Rogers WJ, Smith LR, et al. Clinical effects of glucose-insulin-potassium on left ventricular function in acute myocardial infarction: results from a randomized clinical trial. Am Heart J. 1981;102:313–324. doi: 10.1016/0002-8703(81)90303-3. [DOI] [PubMed] [Google Scholar]
- 25.Rogers J, McDaniel HG, Mantle JA, et al. Glucose-insulin-potassium infusion in acute myocardial infarction-results of a prospective randomized study [abstract] Clin Res. 1982;30:216A. [Google Scholar]
- 26.Oldfield GS, Commerford PJ, Opie LH. Effects of preoperative glucose-insulin-potassium on myocardial glycogen levels and on complications of mitral valve replacement. J Thoracic Cardiovas Surg. 1986;91:874–878. [PubMed] [Google Scholar]
- 27.Coleman GM, Gradinac S, Taegtmeyer H, et al. Efficacy of metabolic support with glucose-insulin-potassium for left ventricular pump failure after aortocoronary bypass surgery. Circulation. 1989;80:I91–I96. [PubMed] [Google Scholar]
- 28.Lazar HL, Philippides G, Fitzgerald C, et al. Glucose-insulin-potassium solutions enhance recovery after urgent coronary artery bypass grafting. J Thoracic Cardiovas Surg. 1997;113:354–360. doi: 10.1016/S0022-5223(97)70333-7. [DOI] [PubMed] [Google Scholar]
- 29.Lazar HL, Chipkin S, Philippides G, et al. Glucose-insulin-potassium solutions improve outcomes in diabetics who have coronary artery operations. Ann Thorac Surg. 2000;70:145–150. doi: 10.1016/s0003-4975(00)01317-5. [DOI] [PubMed] [Google Scholar]
- 30.Lazar HL, Chipkin SR, Fitzgerald CA, et al. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109:1497–1502. doi: 10.1161/01.CIR.0000121747.71054.79. [DOI] [PubMed] [Google Scholar]
- 31.Turkoz A, Toprak HI, Sari S, et al. Glucose-insulin-potassium solution before cardiopulmonary bypass in coronary artery surgery. Turk Anest Rean Cem Mecnuiasi. 2000;28:361–365. [Google Scholar]
- 32.Lindholm L, Bengtsson A, Hansdottir V, et al. Insulin (GIK) improves central mixed and hepatic venous oxygenation in clinical cardiac surgery. Scand Cardiovasc. 2001;35:347–352. doi: 10.1080/140174301317116334. [DOI] [PubMed] [Google Scholar]
- 33.Smith A, Grattan A, Harper M, et al. Coronary revascularization: a procedure in transition from on-pump to off-pump? The role of glucose-insulin-potassium revisited in a randomized, placebo-controlled study. J Cardiothorac Vasc Anesth. 2002;16:413–420. doi: 10.1053/jcan.2002.125151. [DOI] [PubMed] [Google Scholar]
- 34.Lell WA, Nielsen VG, McGiffin DC, et al. Glucose-insulin-potassium infusion for myocardial protection during off-pump coronary artery surgery. Ann Thorac Surg. 2002;73:1246–1251. doi: 10.1016/s0003-4975(01)03619-0. [DOI] [PubMed] [Google Scholar]
- 35.Pache J, Kastrati A, Mehilli J, et al. A randomized evaluation of the effects of glucose-insulin-potassium infusion on myocardial salvage in patients with acute myocardial infarction treated with reperfusion therapy. Am Heart J. 2004;148:e3. doi: 10.1016/j.ahj.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Krljanac G, Vasiljevic Z, Radovanovic M, et al. Effects of glucose-insulin-potassium infusion on ST-elevation myocardial infarction in patients treated with thrombolytic therapy. Am J Cardiol. 2005;96:1053–1058. doi: 10.1016/j.amjcard.2005.05.068. [DOI] [PubMed] [Google Scholar]
- 37.Koskenkari JK, Kaukoranta PK, Kiviluoma KT, et al. Metabolic and hemodynamic effects of high-dose insulin treatment in aortic valve and coronary surgery. Ann Thorac Surg. 2005;80:511–517. doi: 10.1016/j.athoracsur.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 38.Ranasinghe AM, Quinn DW, Pagano D, et al. Glucose-insulin-potassium and tri-iodothyronine individually improve hemodynamic performance and are associated with reduced troponin I release after on-pump coronary artery bypass grafting. Circulation. 2006;114:I245–I250. doi: 10.1161/CIRCULATIONAHA.105.000786. [DOI] [PubMed] [Google Scholar]
- 39.Quinn DW, Pagano D, Bonser RS, et al. Improved myocardial protection during coronary artery surgery with glucose-insulin-potassium: a randomized controlled trial. J Thorac Cardiovasc Surg. 2006;131:34–42. doi: 10.1016/j.jtcvs.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 40.Yanhui L, Zhang L, Zhang H, et al. The effects of high concentration glucose-insulin-potassium (GIK) on the hemodynamics of patients with acute myocardial infarction. Chin J Emerg Med. 2006;15:152–155. [Google Scholar]
- 41.Zuurbier CJ, Hoek FJ, van DJ, et al. Perioperative hyperinsulinaemic normoglycaemic clamp causes hypolipidaemia after coronary artery surgery. Br J Anaesth. 2008;100:442–450. doi: 10.1093/bja/aen018. [DOI] [PubMed] [Google Scholar]
- 42.Clowes GH., Jr Energy metabolism in sepsis: treatment based on different patterns in shock and high output stage. Ann Surg. 1974;179:684–696. doi: 10.1097/00000658-197405000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weisul JP, O’Donnell TF, Jr, Stone MA, et al. Myocardial performance in clinical septic shock: effects of isoproterenol and glucose potassium insulin. J Surg Res. 1975;18:357–363. doi: 10.1016/0022-4804(75)90094-3. [DOI] [PubMed] [Google Scholar]
- 44.Vincent J-L, Dufaye P, Berre J, et al. Infusion of glucose and insulin in circulatory shock. Crit Care Med. 1981;9:209. [Google Scholar]
- 45.Bronsveld W, van den Bos G. Use of glucose-insulin-potassium (GIK) in human septic shock. Crit Care Med. 1985;13:566–570. doi: 10.1097/00003246-198507000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Mauritz W, Schindler I, Zadrobilek E, et al. Glucose-potassium-insulin in hypodynamic septic shock. Anaesthesist. 1986;35:623–627. [PubMed] [Google Scholar]
- 47.Hamdulay SS, Al-Khafaji A, Montgomery H. Glucose-insulin and potassium infusions in septic shock. Chest. 2006;129:800–804. doi: 10.1378/chest.129.3.800. [DOI] [PubMed] [Google Scholar]
- 48.Archer LT, Beller BK, Drake JK, et al. Reversal of myocardial dysfunction in endotoxin shock with insulin. Can J Physiol Pharmacol. 1978;56:132–138. doi: 10.1139/y78-017. [DOI] [PubMed] [Google Scholar]
- 49.Manny J, Rabinovici N, Manny N, et al. Effect of glucose-insulin-potassium on survival in experimental endotoxic shock. Surg Gynecol Obstet. 1978;147:405–409. [PubMed] [Google Scholar]
- 50.Bronsveld W, van Lambalgen AA, van den Bos GC, et al. Ventricular function, hemodynamics, and oxygen consumption during infusions of blood and glucose-insulin-potassium (GIK) in canine endotoxin shock. Circ Shock. 1982;9:145–156. [PubMed] [Google Scholar]
- 51.Tuynman HA, Thijs LG, Straub JP, et al. Effects of glucose-insulin-potassium (GIK) on the position of the oxyhemoglobin dissociation curve, 2.3-diphosphoglycerate, and oxygen consumption in canine endotoxin shock. J Surg Res. 1983;34:246–253. doi: 10.1016/0022-4804(83)90067-7. [DOI] [PubMed] [Google Scholar]
- 52.Bronsveld W, van Lambalgen AA, van den Bos GC, et al. Effects of glucose-insulin-potassium (GIK) on myocardial blood flow and metabolism in canine endotoxin shock. Circ Shock. 1984;13:325–340. [PubMed] [Google Scholar]
- 53.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]