Abstract
Objective:
To prospectively investigate the relationship between physical activity and Parkinson disease (PD).
Methods:
We evaluated physical activity in relation to PD among 213,701 participants of the NIH-AARP Diet and Health Study cohort. Physical activities over 4 periods (ages 15-18, 19-29, and 35-39, and in the past 10 years) were noted in 1996-1997, and physician-diagnosed PD was reported on the 2004-2006 follow-up questionnaire. Only cases diagnosed after 2000 (n = 767) were included in the analyses.
Results:
Higher levels of moderate to vigorous activities at ages 35-39 or in the past 10 years as reported in 1996-1997 were associated with lower PD occurrence after 2000 with significant dose-response relationships. The multivariate odds ratios (OR) between the highest vs the lowest levels were 0.62 (95% CI confidence interval [CI] 0.48-0.81, p for trend 0.005) for ages 35-39 and 0.65 (95% CI 0.51-0.83, p for trend 0.0001) for in the past 10 years. Further analyses showed that individuals with consistent and frequent participation in moderate to vigorous activities in both periods had approximately a 40% lower risk than those who were inactive in both periods. Moderate to vigorous activities at earlier ages or light activities were not associated with PD. Finally, the association between higher moderate to vigorous physical activities and lower PD risk was demonstrated in a metaanalysis of prospective studies.
Conclusions:
Although we cannot exclude the possibility that less participation in physical activity is an early marker of PD, epidemiologic evidence suggests that moderate to vigorous exercise may protect against PD.
GLOSSARY
- CI
= confidence interval;
- CPS-II
= Cancer Prevention Study II Nutrition Cohort;
- HAHS
= Harvard Alumni Health Study;
- HPFS
= Health Professionals Follow-up Study;
- NHS
= Nurses Health Study;
- OR
= odds ratio;
- PD
= Parkinson disease.
Benefits of physical activities have been documented for many health conditions, including obesity, type 2 diabetes, cardiovascular diseases, certain types of cancers, and overall mortality.1,2 The preventive benefit of exercise has been recently extended to neurodegenerative diseases.3–6 A recent metaanalysis showed consistent epidemiologic evidence that higher physical activity was associated with lower risk of dementia or Alzheimer disease.7 Parkinson disease (PD) is the second most prevalent neurodegenerative disease and affects over 1% of the elderly population. In contrast to dementia, the epidemiologic evidence on physical activity and PD has been sparse and inconsistent,8–11 despite fairly consistent evidence from animal experiments that forced exercise may protect the nigrostriatal dopaminergic system.12–15 We therefore examined physical activities at various time periods in relation to PD risk in a large cohort of US older adults: the NIH-AARP Diet and Health Study.
METHODS
Study population and PD case identification.
The cohort was assembled in 1995-1996 by the National Cancer Institute to investigate roles of diet and lifestyle in cancer etiology.16 The cohort comprised 566,402 AARP (formerly known as the American Association of Retired Persons) members (ages 50-71) from 6 US states and 2 metropolitan areas who completed a comprehensive dietary survey on diet and answered some brief questions on lifestyle (referred to as dietary survey).16 In late 1996-1997, 334,908 participants of the original cohort answered a second questionnaire to provide more detail on lifestyle (referred to as risk factor survey), including physical activity levels over 4 time periods. From 2004 to 2006, a follow-up survey was conducted among surviving participants to update lifestyle exposures and to ascertain the occurrence of major chronic diseases, including PD. A total of 220,934 participants participated in both the risk factor and the follow-up surveys and were thus eligible for the current analyses. On the follow-up questionnaire, participants were asked whether they had been diagnosed by a doctor with PD and the year of diagnosis as before 1985, 1985-1994, 1995-1999, or 2000 to present. Because physical activities were asked at the risk factor survey in 1996-1997, we excluded 764 cases diagnosed before 2000 to reduce the potential impact of physical limitations of patients with early PD on the analyses, leaving 917 cases diagnosed in or after 2000 eligible for the current analyses. We further excluded 143 individuals whose self-reports were later denied by themselves or by their treating physicians, and 7 cases missing on physical activities. Of those who did not report a PD diagnosis, we excluded 4,916 participants with invalid or missing PD status on the follow-up questionnaire and 1,403 with missing information on physical activity. Therefore, the final primary analyses included 767 PD cases diagnosed after 2000 and 212,934 participants without PD.
We began in 2007 to validate the self-reported PD diagnoses as part of an effort to collect genetic materials for PD research. This effort was made for all surviving patients with PD, not just cases diagnosed after 2000. Briefly, after obtaining consent from the patients, we asked their treating neurologists to complete a diagnostic questionnaire and to send us a copy of the patient's medical records. The questionnaire collects information on PD cardinal signs (rest tremor, rigidity, bradykinesia, and postural instability), response to dopaminergic treatments, and clinical features that help differential diagnosis. The medical records were reviewed by a movement disorders specialist (X.H.). A diagnosis was validated if the treating neurologist considered it as clinically definite or probable, or if the medical record included a final PD diagnosis or evidence of 2 or more cardinal signs with 1 being rest tremor or bradykinesia, a progressive course, responsiveness to dopaminergic treatments, and absence of features that suggest an alternative diagnosis. This protocol has been successfully implemented in other large cohorts.17,18 To date, we have received a total of 1,069 responses from physicians and 940 (87.9%) PD diagnoses were confirmed. The confirmation rate was similar across years of diagnosis: 83.3% for cases diagnosed before 1985, 92.8% for cases diagnosed in 1985-1994, 88% for cases diagnosed in 1995-1999, and 87.2% for cases diagnosed after 2000.
Exposure assessment.
On the 1996-1997 risk factor questionnaire, participants were asked to report how often they participated in light or moderate to vigorous activities at 4 ages: 15-18 years, 19-29 years, 35-39 years, and in the past 10 years. Frequency categories included never, rarely, hours/week: <1, 1-3, 4-7, and >7. Examples of light activities (such as bowling, slow walking or slow dancing, and light housework) or moderate to vigorous activities (such as tennis, biking, swimming, and heavy housework) were provided. In addition, 3 physical activity-related questions were also asked in the 1995-1996 dietary survey approximately 6 months prior to the risk factor survey. Participants were asked to describe their daily routines at work as mostly sitting, sitting and walking a bit, standing or walking around without carrying loads or lifting, lifting or carrying light loads, or doing heavy work or carrying heavy loads. The second question inquired whether in the prior 12 months there were activities at work or home that lasted at least 20 minutes or increased breath or heart rate or caused sweat. Six possible answers were allowed: never, rarely, times/week: <1, 1-2, 3-4, and ≥5. The third question asked about how often the participants exercised or participated in sports at ages 15-18 years with the same categories for activities in the past year.
In the 1995-1996 dietary survey, participants were asked about their smoking status, the typical number of cigarettes smoked per day, and the number of years since last smoking for former smokers. In addition to smoking, the questionnaire collected information on date of birth, sex, race, education level, and coffee consumption.16
Statistical analysis.
As the physical activity data were structurally collected at the risk factor survey, we limited our primary analysis to participants of the risk factor survey and follow-up survey. Further, we used PD diagnosis after 2000 as the primary outcome to reduce the possibilities of reverse causality and recall bias. Odds ratios (OR) and 95% confidence intervals (CI) were derived from logistic regression models, adjusting for age in 5-year categories, sex, race (non-Hispanic white vs others), smoking (never smokers, past smokers [years since quitting: ≥35, 30-34, 20-29, 10-19, 5-9, and 1-4], and current smokers [cigarettes per day: 1-10, 11-20, >20]), education (<8 years, 8-11 years, high school, post-high/some college, and college and above), and coffee consumption (cups/day: none, <1, 1, 2-3, and >3). Analyses were conducted separately for light activities and moderate to vigorous activities and first in both men and women and then by sex. The statistical significance for a linear trend was tested by including a continuous variable with assigned midpoint of each exposure category in the regression model. We also examined whether changing activity levels between ages 35-39 and in the past 10 years was associated with PD risk after 2000. For this analysis, we considered never or rarely as low, >7 hours/week as high, and those in-between as medium. Further, we also examined the relationship between the 3 physical activity-related questions collected at the dietary survey in 1995-1996 and PD risk after 2000.
To our knowledge, 3 prospective studies8–10 have published data on physical activity and risk of PD, including the Health Professionals Follow-up Study (HPFS, men only, 252 cases) and the Nurses Health Study (NHS, women only, 135 cases),8 the Harvard Alumni Health Study (HAHS, men only, 101 cases),9 and the Cancer Prevention Study II Nutrition Cohort (CPS-II, cases: 264 men and 145 women).10 We conducted a meta-analysis of these studies with the current one. To reduce the possibility of reverse causality, for HPFS, NHS, and the current study, we used the risk estimates from lag analyses that excluded the first several years of follow-up. The meta-analysis was conducted using Review Manager (RevMan) (Version 5.0, Copenhagen, Denmark).19 Other analyses were conducted using SAS software (version 9.1, Cary, NC) with 2-tailed significance level at α = 0.05.
Standard protocol approvals, registrations, and patient consents.
Participants consented to the study by returning survey questionnaires. The study protocol was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences and the Special Studies Institutional Review Board of the National Cancer Institute.
RESULTS
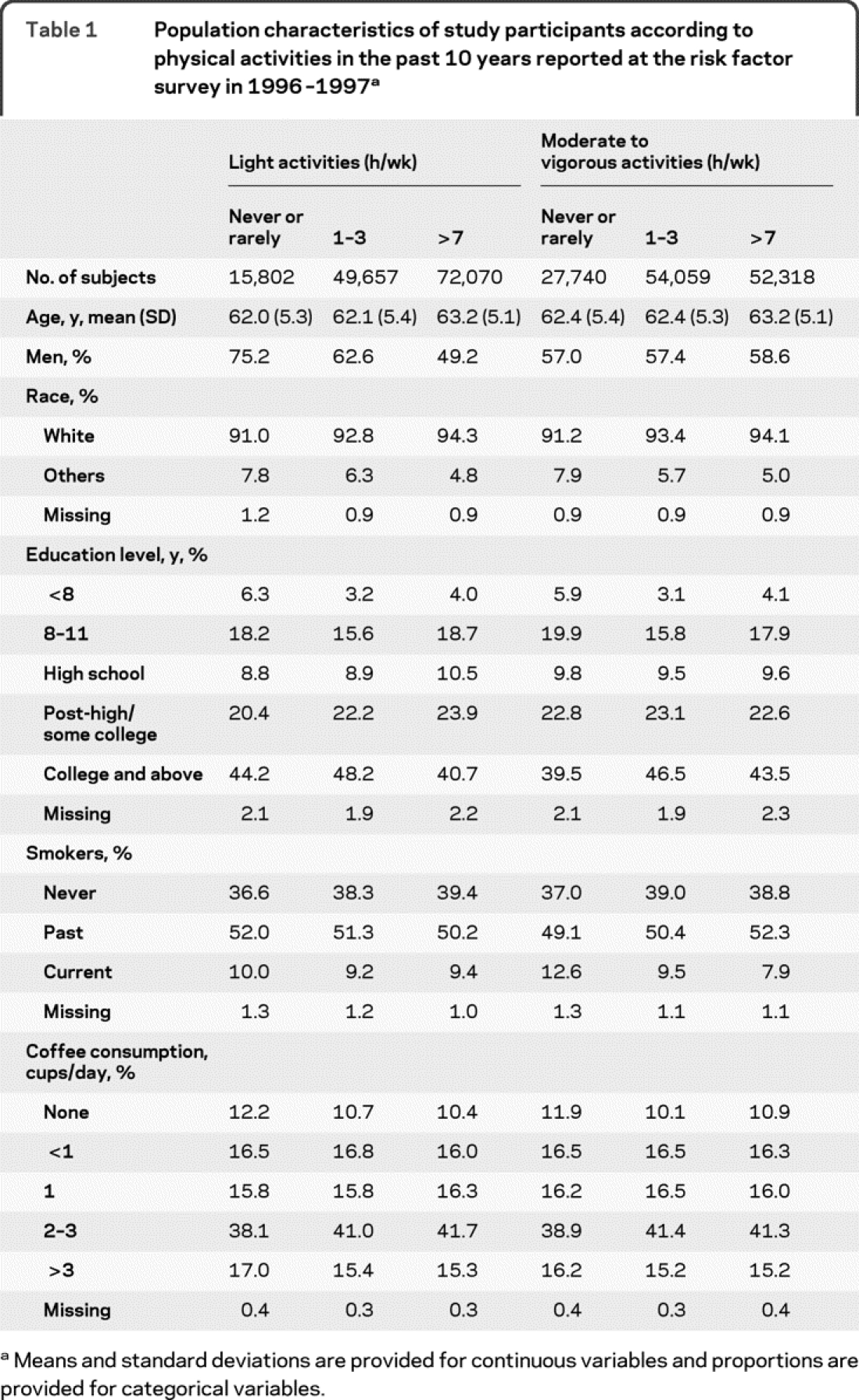
Table 1 showed population characteristics according to levels of light or moderate to vigorous activities in the past 10 years as reported on the 1996-1997 questionnaire. Participants involved in frequent light activities were older, and were more likely to be women and white than those who never or rarely participated in such activities. For moderate to vigorous activities, frequent participants were slightly older, more likely to be men and white, and have a higher education level. They were also less likely to be current smokers than those who never or rarely did moderate to vigorous activities.
Table 1 Population characteristics of study participants according to physical activities in the past 10 years reported at the risk factor survey in 1996-1997
Higher levels of moderate to vigorous activities at ages 35-39 and in the past 10 years as reported in 1996-1997 were associated with lower PD risk after 2000 (table 2). Compared with participants who never or rarely did moderate to vigorous activities at ages 35-39, those with >7 hours/week of such activities had a 38% lower odds of having PD (OR = 0.62, 95% CI 0.48-0.81, p for trend = 0.005). Similar results were obtained for moderate to vigorous activities in the past 10 years (corresponding OR = 0.65, p for trend = 0.0001). Moderate to vigorous activities at earlier ages were, in general, not associated with the risk of PD. Light activities at any time periods were not related to PD risk, the multivariate ORs comparing >7 hours/week of light activities with never or rarely were 1.11 (95% CI 0.88-1.38) for ages 15-18, 1.12 (95% CI 0.85-1.47) for ages 19-29, 0.91 (95% CI 0.68-1.21) for ages 35-39, and 1.06 (95% CI 0.78-1.44) for in the past 10 years.
Table 2 Odds ratios (OR) and 95% confidence intervals (CI) of Parkinson disease after 2000 according to levels of moderate to vigorous physical activities at various life periods reported at the risk factor survey in 1996-1997

We further evaluated change of participation in moderate to vigorous activities between ages 35-39 and in the past 10 years in relation to PD after 2000 (figure 1). Compared with individuals with consistently low level of such activities, the OR was 0.57 (95% CI 0.41-0.79) for those consistently at high levels, 0.58 (0.40-0.84) for those who increased from medium to high, 0.63 (0.44-0.89) for those who decreased from high to medium, and 0.79 (0.60-1.04) for those who remained medium. Analyses for other groups were based on small numbers and were statistically less stable. Decreasing activity levels over this period (from high to low, high to medium, and medium to low) apparently was not related to higher odds of PD as compared with those who remained at low or even at moderate levels of activities.
Figure 1 Changes of physical activities in relation to risk of Parkinson disease (PD)
Odds ratios (OR) and 95% confidence intervals (CI) of PD after 2000 according to changes of moderate to vigorous physical activities between ages 35-39 and in the past 10 years as reported at the risk factor survey in 1996-1997. The analysis adjusted for age at risk factors survey, gender, race, education levels, smoking status, and coffee consumption.
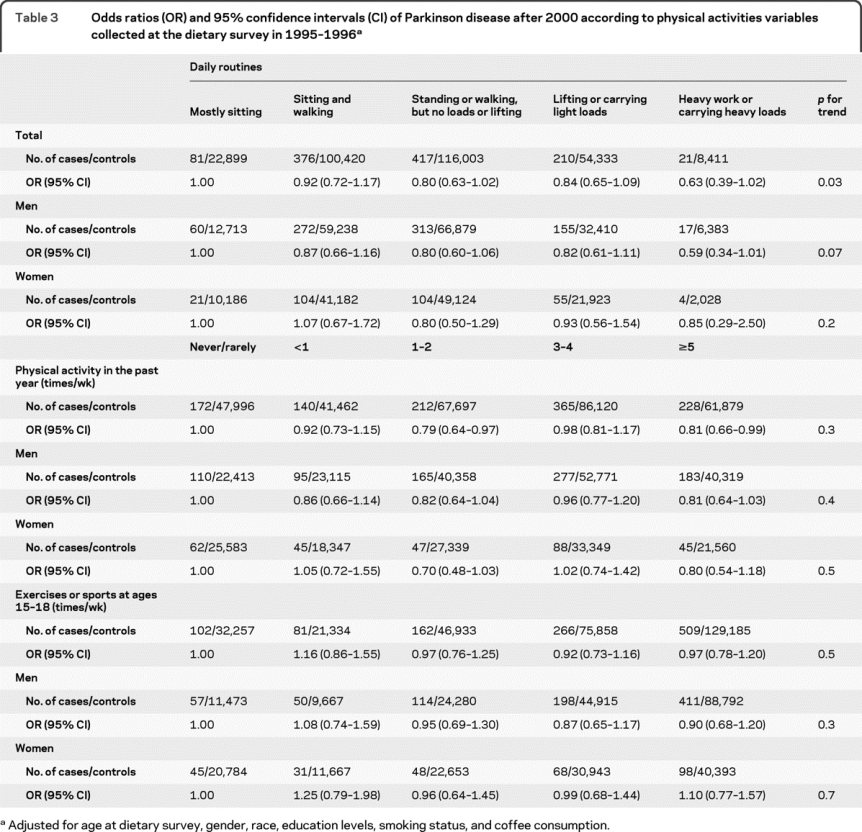
Having a physically demanding daily routine as asked in the dietary survey was also related to lower PD risk after 2000 (table 3). Compared with individuals who sit most of the day, those carrying heavy loads or doing heavy work had a 37% lower risk of PD after 2000 (OR = 0.63, p for trend = 0.03). An approximately 20% lower risk was also found for participants who reported ≥5 times/week of activities from work or home when compared with those who were never or rarely physically active (OR = 0.81, 95% CI 0.66-0.99); however, the linear trend test was not significant. Recalled frequency of participating in physical activity or sports at ages 15-18 was not related to PD risk.
Table 3 Odds ratios (OR) and 95% confidence intervals (CI) of Parkinson disease after 2000 according to physical activities variables collected at the dietary survey in 1995-1996
The meta-analysis showed consistently that higher moderate to vigorous physical activities were associated with lower PD risk in both men and women (figure 2). The OR comparing the highest activity level with the lowest were 0.67 (95% CI 0.56-0.80) for all participants, 0.66 (95% CI 0.53-0.82) for men, and 0.67 (95% CI 0.48-0.94) for women. The HAHS did not differentiate light from moderate/vigorous activities. However, excluding this study did not change the results (data not shown).
Figure 2 A mini meta-analysis of prospective studies on physical activities and risk of Parkinson disease (PD) in men and women
Odds ratios (OR) and 95% confidence intervals (CI) were between the highest activity levels and the lowest. Squares indicate the individual OR in each study. The size of each square is proportional to the percent weight of that individual study in the meta-analysis, and the horizontal line represents the 95% CI. Pooled ORs and 95% CIs are indicated by the solid diamonds. The pooled p values were 0.0002 for men, 0.02 for women, and <0.0001 for men and women combined.
DISCUSSION
In this large population of US older adults, higher levels of moderate to vigorous physical activities at ages 35-39 or in the past 10 years were associated with future lower risk of PD. This association was present in both men and women. The meta-analysis of prospective studies confirmed this relationship, and therefore the totality of epidemiologic evidence seems to support that higher levels of moderate to vigorous activities in mid or later life are associated with lower risk of PD.
The hypothesis that higher physical activity protects against PD originated from animal experiments in which forced exercise prior to or after dopamine-selective neurotoxicant treatments spared dopaminergic neurons and attenuated movement abnormalities.12–14 Examining this hypothesis in epidemiologic studies is difficult because the underlying PD pathogenesis might affect one's ability to exercise even in its preclinical stages. Previous analysis showed a persistent trend of decreasing activity level among patients with PD starting about 2-4 years prior to the diagnosis.8 Interestingly, the levels of physical activity among patients with PD were rather stable before that point. Therefore, etiologic research on physical activity and PD should be preferably conducted in large prospective cohorts with activity data collected years before the diagnosis.
To date, only a few prospective studies on physical activity and PD are available.8–11 In the HPFS and NHS cohorts,8 higher baseline vigorous activity was associated with lower PD risk in men, but not in women. Recalled participation in strenuous exercises in early life was, however, associated with lower PD risk in both men and women. In the male-only HAHS cohort,9 neither baseline nor recalled physical activity in earlier life was significantly associated with PD risk, although men with the highest energy expenditure had 37% lower PD risk than those in the lowest (RR = 0.63, 95% CI 0.36-1.12, p for trend = 0.1). In the CPS-II Nutrition cohort,10 higher levels of moderate to vigorous activities were associated with lower future PD risk with a borderline statistical significance. No gender difference was found, but the gender-specific results were not significant due to small sample sizes. A fourth study in Finland reported in the text that leisure time physical activity was nonsignificantly associated with lower PD risk, but did not provide the data.11
Compared with previous studies, the current study is substantially larger with uniformly collected physical activity data for various time periods and includes substantially more female cases. The meta-analysis shows consistent results across studies and gender, suggesting the association between physical activity and PD is not likely due to chance. We could not exclude the possibility of reverse causality that low physical activity was a result of preclinical PD pathogenesis. However, several findings may argue against this as a primary explanation for the relationship between higher physical activity and lower PD risk. First, analysis with repeatedly measured physical activities showed no decrease in activity level among patients with PD until 2-4 years before the diagnosis.8 Second, the HPFS/NHS, CPS-II, and the current study all conducted analyses excluding the first several years of follow-up.8,10 Third, in the current analysis, recalled moderate to vigorous activities at ages 35-39 showed association with lower PD risk, as did strenuous exercises in early life in the HPFS and NHS studies.8 Finally, we demonstrated that decreasing physical activity between ages 35-39 and in the past 10 years was not related to a higher PD risk, suggesting activity levels in these life periods did not yet reflect changes as a result of undiagnosed PD.
The potential mechanisms that underlie the physical activity-PD relationship are yet to be elucidated. In animal experiments, forced exercise induced the secretion of neurotrophic factors which might in turn contribute to the neuroplasticity and survival of dopamine neurons.20,21 Further, exercise downregulates dopamine transporter14 and decreases its ratio to vesicular monoamine transporter. This may reduce cytosolic dopamine turnover and the susceptibility of dopaminergic neurons to neurotoxicants.12,22 Finally, vigorous, but not light, exercise induces lasting elevation of plasma urate,23 which in turn predicts lower PD risk24–27 and slower progression.28 The relevance of these proposed mechanisms needs to be evaluated in future clinical and experimental studies.
The study has several limitations. In this large prospective cohort, we had to rely on self-reports to identify patients with PD, and this inevitably introduced diagnostic and reporting errors. In our diagnostic confirmation effort, about 88% of the self-reported diagnoses were verified with medical information from their treating neurologists. Further, we excluded identified erroneous reports or misdiagnoses from the analysis. Physical activities were self-reported via a structured survey. Although the questionnaire was not directly validated, it contained key elements of the Physical Activity Scale for the Elderly that showed reasonable reliability and validity when compared against objectively measured energy expenditure using doubly labeled water.29,30 Physical activity assessed in this study has been previously linked to lower overall mortality31 and lower risk of colorectal cancer.32 Further, information on physical activity was collected prior to case identification and therefore the recall errors should be nondifferential and might have attenuated the true strengths of associations. Nevertheless, exposure misclassification is a concern as the accuracy of reporting physical activities was subject to participants' abilities of observation and memory. In particular, our findings that early life physical activities were not related to PD risk need cautious interpretations as the recall of activities in the distant past might have had more errors than that of recent activities. Despite the fact that we only included cases diagnosed at least 3 years after the exposure assessment and we presented several lines of evidence against the possibility of reverse causality, we could not entirely exclude the likelihood that our finding was due to decreased physical activities of patients with PD in early preclinical stages. Finally, most of our study participants were white; therefore the generalizability of our results to other ethnicities needs to be evaluated in future studies.
ACKNOWLEDGMENT
The authors thank the participants of the NIH-AARP Diet and Health Study for their contributions.
DISCLOSURE
Dr. Xu and Dr. Park report no disclosures. Dr. Huang has served as a consultant for Easton Associate, Public Healthcare, Teva Pharmaceutical Industries Ltd., and the National Institute of Environmental Health Sciences; holds patent US 6,916,823 (issued 2005): Method of treatment of dopamine-related dysfunction (plus foreign patents) and has filed a patent regarding Early detection of Parkinson's disease using novel motor signs; receives research support from the NIH/NINDS (NS060722 [PI]), the Pennsylvania Tobacco Settlement Fund, and Huck Institute of Penn State University; and holds stock in BioValve Technologies, Inc. Dr. Hollenbeck serves on the Scientific Advisory Committee of the Love/Avon Army of Women and is a full-time salaried employee of AARP. Dr. Blair is a Scientist Emeritus at the National Cancer Institute and serves on the editorial advisory boards of the Scandinavian Journal of Work Environment and Health, the American Journal of Industrial Medicine, and the Journal of Agricultural Safety and Health; and served as the Interim Director of the Occupational Cancer Research Centre in Toronto, Canada. Dr. Schatzkin is an employee of the NIH National Cancer Institute and serves as Principal Investigator of the NIH-AARP Diet and Health Study. Dr. Chen receives NIH intramural funding (Z01-ES-101986) and serves on the editorial board of the International Journal of Molecular Epidemiology and Genetics.
Address correspondence and reprint requests to Dr. Honglei Chen, Epidemiology Branch, National Institute of Environmental Health Sciences, 111 T.W. Alexander Dr., PO Box 12233, Mail Drop A3-05, Research Triangle Park, NC 27709 chenh2@niehs.nih.gov
Study funding: Supported by the intramural research program of the NIH, the National Institute of Environmental Health Sciences (Z01-ES-101986), and the National Cancer Institute (Z01 CP010196-02).
Disclosure: Author disclosures are provided at the end of the article.
Received November 18, 2009. Accepted in final form March 31, 2010.
REFERENCES
- 1.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995;273:402–407. [DOI] [PubMed] [Google Scholar]
- 2.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 2004;351:2694-2703. [DOI] [PubMed] [Google Scholar]
- 3.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA 2004;292:1447–1453. [DOI] [PubMed] [Google Scholar]
- 4.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol 2005;4:705–711. [DOI] [PubMed] [Google Scholar]
- 5.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008;300:1027–1037. [DOI] [PubMed] [Google Scholar]
- 6.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009;302:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med 2009;39:3–11. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology 2005;64:664–669. [DOI] [PubMed] [Google Scholar]
- 9.Logroscino G, Sesso HD, Paffenbarger RS, Jr., Lee I-M. Physical activity and risk of Parkinson's disease: a prospective cohort study. J Neurol Neurosurg Psychiatry 2006;77:1318–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thacker EL, Chen H, Patel AV, et al. Recreational physical activity and risk of Parkinson's disease. Mov Disord 2008;23:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu G, Jousilahti P, Nissinen A, Antikainen R, Kivipelto M, Tuomilehto J. Body mass index and the risk of Parkinson disease. Neurology 2006;67:1955–1959. [DOI] [PubMed] [Google Scholar]
- 12.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience 2003;119:899–911. [DOI] [PubMed] [Google Scholar]
- 13.Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci 2001;21:4427–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petzinger GM, Walsh JP, Akopian G, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci 2007;27:5291–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith AD, Zigmond MJ. Can the brain be protected through exercise? Lessons from an animal model of parkinsonism. Exp Neurol 2003;184:31–39. [DOI] [PubMed] [Google Scholar]
- 16.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 2001;154:1119–1125. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Jacobs E, Schwarzschild MA, et al. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol 2005;58:963–967. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Zhang SM, Hernan MA, Willett WC, Ascherio A. Diet and Parkinson's disease: a potential role of dairy products in men. Ann Neurol 2002;52:793–801. [DOI] [PubMed] [Google Scholar]
- 19.Review Manager (RevMan) [Computer program] Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2008.
- 20.Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem 2003;85:299–305. [DOI] [PubMed] [Google Scholar]
- 21.Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 1996;726:49–56. [PubMed] [Google Scholar]
- 22.Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transporters and neuronal injury. Trends Pharmacol Sci 1999;20:424–429. [DOI] [PubMed] [Google Scholar]
- 23.Green HJ, Fraser IG. Differential effects of exercise intensity on serum uric acid concentration. Med Sci Sports Exerc 1988;20:55–59. [DOI] [PubMed] [Google Scholar]
- 24.Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM. Observations on serum uric acid levels and the risk of idiopathic Parkinson's disease. Am J Epidemiol 1996;144:480–484. [DOI] [PubMed] [Google Scholar]
- 25.Weisskopf M, O'Reilly E, Chen H, Schwarzschild M, Ascherio A. Plasma urate and risk of Parkinson's disease. Am J Epidemiol 2007;166:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol 2005;58:797–800. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Mosley TH, Alonso A, Huang X. Plasma urate and Parkinson's disease in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol 2009;169:1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol 2008;65:698–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46:153–162. [DOI] [PubMed] [Google Scholar]
- 30.Schuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the Physical Activity Scale for the Elderly (PASE): according to energy expenditure assessed by the doubly labeled water method. J Clin Epidemiol 1997;50:541–546. [DOI] [PubMed] [Google Scholar]
- 31.Leitzmann MF, Park Y, Blair A, et al. Physical activity recommendations and decreased risk of mortality. Arch Intern Med 2007;167:2453–2460. [DOI] [PubMed] [Google Scholar]
- 32.Howard RA, Freedman DM, Park Y, Hollenbeck A, Schatzkin A, Leitzmann MF. Physical activity, sedentary behavior, and the risk of colon and rectal cancer in the NIH-AARP Diet and Health Study. Cancer Causes Control 2008;19:939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]




