Abstract
Objective:
To test the validity and reliability of a new measure of clinical impairment in primary progressive aphasia (PPA), the Progressive Aphasia Severity Scale (PASS), and to investigate relationships with MRI-based cortical thickness biomarkers for localizing and quantifying the severity of anatomic abnormalities.
Methods:
Patients with PPA were rated using the PASS and underwent performance-based language testing and MRI scans that were processed for cortical thickness measures.
Results:
The level of impairment in PASS fluency, syntax/grammar, and word comprehension showed strong specific correlations with performance-based measures of these domains of language, and demonstrated high interrater reliability. Left inferior frontal thinning correlated with impairment in fluency and grammar/syntax, while left temporopolar thinning correlated with impairment in word comprehension. Discriminant function analysis demonstrated that a combination of left inferior frontal, left temporopolar, and left superior temporal sulcal thickness separated the 3 PPA subtypes from each other with 100% accuracy (87% accuracy in a leave-one-out analysis).
Conclusions:
The PASS, a novel measure of the severity of clinical impairment within domains of language typically affected in PPA, demonstrates reliable and valid clinical-behavioral properties. Furthermore, the presence of impairment in individual PASS domains demonstrates specific relationships with focal abnormalities in particular brain regions and the severity of impairment is strongly related to the severity of anatomic abnormality within the relevant brain region. These anatomic imaging biomarkers perform well in classifying PPA subtypes. These data provide robust support for the value of this novel clinical measure and the new imaging measure as markers for potential use in clinical research and trials in PPA.
GLOSSARY
- AD
= Alzheimer disease;
- BDAE
= Boston Diagnostic Aphasia Examination;
- CDR
= Clinical Dementia Rating;
- CSB
= Cambridge Semantic Battery;
- ICC
= intraclass correlation coefficient;
- NACC UDS
= National Alzheimer's Coordinating Center Uniform Data Set;
- OC
= older control participants;
- PASS
= Progressive Aphasia Severity Scale;
- PPA
= primary progressive aphasia;
- PPA-G
= agrammatic primary progressive aphasia;
- PPA-L
= logopenic primary progressive aphasia;
- PPA-S
= semantic primary progressive aphasia;
- ROI
= region of interest;
- WAB
= Western Aphasia Battery.
Planning clinical trials for primary progressive aphasia (PPA)1 is challenging, in part because of clinicopathologic heterogeneity2,3 and in part because of a paucity of clinical assessment instruments specifically tailored to this population.4 Although quantitative performance-based linguistic measures are valuable,5 research in Alzheimer disease (AD) has demonstrated that performance-based measures provide only one facet of the clinical picture, while clinician judgment–based ratings of symptom severity provide complementary information that is useful in grading illness severity and prognostication.6 The Clinical Dementia Rating (CDR) scale7 is such an instrument of widely acknowledged utility in AD clinical research and trials.
At present, PPA symptom rating instruments are lacking—language ratings are not part of the original CDR, and the new language supplement in the National Alzheimer's Coordinating Center Uniform Data Set (NACC UDS)8 is a single global rating that captures overall level of language impairment but does not differentiate specific language domains. Since the predominant domain of language impairment relates to the localization of atrophy/hypometabolism and this localization appears to relate probabilistically to molecular neuropathology,9 it would likely be of value for both clinical and pathologic investigations to develop additional methods for quantifying impairment in different language domains.
Building on the CDR supplemental language box, we developed and piloted an approach to the rating of symptoms in 3 language domains that have traditionally been employed in PPA clinical characterization—speech fluency, syntax and grammar, and single word comprehension. We also investigated the anatomic correlates of impairment in these domains. These data were then analyzed to determine how well the measures perform in diagnostic sensitivity and specificity. The overall goal of these efforts was to determine whether anatomic measures are a sensitive and specific reflection of the presence and severity of various types of language impairment in PPA, with the aim of using both of these kinds of measures in PPA clinical research and trials.
METHODS
Participants, clinical assessment, and MRI data acquisition.
Forty right-handed participants were studied. Patients with PPA (n = 23) were recruited from an ongoing longitudinal study being conducted in the Progressive Aphasia Program in the Massachusetts General Hospital Frontotemporal Dementia Unit, and were evaluated using a structured clinical assessment performed by a behavioral neurologist (B.C.D.) and speech and language pathologist (D.S.). The clinical evaluation of each patient by the neurologist included 1) behavioral observations of the patient's speech and language during a semi-structured interview regarding history of illness, 2) office-based cognitive assessment, 3) neurologic examination, and 4) history from informant. The neurologist's evaluation did not include the use of any formal psycholinguistic instruments (Western Aphasia Battery [WAB], Boston Diagnostic Aphasia Examination [BDAE], Cambridge Semantic Battery [CSB]) and was performed independently. The clinical evaluation of each patient by the speech pathologist included 1) behavioral observations of the patient's speech and language during a semi-structured interview regarding history of illness; 2) speech-language examination, which included structured conversation (e.g., “What did you do for work?” “How do you spend your weekends?”), picture descriptions, and a brief language assessment similar to that of the Addenbrooke's Cognitive Examination including tasks of naming, repetition, word comprehension, and a motor speech examination; and 3) history from informant. In addition, elements of the WAB, BDAE, and CSB were performed as part of the speech pathologist's evaluation; these quantitative measures were not used in generating Progressive Aphasia Severity Scale (PASS) ratings (see below). The diagnosis of PPA was made if 1) a gradually progressive language disturbance was the most salient symptom prompting the patient/family to seek clinical evaluation; 2) the progressive nature of the deficits and the fact that the language disorder was the chief problem during the initial few years of the disease were documented by the history obtained from the patient and an informant who knows the patient well; and 3) the presence of aphasia was documented by a structured clinical evaluation which also demonstrated the absence of other salient deficits. PPA subtypes (agrammatic [PPA-G], semantic [PPA-S], logopenic [PPA-L], and mixed/other) were diagnosed using an approach similar to that previously described.5
In addition, older control participants (OC, n = 17; mean age = 70.6 years, SD = 9; 11 female) were included for MRI analyses. As participants in the Massachusetts Alzheimer's Disease Research Center Longitudinal Cohort, they undergo a comprehensive annual evaluation by experienced clinicians and were selected for this analysis based on a normal clinical status (CDR = 0).
In this sample, MRI data were acquired using a Siemens Trio 3.0 Tesla scanner (Siemens Medical Systems, Erlangen, Germany). Procedures for data collection included head movement restriction using expandable foam cushions and automated scout and shimming procedures. Two 3-dimensional magnetization-prepared rapid gradient echo sequences were acquired (repetition time/inversion time/echo time 2,300/900/2.98 msec, field of view 256, flip angle 7°, 192 sagittal 1-mm-thick slices, matrix 240 × 256). A fluid-attenuated inversion recovery sequence was visually inspected to rule out nondegenerative pathologies.
Standard protocol approvals, registrations, and patient consents.
All participants gave written informed consent in accordance with guidelines established by the Partners Human Research Committee.
Progressive Aphasia Severity Scale.
The PASS is a structured clinical instrument, currently under development and modeled after the CDR scale,7,10 that aims to provide a semiquantitative grading of the severity of impairment within a variety of speech and language domains. This 5-point scale enables the clinician to capture change from the patient's premorbid baseline. Ratings are made from normal (0) to questionable/very mild (0.5), mild (1.0), moderate (2.0), or severe (3.0) impairment.
The version of the PASS described here is meant to provide an initial elaboration upon the Supplemental Language Box currently in use in the NACC UDS 2.0, which provides a single global rating of language impairment. Based on the hallmark features of progressive aphasia, the first 3 domains of language for which ratings have been developed are fluency of speech, syntax and grammar, and single word comprehension. Scoring criteria are provided in table 1.
Table 1 Abbreviated Progressive Aphasia Severity Scale, version 5.1
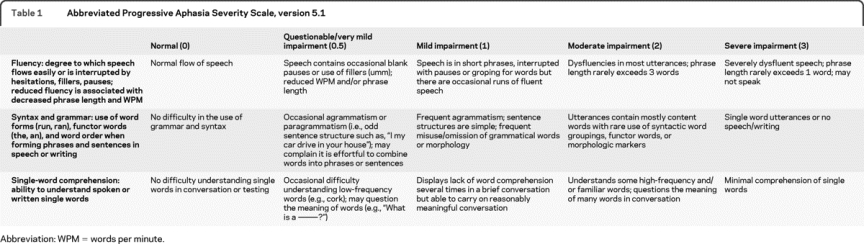
Ratings are made by a clinician after a structured clinical assessment, including interviews separately with the patient and informant and an examination of the patient. These ratings are meant to reflect the clinician's best judgment of the overall level of impairment in each domain based on history of problems in daily life and deficits on examination. As with the CDR, although a number of specific approaches can be used to obtain the information needed to derive this rating, we are working to standardize the questions asked during the interviews and to provide examples of useful examination instruments (material available upon request). Our standard protocol dictates that ratings are first made independently by each clinician (blind to each others' ratings), thus enabling interrater reliability analysis. Once all data are collected and scored, a consensus discussion is employed to adjudicate differences.
We investigated relationships of the PASS ratings to the WAB fluency measure, BDAE grammatic form measure, and CSB word-picture matching measure. Since this analysis was a goal of the present study, PASS ratings were made by the speech pathologist using information from the diagnostic evaluation described above without using the scores on these tests. For the analyses presented here, the neurologist was blind to both the speech pathologist's PASS ratings and the scores on these psycholinguistic measures, using the information obtained from the diagnostic evaluation to determine ratings. Our intent is that, in general use, PASS ratings would be made using the clinician's impression from all data gathered during a diagnostic evaluation. Although all available information should be considered in rating these domains of impairment, the clinician's overall impression of the severity of symptoms in everyday life and of impairment in conversation and examination in the office should be the main contributors to the ratings, with less emphasis on specific language test scores.
MRI morphometric data analysis.
The methods employed here have been previously described in detail, including cortical thickness processing and spherical registration to align subjects' cortical surfaces (Freesurfer 4.5.0 [http://surfer.nmr.mgh.harvard.edu]).11,12
Based on previous literature,13,14 a hypothesis-driven approach using a priori regions of interest (ROIs) was used to investigate whether inferior frontal (pars opercularis) cortical thinning relates to impaired speech fluency with agrammatism while temporopolar thinning relates to impaired word comprehension; ROIs were derived from an automated cortical parcellation15 and thickness was normalized to the OC sample by calculating a Z score: Z = (x − μage-matched OC-s)/σage-matched OCs.
Next, we performed an exploratory analysis in which the PASS ratings described above were used as variables in a general linear model correlating each PASS measure to cortical thickness across the entire cortical mantle, as described previously16 using a threshold of p < 0.01, false discovery rate corrected. Finally, we also performed similar exploratory analyses of each of the 3 clinical subtypes of PPA vs the control group.
Statistical analyses.
Interrater reliability of the 2 clinicians' independent PASS measures was assessed using intraclass correlation coefficient (ICC). Brain–behavior relationships were investigated using Pearson correlation analysis. Discriminant function analysis (Wilks lambda) was performed to determine how well the anatomic measures would separate PPA subtypes. Statistical analyses were performed using SPSS 16.0 (Chicago, IL) and results were considered significant if p < 0.01.
RESULTS
Word-finding difficulty was reported as the presenting symptom in about half the participants. Other presenting symptoms included slurring or mispronunciation of words or stuttering or false starts (often phonologic errors on examination), problems using grammar, confusion about word meanings, difficulty understanding idioms, or difficulty reading. Subtype diagnoses included PPA-G, PPA-S, and PPA-L variants. Demographic and clinical characteristics are presented in table 1. Despite deficits in confrontation naming, all patients with PPA-L demonstrated substantial improvements with phonemic cueing or 3-choice recognition, indicating intact semantic knowledge of word meaning.
PASS characteristics.
Interrater reliability (between neurologist and speech pathologist) of PASS ratings was high, with ICC >0.9 for fluency (0.99), grammar/syntax (0.99), word comprehension (0.91), and global CDR language (1.0). For subsequent analyses, we use a consensus PASS score of the 2 raters. The degree of impairment reflected by PASS scores was closely related to specific performance deficits, supporting the validity of the scale (table 2). Correlations were present between PASS fluency and WAB fluency (r = −0.92) and BDAE grammar (r = −0.94), PASS syntax/grammar and WAB fluency (r = −0.81) and BDAE grammar (r = −0.82), and PASS word comprehension and CSB word-picture matching (r = −0.87). The NACC UDS Global Language measure correlated with WAB fluency (r = −0.59) and BDAE grammar (r = −0.66) but not CSB.
Table 2 Demographic and clinical characteristics
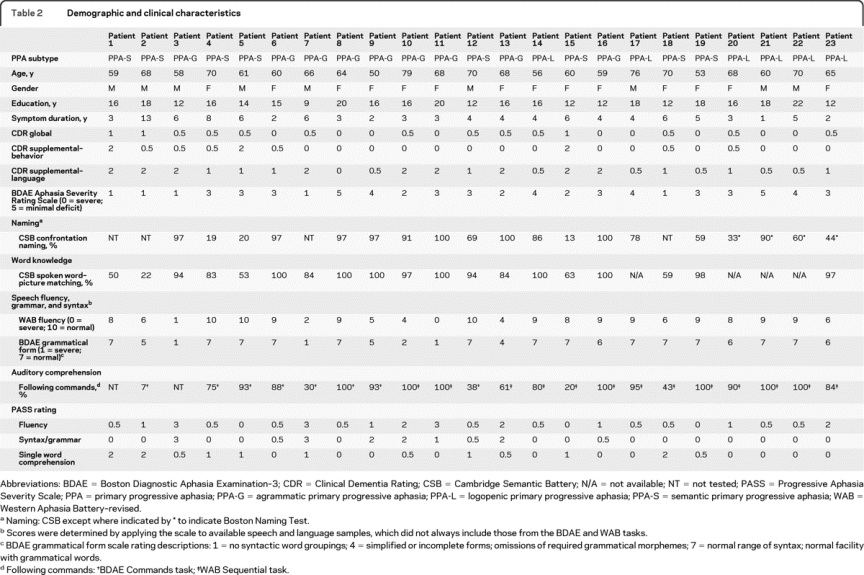
The fluency and grammar/syntax measures for patients in this study were correlated (r = 0.76), but word comprehension was not correlated with fluency or grammar/syntax (p > 0.3).
Anatomic findings related to symptom severity.
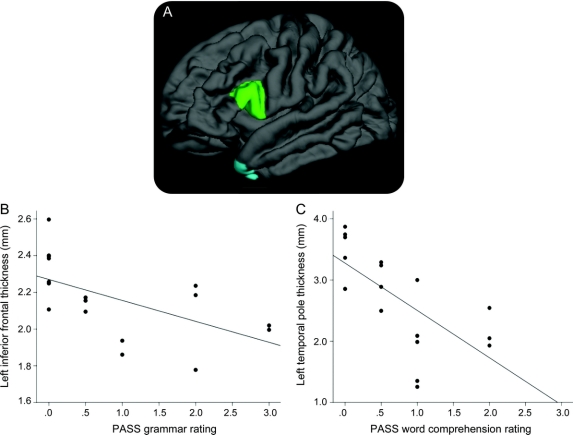
Hypothesis-driven analyses demonstrated that left inferior frontal cortical thickness correlated with severity of impairment in fluency (r = −0.71) and grammar/syntax domains (r = −0.57) but these clinical measures did not correlate with temporal polar thickness (figure 1). Severity of impairment in the comprehension domain correlated with left temporopolar cortical thickness (r = −0.68) but not inferior frontal thickness. The CDR global language rating was not correlated with either ROI (p > 0.1).
Figure 1 Hypothesis-driven analysis of the relationship between specific regions of interest (ROIs) and the severity of specific symptoms
(A) A priori anatomically defined caudal left inferior frontal gyrus (LIFG) ROI and left temporopolar (LTP) ROI were used in this analysis. (B) Correlation between LIFG ROI thickness and grammatic/syntactic impairment (r = −0.71, p < 0.005). (C) Correlation between LTP ROI thickness and word comprehension impairment (r = −0.68, p < 0.003). PASS = Progressive Aphasia Severity Scale.
Exploratory maps across the entire cortex focusing on specific symptoms revealed that grammar/syntax was most strongly correlated with thickness in caudal inferior and middle frontal regions, with strong but not complete left lateralization (figure e-1A on the Neurology® Web site at www.neurology.org). Since the fluency measure was correlated with the grammar/syntax measure, the exploratory map looks very similar. Comprehension was most strongly correlated with temporopolar thickness (figure e-1B).
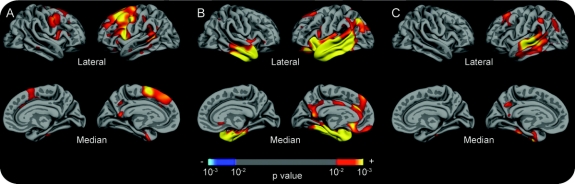
Finally, exploratory maps comparing each of the 3 PPA subtypes to controls demonstrated strongly left-lateralized inferior, middle, and superior frontal, precentral, and caudal superior temporal sulcal thinning in PPA-G, strongly left-lateralized superior and middle temporal and inferior parietal thinning in PPA-L, and left-lateralized temporal pole thinning in PPA-S (figure 2).
Figure 2 Regionally specific cortical thinning in primary progressive aphasia (PPA) subtypes
Exploratory analyses of the localization of cortical thinning in (A) PPA-agrammatic patients compared to controls, (B) PPA-semantic patients compared to controls, and (C) PPA-logopenic patients compared to controls. The color scale at the bottom represents the p value of the effects (p < 0.01).
Diagnostic classification.
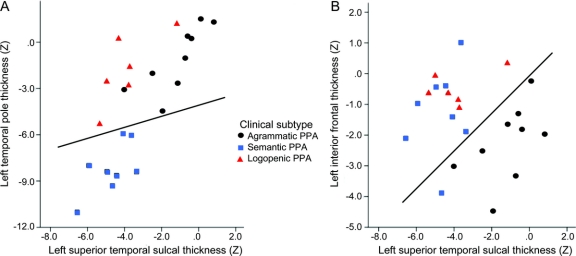
A stepwise discriminant function analysis aiming to separate PPA subtypes from each other was performed using the a priori ROIs in this study—left inferior frontal gyrus and left temporal pole—as well as 2 ROIs defined in the logopenic subtype in this study—left superior temporal sulcus and left supramarginal gyrus. This stepwise analysis demonstrated that a combination of the left temporal pole, left superior temporal sulcus, and the left inferior frontal gyrus was best at discriminating the 3 PPA subtypes from each other (χ2 = 18.2, p < 0.01), with an accuracy of 100%. Leave-one-out analysis demonstrated an accuracy of 87% (100% PPA-S, 89% PPA-G [1 patient classified as logopenic], and 66% of PPA-L [1 patient classified as semantic and 1 as agrammatic]; figure 3).
Figure 3 Anatomic measures can accurately discriminate primary progressive aphasia (PPA) subtypes
Discriminant plot of each PPA participant in this study as a function of (A) left temporopolar thickness (y axis) and left superior temporal thickness (x axis) and (B) left inferior frontal thickness (y axis) and left superior temporal thickness (x axis). The use of these 3 anatomic measures can accurately separate PPA-semantic variant patients (squares) from PPA-agrammatic variant patients (circles) from PPA-logopenic variant patients (triangles). Lines are drawn to approximate the discriminability matrix but actual discriminant functions are more complex.
DISCUSSION
Impairments in patients with PPA are heterogenous, and quantification is essential for monitoring and the ultimate evaluation of potential treatments. PPA impairments are typically quantified using language test performance measures.5,13,17,18 Here we have extended recent work to quantify the overall impairment of language functions through the judgment of trained clinicians, an approach that has traditionally played an important role in AD clinical research and trials. We developed a method for clinician judgment-based grading of overall impairment in fluency, grammar/syntax, and single word comprehension, since these are hallmark language abnormalities in PPA and are often dissociated from each other. The present study demonstrated that this approach is reliable between raters and is valid against performance-based measures. Since performance-based and clinician-rated measures of cognitive impairment are not completely redundant in AD,6 it stands to reason that they may provide complementary information in PPA, although this deserves further study. Furthermore, the clinician-graded impairments in fluency/grammar/syntax and comprehension are strongly dissociated with respect to neuroanatomic abnormalities in cortical thickness, also supporting their validity. Both hypothesis-driven and exploratory analyses demonstrated sensitive and specific relationships between impaired syntax/grammar and ventrolateral prefrontal atrophy, and between impaired word comprehension and temporopolar atrophy.
We have previously shown through longitudinal clinical research in prodromal AD that measures grading both symptom severity in daily life and in the office and performance abilities on psychometric testing can provide complementary information with respect to present level of impairment as well as prognosis.6 Every clinician has worked with a patient who exhibits prominent symptoms in daily life yet who performs relatively well on office-based tests. Conversely, there are many patients who have relatively subtle symptoms, maintaining generally good function in complex activities of daily life, yet who perform strikingly poorly on tests. This can be particularly true in some patients with fluent, word comprehension-centered forms of PPA. Thus, we believe it is critical for the field to continue to develop and apply a rich set of instruments for clinical assessment,4 ideally tailored to the PPA population and including patient- and informant-rated questionnaires, performance-based instruments, and clinician-graded measures. A comprehensive battery of such measures will likely be of great value for clinical research and ultimately treatment trials. We hope that the PASS instrument described here is of use for that goal, and that the specific anatomic relationships found here provide data to support its validity.
Previous studies of PPA have demonstrated the relationships between semantic deficits and left temporopolar atrophy19,20 as well as deficits in syntactic processing and inferior frontal atrophy.21 Yet there have been few investigations of these dissociable relationships within a single sample of patients with PPA.5,13,22 The investigation of dissociated deficits in single samples of patients with PPA has revealed valuable findings regarding some aspects of language dysfunction in PPA, particularly naming deficits with temporopolar atrophy and nonfluent/agrammatic speech with middle and inferior frontal atrophy.13 A recent study employed cortical thickness analysis in comparison with performance-based measures of grammar and semantic processing and found that the patterns of localization of cortical thinning for the 3 subtypes were remarkably similar to those identified here.5 Notably, the caudal middle temporal gyrus/superior temporal sulcus—a critical region for linking speech sounds to word meaning23—is involved in all 3 variants. Another recent study employing cortical thickness analysis showed a similar pattern of thinning for the PPA-semantic subtype but a somewhat different pattern for PPA-nonfluent subtype, raising questions about whether that sample was truly clinically comparable to the present one.24 One advantage of cortical thickness analysis in this type of work is that the measures can be obtained from single individuals using an a priori ROI approach,12 and cortical thickness measures are directly related to morphometric measures that can be made in postmortem brain specimens.11,12 Although the cellular and pathologic correlates of cortical thickness have received little study,25 some histologic data indicate that cortical thinning is present in regions that harbor AD pathology.26,27 Yet while regional cortical thinning identified via in vivo MRI measures in patients with presumed neurodegenerative syndromes is undoubtedly valuable in localizing pathologic change, it seems much less likely that these regional measures will provide specific evidence regarding the molecular nature of that pathology.28
The congruence between the anatomic classification of patients with PPA using inferior frontal, temporopolar, and superior temporal sulcal thickness and clinical subtyping is impressively good (figure 3), as has similarly been demonstrated using atrophy pattern classification analyses.29 Such findings suggest that these types of quantitative MRI-based measures deserve a place in new clinical diagnostic criteria since they can be applied at the individual level, similarly to recently reported performance-based measures.5
Limitations of the present study include the lack of additional domains in the PASS rating scale. It is clear that patients with PPA can exhibit a variety of other relevant symptoms beyond impaired fluency, grammar/syntax, and comprehension. Fluency can be impaired for a variety of reasons (such as impairments in grammar/syntax, phonology, or word retrieval), so this measure may ultimately need to be refined or replaced, but it is useful at present. We have developed ratings for other domains of language function, including word retrieval, repetition, articulation, and others, and are currently testing these measures (the current scale is available upon request). It is also not clear how readily the PASS approach used here will generalize within the community of PPA investigators, but studies are being planned to evaluate its performance at multiple centers. It is not clear how well the PASS will perform in differentiating types of impairment in patients with more advanced stages of illness; it seems to perform very well in mildly to moderately impaired patients. Finally, we do not yet know how well the clinical or anatomic measures employed here will perform in longitudinal analyses, but these investigations are underway. Ultimately, we hope that further investigation will demonstrate that the types of measures studied here can be translated into clinical and imaging markers for use in diagnosis, monitoring, and prognostication, and will prove useful in clinical trials of novel therapeutics for these devastating diseases.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. B.C. Dickerson.
ACKNOWLEDGMENT
The authors thank the faculty and staff of the Massachusetts ADRC and the MGH Cognitive and Behavioral Neurology Unit, the MGH Speech and Language Pathology Department, MGH Psychology Assessment Center, the Division of Cognitive and Behavioral Neurology at Brigham and Women's Hospital, the Beth Israel Behavioral Neurology Unit, the Harold Goodglass Aphasia Research Center, and other providers who referred patients. The authors thank the staff at the Martinos Center for Biomedical Imaging, particularly Mary Foley and Larry White, for their technical expertise, and the staff of the MGH ADRC for their expertise in coordinating and evaluating participants in the Longitudinal Cohort. The authors also thank the participants in this study and their families for their contributions.
DISCLOSURE
D. Sapolsky, A. Bakkour, A. Negreira, and P. Nalipinski report no disclosures. Dr. Weintraub serves on the editorial boards of Alzheimer's and Dementia, the Turkish Journal of Neurology, and Dementia & Neuropsychologia and receives research support from the NIH (NIA NS1P30 AG13854-06 [Co-I, Clinical Core Leader], NIA 1 T32 AG20506-01-05 [Associate Director], NIDCD K08 DC07653-01 [Mentor], and NIDCD AG-260-06-01 [Co-I]). Dr. Mesulam serves on scientific advisory boards for the Cure Alzheimer Fund and the Association on Frontotemporal Dementia; serves on the editorial boards of Brain, Annals of Neurology, Human Brain Mapping, and the Journal of Cognitive Neuroscience; receives royalties from the publication of Principles of Behavioral and Cognitive Neurology (Oxford University Press, 2000); and receives research support from the NIH (AG13854 [PI] and DC008552 [PI]). Dr. Caplan serves on the editorial board of Language and Cognitive Processes and receives research support from the NIH (5 RO1 DC00942-12 [PI], NIDCD 5 R01 DC02146-12 [PI], NIDCD 1R21DC010461-01 [PI of MGH subcontract], NIDCD 2 R01 DC003108-11A1 [Investigator], R305G050083 [Investigator], 1R01DC011032-01 [PI], R01 MH087569 [Investigator], 1R01AG033583-01A2 [Co-I], and R305G050083 [Investigator]). Dr. Dickerson serves on the editorial board of Hippocampus and receives research support from the NIH (R01-AG29411 [PI] and R21-AG29840 [PI]) and from the Alzheimer's Association.
Supplementary Material
Address correspondence and reprint requests to Dr. Brad Dickerson, MGH Frontotemporal Dementia Unit, 149 13th St., Suite 2691, Charlestown, MA 02129 bradd@nmr.mgh.harvard.edu
Supplemental data at www.neurology.org
Study funding: Supported by NIA R01-AG29411, R21-AG29840, P50-AG005134, NCRR P41-RR14075, U24-RR021382, the Alzheimer's Association, and the Mental Illness and Neuroscience Discovery (MIND) Institute.
Disclosure: Author disclosures are provided at the end of the article.
Received December 21, 2009. Accepted in final form April 8, 2010.
REFERENCES
- 1.Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol 1982;11:592–598. [DOI] [PubMed] [Google Scholar]
- 2.Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol 2008;63:709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Josephs KA, Whitwell JL, Duffy JR, et al. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology 2008;70:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knopman DS, Boeve BS, Caselli RJ, et al. Longitudinal tracking of FTLD: toward developing clinical trial methodology. Alzheimer Dis Assoc Disord 2007;21:S58–S63. [DOI] [PubMed] [Google Scholar]
- 5.Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Arch Neurol 2009;66:1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickerson BC, Sperling RA, Hyman BT, Albert MS, Blacker D. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch Gen Psychiatry 2007;64:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris JC, Ernesto C, Schafer K, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer's Disease Cooperative Study experience. Neurology 1997;48:1508–1510. [DOI] [PubMed] [Google Scholar]
- 8.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006;20:210–216. [DOI] [PubMed] [Google Scholar]
- 9.Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol 2010;6:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 11.Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology 2009;72:1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex 2009;19:497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004;55:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galton CJ, Patterson K, Graham K, et al. Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology 2001;57:216–225. [DOI] [PubMed] [Google Scholar]
- 15.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 16.Dickerson BC, Fenstermacher E, Salat DH, et al. Detection of cortical thickness correlates of cognitive performance: reliability across MRI scan sessions, scanners, and field strengths. Neuroimage 2008;39:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorno-Tempini ML, Brambati SM, Ginex V, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology 2008;71:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodges JR, Patterson K. Nonfluent progressive aphasia and semantic dementia: a comparative neuropsychological study. J Int Neuropsychol Soc 1996;2:511–524. [DOI] [PubMed] [Google Scholar]
- 19.Butler CR, Brambati SM, Miller BL, Gorno-Tempini ML. The neural correlates of verbal and nonverbal semantic processing deficits in neurodegenerative disease. Cogn Behav Neurol 2009;22:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol 2000;47:36–45. [PubMed] [Google Scholar]
- 21.Amici S, Brambati SM, Wilkins DP, et al. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. J Neurosci 2007;27:6282–6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amici S, Ogar J, Brambati SM, et al. Performance in specific language tasks correlates with regional volume changes in progressive aphasia. Cogn Behav Neurol 2007;20:203–211. [DOI] [PubMed] [Google Scholar]
- 23.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci 2007;8:393–402. [DOI] [PubMed] [Google Scholar]
- 24.Rohrer JD, Warren JD, Modat M, et al. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology 2009;72:1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman SH, Kandel R, Cruz L, et al. Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without Alzheimer disease. J Neuropathol Exp Neurol 2008;67:1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez-Isla T, Price JL, McKeel DW Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci 1996;16:4491–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regeur L. Increasing loss of brain tissue with increasing dementia: a stereological study of post-mortem brains from elderly females. Eur J Neurol 2000;7:47–54. [DOI] [PubMed] [Google Scholar]
- 28.Weintraub S, Mesulam M. With or without FUS, it is the anatomy that dictates the dementia phenotype. Brain 2009;132:2906–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson SM, Ogar JM, Laluz V, et al. Automated MRI-based classification of primary progressive aphasia variants. Neuroimage 2009;47:1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.