Abstract
Objectives:
Quality of life (QOL) after stroke is poorly characterized. We sought to determine long-term natural history and predictors of QOL among first ischemic stroke survivors without stroke recurrence or myocardial infarction (MI).
Methods:
In the population-based, multiethnic Northern Manhattan Study, QOL was prospectively assessed at 6 months and annually for 5 years using the Spitzer QOL index (QLI), a 10-point scale. Functional status was assessed using the Barthel Index (BI) at regular intervals, and cognition using the Mini-Mental State Examination at 1 year. Generalized estimating equations estimated the association between patient characteristics and repeated QOL measures over 5 years. Follow-up was censored at death, recurrent stroke, or MI.
Results:
There were 525 incident ischemic stroke patients ≥40 years (mean age 68.6 ± 12.4 years). QLI declined after stroke (annual change −0.10, 95% confidence interval −0.17 to −0.04), after adjusting for age, sex, race-ethnicity, education, insurance, depressed mood, stroke severity, bladder continence, and stroke laterality. This decline remained when BI ≥95 was added to the model as a time-dependent covariate, and functional status also predicted QLI. Changes in QLI over time differed by insurance status (p for interaction = 0.0017), with a decline for those with Medicaid/no insurance (p < 0.0001) but not Medicare/private insurance (p = 0.98).
Conclusions:
In this population-based study, QOL declined annually up to 5 years after stroke among survivors free of recurrence or MI and independently of other risk factors. QLI declined more among Medicaid patients and was associated with age, mood, stroke severity, urinary incontinence, functional status, cognition, and stroke laterality.
GLOSSARY
- BI
= Barthel Index;
- CAD
= coronary artery disease;
- CHF
= congestive heart failure;
- CI
= confidence interval;
- CUMC
= Columbia University Medical Center;
- DM
= diabetes mellitus;
- GEE
= generalized estimating equation;
- HTN
= hypertension;
- MI
= myocardial infarction;
- MMSE
= Mini-Mental State Examination;
- NIHSS
= NIH Stroke Scale;
- NOMAS
= Northern Manhattan Study;
- QOL
= quality of life;
- QLI
= quality of life index.

e–Pub ahead of print

CME
Stroke causes a significant decrease in quality of life (QOL), even among those who have no poststroke disability.1 Prior studies examining QOL in stroke survivors have been limited by hospitalized samples,2 cross-sectional design,3 small sample sizes,2 and samples with both hemorrhagic and ischemic strokes.1,2,4,5 Longitudinal studies have been limited by short follow-up and enrollment of patients in different stages of early poststroke recovery. Few long-term population-based studies have systematically examined QOL after stroke. Those studies have been limited by few follow-up assessments5,6 and significant loss to follow-up.5 No study, to our knowledge, has censored recurrent vascular events, and the natural history and determinants of long-term QOL after a single ischemic stroke are not known. Finally, the effect of access to health care on long-term QOL after stroke is not well-characterized.7
We sought, in a prospective, population-based, multiethnic, urban stroke cohort study, to determine the long-term natural history and predictors of QOL among participants who experienced a first ischemic stroke. Our prior research provided evidence that disability increased over time after stroke independently of recurrence.8 We hypothesized that ischemic stroke patients would similarly experience decline in QOL over 5 years independent of recurrent stroke and other risk factors. Since validated stroke-specific QOL scales were not available at the time of the design of this study, we used a validated generic QOL scale, the Spitzer QLI,9 which has been used extensively in prior research in various diseases.10–12
METHODS
The Northern Manhattan Study (NOMAS) includes a population-based incident ischemic stroke follow-up study designed to determine predictors of stroke recurrence and prognosis in a multiethnic, urban population consisting of 63% Hispanic, 20% black, and 15% white residents.13,14
Selection of NOMAS cohort.
Methods of patient identification and enrollment have been described.15,16 Briefly, patients were enrolled if they were 1) diagnosed with a first ischemic stroke, 2) age ≥40 years, and 3) resident in Northern Manhattan for ≥3 months in a household with a telephone. Case ascertainment occurred between July 1993 and June 1997, and assessments were completed in August 2001. Over 80% of patients with acute ischemic stroke in northern Manhattan are hospitalized at Columbia University Medical Center (CUMC). Subjects hospitalized at other local hospitals were identified through active surveillance of admissions to those hospitals and through agreements with local physicians. Approximately 5% of incident ischemic stroke patients in northern Manhattan were not hospitalized but were also included.13,16 Evaluation of patients occurred at the hospital; those subjects either not hospitalized or hospitalized elsewhere were evaluated in the outpatient research clinic. Among participants not hospitalized at CUMC, only those with an initial evaluation and standardized neurologic examination within 20 days of first stroke were included in analyses involving the NIH Stroke Scale (NIHSS).
Standard protocol approvals, registrations, and patient consents.
The CUMC Institutional Review Board approved the study. All participants gave written informed consent directly or through a surrogate when appropriate.
Index evaluation of subjects.
Data were collected through interviews of the participant (or proxy, among those who could not be interviewed) by trained research assistants in English or Spanish (depending on the participant's primary language), and physical and neurologic examinations were conducted by study neurologists, as previously described.15,16 Race-ethnicity was self-identified. Standardized questions were adapted from the Behavioral Risk Factor Surveillance System17 by the Centers for Disease Control and Prevention to identify the following: hypertension (HTN), diabetes mellitus (DM), hypercholesterolemia, peripheral vascular disease, TIA, cigarette smoking, and cardiac conditions such as myocardial infarction (MI), coronary artery disease (CAD), angina, congestive heart failure (CHF), atrial fibrillation, other arrhythmias, and valvular heart disease. HTN was defined as in prior publications,15,16 and DM was defined by fasting blood glucose level ≥126 mg/dL, self-report of DM, or insulin/oral hypoglycemic use. Presence or absence of urinary incontinence within 7–10 days of the index stroke was determined. Depressed mood in the week prior to stroke was assessed by a standardized questionnaire at enrollment. Physical activity was assessed using a questionnaire18 adapted from the National Health Interview Survey of the National Center for Health Statistics, and classified as light-moderate and heavy, as previously described.15 Alcohol consumption was assessed as in previous publications.19
Stroke severity was assessed using the NIHSS score derived from a standardized neurologic examination, and was categorized as mild (NIHSS <6), moderate (NIHSS 6–13), and severe (NIHSS ≥14). This categorization was based on previous analyses of stroke severity in relation to stroke outcome from our population and a clinical trial.20,21 Stroke diagnostic evaluation included computerized tomography, brain MRI, or both; ultrasound evaluation, MRI, or both; vascular imaging; and echocardiography as appropriate. A consensus of stroke neurologists assessed stroke subtype using modified Stroke Data Bank criteria and all available information, as described in previous publications.22,23 Stroke neurologists also determined brain side of index stroke, presence of aphasia, and parietal lobe dysfunction.
Follow-up and outcomes assessment.
Follow-up evaluations were conducted at 6 months, 1 year, 1.5 years, and then annually to 5 years. Each evaluation was conducted at CUMC, by telephone, or, for patients unable or unwilling to come to CUMC, by an in-person home visit by a research staff member. Evaluations were conducted with the patient, family member, and caregiver if available. Information on vital status, QOL as measured by QLI, and intercurrent symptoms, illness, or hospitalization was collected. In-person assessments additionally included measurement of vital signs and physical and neurologic examination.
The QLI is a 10-point scale, with higher numbers denoting better QOL. The patient, family member, or health care provider rated the patient's QOL in 5 domains—activity, daily living, health, support, and outlook—assigning a score of 0, 1, or 2 in each domain. The QLI has previously been used in Spanish-speaking populations.24 Of 2,526 total QLI assessments in this study, 75% were assessed from the subject directly, and 52% were assessed during in-person visits.
Functional status measured by the Barthel Index (BI) was determined at all follow-up assessments; previous research demonstrated the reliability of phone assessments of the BI.25 Mini-Mental State Examination (MMSE) was performed at 1-year in-person assessments.26
An ongoing surveillance system of admissions to CUMC and other local hospitals, described in a previous publication,13 was also used to identify study participants who experienced a clinically evident recurrent stroke, MI, hospitalization, or death. When available, medical records were reviewed for all outcome events including death by a specially trained research assistant and adjudicated by study neurologists or cardiologists, as appropriate.
Statistical analyses.
Statistical analyses were conducted using SAS version 9.1.3 (SAS Institute, Cary, NC). For descriptive purposes, means were calculated for continuous variables and proportions for categorical variables. Follow-up was censored at the time of death, clinically evident recurrent stroke, or MI because of the anticipated adverse effect of these events on QOL.
QLI was analyzed first as a continuous variable. We used generalized estimating equations (GEE) to assess the relationship between several predictor variables, including time of follow-up at regular intervals, with repeated measurements of QLI. GEE models provided parameter estimates (β) and 95% confidence intervals (95% CI) for the association between predictors and QLI across 5 years of follow-up, after adjusting for baseline demographic (age, sex, race/ethnicity, education level, marital status, insurance status) and medical risk factors (HTN, coronary artery disease or MI, DM, smoking, physical activity, alcohol consumption), depressed mood, as well as relevant stroke characteristics (initial stroke severity, aphasia, parietal lobe dysfunction including neglect, side of stroke, and presence of urinary incontinence). As a working correlation structure we chose the exchangeable (intraclass) structure since it provided the best fit. Final multivariate models were constructed in a backward stepwise manner using those variables with p value <0.10. In a separate model, we additionally adjusted for functional status as a time-dependent covariate, using categories of functional independence (BI ≥95) and dependence (BI <95), as in our prior analysis.8 Insurance status was characterized as Medicare/private insurance vs Medicaid/no insurance.
Subgroup analysis was conducted among those with BI ≥95 6 months after stroke to assess the course and predictors of QOL among those deemed recovered after the acute phase. Among this cohort, the effect of cognitive status was assessed by including the 1-year MMSE in the multivariate model.
Preliminary graphs of QLI over time showed a decline for those with Medicaid/no insurance. Interaction between time of follow-up assessment and each variable in the final model (including insurance) was therefore included in multivariate models to assess whether change in QLI differed by insurance status. A separate analysis with only self-reported QLI scores was conducted to assess whether proxy responses produced any bias.
Time of follow-up assessment was analyzed as a continuous variable as well as a discrete variable (at 1-, 1.5-, 2-, 3-, 4-, and 5-year time points, with 0.5 year as reference). Analysis of discrete time points assumed that the time trend was a flexible step function allowed to change at each time point. A lack of linearity test was conducted that allowed the time trend to take any functional form; this test determined whether the fit of the model was significantly improved over the linear model.
In order to assess the change in each domain of the QLI over time, each of the 5 domains was analyzed in a separate multivariate model, with time treated as a continuous variable and adjusted as above. The odds of having a maximum score (2 vs 0 or 1) was calculated for each domain. Tests for interactions between time to follow-up and other covariates were performed.
RESULTS
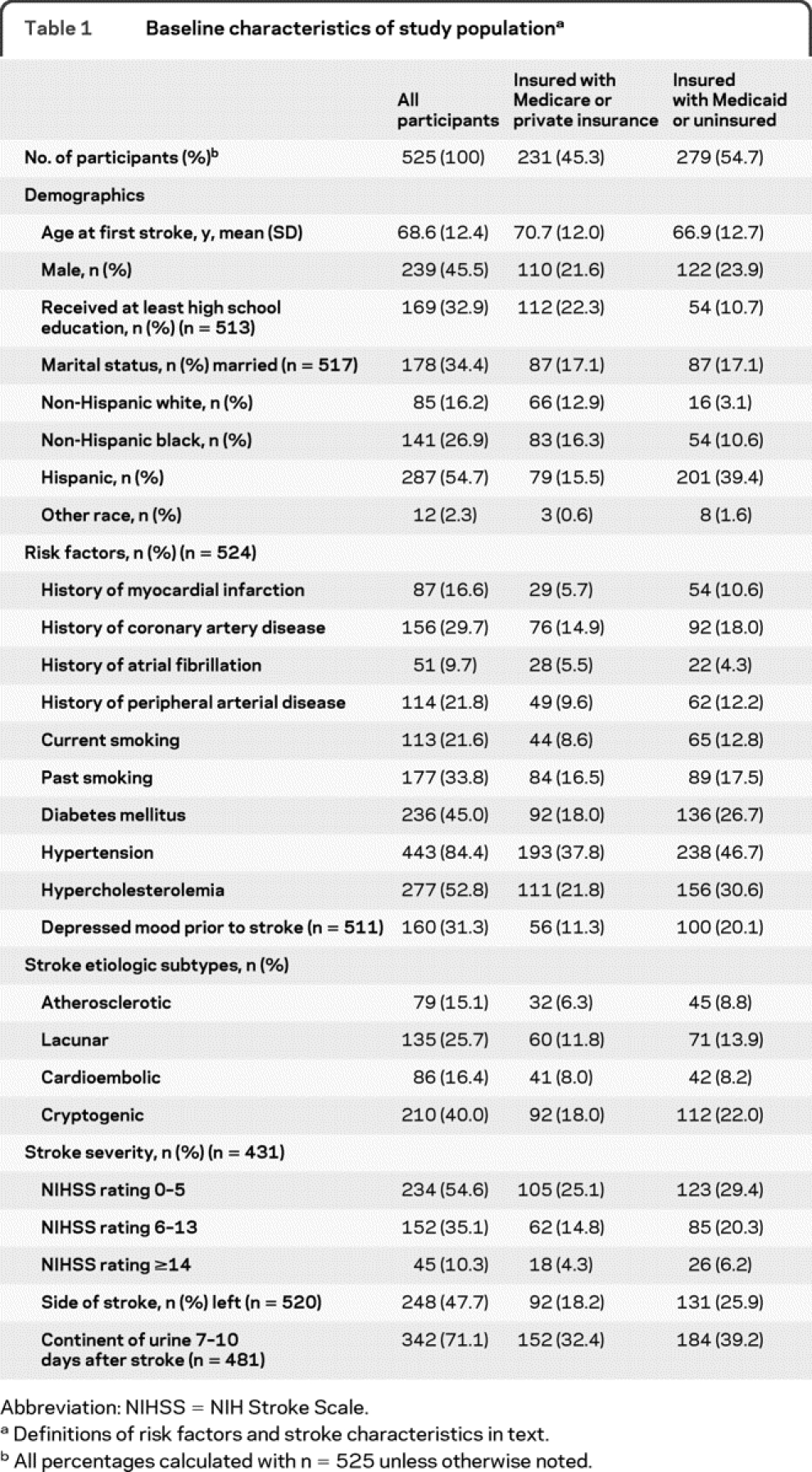
There were 655 patients enrolled in the NOMAS stroke incidence and follow-up study. No follow-up data were available for participants who died within 6 months (n = 83) and for those who died between 6 months and 1 year (n = 14). QLI data were not collected for a further 9 participants. Twenty-four participants had recurrent stroke before first QLI assessment and were excluded. A total of 525 patients were thus available for analysis. During follow-up, 84 patients had recurrent stroke or MI, and there were 133 deaths. Table 1 lists baseline characteristics of the study population. The median QLI score at 6 months was 8 (interquartile range 6–10).
Table 1 Baseline characteristics of study population
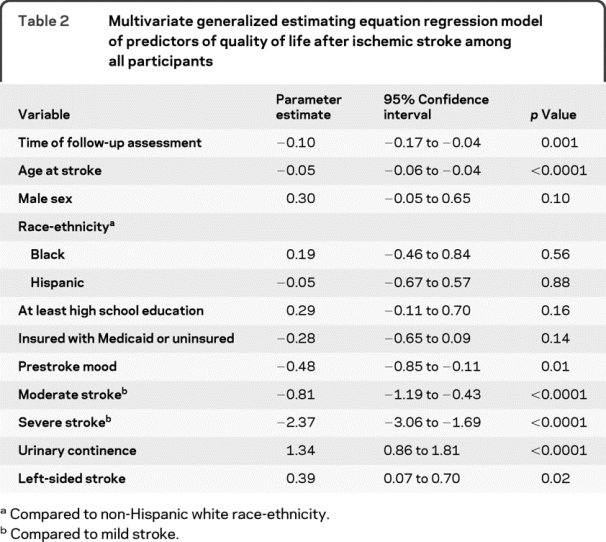
There was an annual decline in QLI in an unadjusted regression model considering time to follow-up assessment as a continuous variable (−0.09 per year, 95% CI −0.15 to −0.04). When adjusted for demographic variables, the annual decline was similar (−0.09 per year, 95% CI −0.15 to −0.03), and did not change further when adjusted for demographic variables, medical risk factors, and depressed mood (−0.10 per year, 95% CI −0.16 to −0.04). Further adjusting for stroke characteristics, such as stroke severity, side of stroke, and urinary incontinence, also did not change the extent of decline (−0.10 per year, 95% CI −0.17 to −0.04; table 2). Other predictors of QLI among all participants were age at stroke, prestroke mood, stroke severity, urinary continence, and left-sided stroke.
Table 2 Multivariate generalized estimating equation regression model of predictors of quality of life after ischemic stroke among all participants
When BI ≥95 was added to the model as a time-dependent covariate, the extent of decline in QLI lessened slightly but remained significant (−0.07 per year, 95% CI −0.13 to −0.01). Other predictors remained, and BI ≥95 was also a predictor (1.63 per year, 95% CI 1.36 to 1.91).
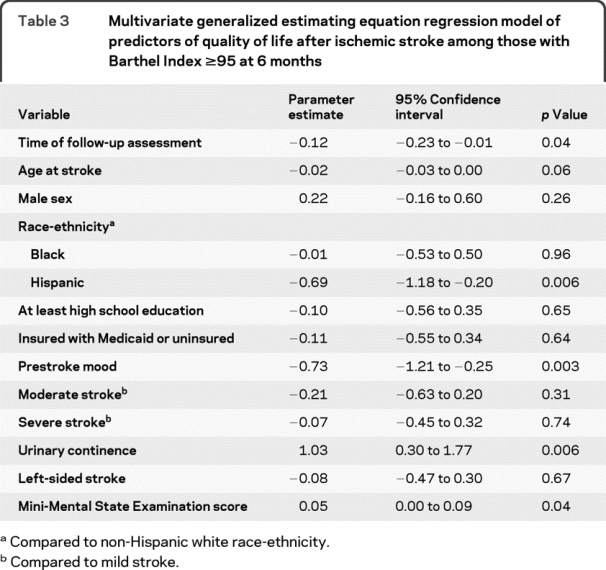
In subgroup analyses among those who had BI ≥95 at 6 months (n = 245), there was still a significant annual decline in QLI over follow-up, even after adjustment for MMSE (table 3). Higher cognitive scores on the MMSE were associated with better QLI.
Table 3 Multivariate generalized estimating equation regression model of predictors of quality of life after ischemic stroke among those with Barthel Index ≥95 at 6 months
Changes in QLI over time differed by insurance status (p for interaction = 0.0017), with a decline in QLI over time for those with Medicaid/no insurance (−0.20 per year in a fully adjusted model, 95% CI −0.29 to −0.11), whereas there was no definite decline among those with Medicare/private insurance (0.001 per year, 95% CI −0.08 to 0.09). The only other 2-way interaction was between time to follow-up and age at stroke onset (p = 0.02). The figure depicts QLI stratified by insurance status, unadjusted for other risk factors. Results were similar when the final multivariate model was restricted to self-reported QLI scores (available in 75% of the cohort), indicating that proxy assessments did not produce bias.
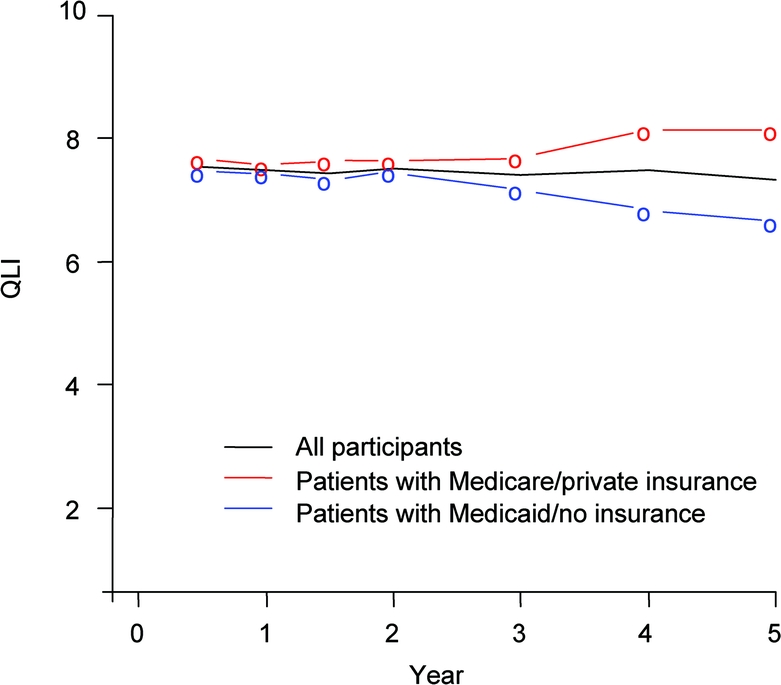
Figure Mean quality of life index (QLI) over 5 years of follow-up, stratified by insurance status
We fitted a model with discrete time points, without assuming a particular functional form for the time trend. A significant difference in QLI was seen at 3 years (−0.29, 95% CI −0.53 to −0.05), 4 years (−0.37, 95% CI −0.63 to −0.12), and 5 years (−0.54, 95% CI −0.87 to −0.21), each compared to the 0.5-year timepoint. Nonsignificant differences were seen for time points earlier than 3 years. In the final regression model, time was treated as a continuous variable with linear form, since there was no evidence that an alternative model better fit the data (lack of linearity χ2 test: df = 4, p = 0.12).
When domains of QLI were analyzed separately, domain scores in activity (OR 0.88 per year, 95% CI 0.82 to 0.95), daily living (OR 0.89 per year, 95% CI 0.83 to 0.96), and support (OR 0.88 per year, 95% CI 0.79 to 0.98) were associated with time of follow-up assessment, after adjusting for demographics, medical risk factors, and stroke characteristics. There were interactions between insurance status and time of follow-up assessment for activity (p for interaction = 0.02), daily living (p for interaction = 0.02), and outlook (p for interaction = 0.01).
DISCUSSION
In this large, prospective, population- based, multiethnic study of first ischemic stroke patients with multiple follow-up assessments, there was a significant linear decline in QOL in the years following stroke among survivors free of clinically evident recurrent stroke or MI. This decline in QOL appeared independent of activities of daily living independence and cognition and began to be apparent at 3 years of follow-up. Furthermore, the decline in QOL was seen exclusively among the uninsured and those with Medicaid, a state-administered insurance only available to low-income residents,27 which has been associated with limited access to health care in New York state.28
A decline in QOL after the acute phase of stroke recovery has been observed in prior hospital-based studies,29 but these have been limited by small sample sizes, limited follow-up, the inclusion of hemorrhagic stroke, and selected samples of hospitalized patients. Few population-based studies have examined QOL after stroke. One examined 304 stroke patients and 234 caregivers and found that Short Form-36 scores declined between 4 months and 16 months after stroke. Our study included a larger sample and assessed QOL multiple times during a longer follow-up period, and hence is able to delineate the natural history and predictors of QOL over the long term in an unselected stroke cohort while adjusting for potential confounders including stroke severity, function, and cognition. Furthermore, we censored recurrent strokes and MI, allowing us to determine the long-term effect on QOL of a single, first ischemic stroke alone.
Few studies have shown an effect of socioeconomic indicators on QOL after stroke.5,6 In our population, baseline demographic and risk factor profiles differed between the 2 insurance groups. However, even after adjusting for these variables, a significant decline in QOL was seen exclusively among the uninsured and those insured with Medicaid. This decline may be due to disparities in access to rehabilitative services, information about health, and ongoing management of risk factors and chronic conditions.7 Prior research has demonstrated an association between limited access to health care and worse medication compliance.30 Also, the progression of white matter changes over time is more pronounced in the setting of poor blood pressure control31 and subclinical infarcts are common.32 In the Northern Manhattan population, 18% of 892 participants free of clinical stroke had subclinical infarcts.33 Silent infarcts have been associated with cognitive function,34 and cerebral leukoaraiosis with QOL in previous studies.35 Accumulation of additional subclinical infarcts and leukoariosis over time may therefore account for the decline in QOL over time after stroke, and this effect may be most prominent in those with limited access to health care resources. Finally, socioeconomic status has an impact on indicators that may mediate QOL, such as disability and handicap after stroke.36 Since we did not assess for the use of rehabilitative services, the development of comorbidities during follow-up, the extent of risk factor control, medical compliance, and subclinical infarcts, further research is needed to clarify the role of these variables on long-term QOL.
Similar to our study, age,1,4 mood or depression,2 stroke severity,5,6 urinary incontinence,37 side of stroke,38 and DM1 were also predictors of QOL in previous studies. Decline in QOL was most prominent in the domains of activity, daily living, and support, which is consistent with prior findings in this population,8 and suggests that decline in QOL may be associated with decline in functional and occupational status. The interaction between time and insurance status in the domain of outlook suggests that limited access to care may have an impact on one's sense of control in matters of health.
A limitation of this study is that the use of the generic QLI might have missed stroke-specific aspects of QOL. However, we observed a significant decline in QLI and found predictors of QOL that are in parallel with prior research in stroke patients, suggesting that the scale is sensitive to the QOL of stroke patients. Another limitation is the absence of data about whether participants were diagnosed with depression. In the absence of a formal diagnosis, we employed a question that assessed mood in the 2 weeks prior to stroke, which was posed to patients after their first stroke and thus may be subject to recall bias. Finally, although this study delineates the natural history of QOL after ischemic stroke, further study is needed in other populations, such as stroke-free individuals, to be able to compare stroke patients with other groups.
Providers who care for poststroke patients may be able to identify those at higher risk for poorer QOL and provide more aggressive rehabilitative and social support interventions. Future research should be directed toward clarifying the relationship between time, aging, and QOL; comparing QOL over time in stroke patients and stroke-free individuals; assessing QOL with stroke-specific scales39,40 not available at the time of the design of this study; and clarifying the role of ongoing risk factor management in the long-term QOL of stroke patients.
DISCLOSURE
Dr. Dhamoon and Y.P. Moon report no disclosures. Dr. Paik serves as Statistical Editor for the Journal of General Internal Medicine and receives royalties from the publication of Statistical Methods for Rates and Proportions (Wiley, 2003). Dr. Boden-Albala serves on a scientific advisory board for Lundbeck, Inc. and receives research support from the American Heart Association and the National Stroke Association. Dr. Rundek serves on the editorial board of Neurology®; served on the speakers' bureau of Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership; and receives research support from the NIH (NINDS R37 NS 029993-11 [Co-I] and NINDS K24 NS 062737 [PI]). Dr. Sacco has served as a consultant to Boehringer Ingelheim, Sanofi-Aventis, and GlaxoSmithKline and receives research support from the NIH [NINDS R37 NS29993 (PI), NINDS R01 NS 040807 (PI), NINDS R01 047655 (Co-I), and NHLBI HC-98-08-HC-83169701 (Co-I)]. Dr. Elkind serves as Resident and Fellow Section Editor for Neurology®; serves as a consultant to Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, GlaxoSmithKline, Jarvik Heart, Tethys Bioscience, Inc., and Daiichi-Sankyo; serves on speakers' bureaus for Boehringer-Ingelheim, Inc. and Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership; and receives research support from diaDexus, Inc., Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, and the NIH/NINDS [ R01 NS050724 (PI), NS048134 (PI), P50 NS049060 (Project PI), R37 NS029993 (Co-PI), R01 NS55809 (Co-I), and R01 NS062820 (Co-I)]; and has given expert testimony on behalf of Merck Serono (Vioxx® litigation), Pfizer Inc. (Shiley valve and Celebrex®/Bextra® litigation), and Novartis (Zelnorm® and stroke litigation).
Address correspondence and reprint requests to Dr. Mandip S. Dhamoon, Neurological Institute, 710 W. 168th St. Room 640, New York, NY 10032 msd2102@mail.cumc.columbia.edu
e-Pub ahead of print on June 23, 2010, at www.neurology.org.
Study funding: Supported by the NIH/NINDS (R01 NS48134, MSVE; R37 29993 to R.L.S. and M.S.V.E.).
Disclosure: Author disclosures are provided at the end of the article.
Received November 17, 2009. Accepted in final form March 13, 2010.
REFERENCES
- 1.Lai SM, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke 2002;33:1840–1844. [DOI] [PubMed] [Google Scholar]
- 2.Haacke C, Althaus A, Spottke A, Siebert U, Back T, Dodel R. Long-term outcome after stroke: evaluating health-related quality of life using utility measurements. Stroke 2006;37:193–198. [DOI] [PubMed] [Google Scholar]
- 3.Duncan PW, Samsa GP, Weinberger M, et al. Health status of individuals with mild stroke. Stroke 1997;28:740–745. [DOI] [PubMed] [Google Scholar]
- 4.Nys GM, van Zandvoort MJ, van der Worp HB, et al. Early cognitive impairment predicts long-term depressive symptoms and quality of life after stroke. J Neurol Sci 2006;247:149–156. [DOI] [PubMed] [Google Scholar]
- 5.Paul SL, Sturm JW, Dewey HM, Donnan GA, Macdonell RA, Thrift AG. Long-term outcome in the North East Melbourne Stroke Incidence Study: predictors of quality of life at 5 years after stroke. Stroke 2005;36:2082–2086. [DOI] [PubMed] [Google Scholar]
- 6.Sturm JW, Donnan GA, Dewey HM, et al. Quality of life after stroke: the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2004;35:2340–2345. [DOI] [PubMed] [Google Scholar]
- 7.Mold F, McKevitt C, Wolfe C. A review and commentary of the social factors which influence stroke care: issues of inequality in qualitative literature. Health Soc Care Community 2003;11:405–414. [DOI] [PubMed] [Google Scholar]
- 8.Dhamoon MS, Moon YP, Paik MC, et al. Long-term functional recovery after first ischemic stroke: The Northern Manhattan Study. Stroke 2009;40:2805–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spitzer WO, Dobson AJ, Hall J, et al. Measuring the quality of life of cancer patients: a concise QL-index for use by physicians. J Chronic Dis 1981;34:585–597. [DOI] [PubMed] [Google Scholar]
- 10.Bonnetain F, Paoletti X, Collette S, et al. Quality of life as a prognostic factor of overall survival in patients with advanced hepatocellular carcinoma: results from two French clinical trials. Qual Life Res 2008;17:831–843. [DOI] [PubMed] [Google Scholar]
- 11.Shor-Posner G, Lecusay R, Miguez-Burbano MJ, et al. Quality of life measures in the Miami HIV-1 infected drug abusers cohort: relationship to gender and disease status. J Subst Abuse 2000;11:395–404. [DOI] [PubMed] [Google Scholar]
- 12.Cooper AB, Ferguson ND, Hanly PJ, et al. Long-term follow-up of survivors of acute lung injury: lack of effect of a ventilation strategy to prevent barotrauma. Crit Care Med 1999;27:2616–2621. [DOI] [PubMed] [Google Scholar]
- 13.Sacco RL, Boden-Albala B, Gan R, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol 1998;147:259–268. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann A, Rundek T, Mast H, et al. Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study. Neurology 2001;57:2000–2005. [DOI] [PubMed] [Google Scholar]
- 15.Sacco RL, Gan R, Boden-Albala B, et al. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke 1998;29:380–387. [DOI] [PubMed] [Google Scholar]
- 16.Sacco RL, Elkind M, Boden-Albala B, et al. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA 1999;281:53–60. [DOI] [PubMed] [Google Scholar]
- 17.Gentry EM, Kalsbeek WD, Hogelin GC, et al. The behavioral risk factor surveys: II: design, methods, and estimates from combined state data. Am J Prev Med 1985;1:9–14. [PubMed] [Google Scholar]
- 18.Moss AJ, Parsons VL. Current estimates from the National Health Interview Survey: United States, 1985. Vital Health Stat 1986;10:i–iv, 1–182. [PubMed] [Google Scholar]
- 19.Elkind MS, Sciacca R, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Moderate alcohol consumption reduces risk of ischemic stroke: the Northern Manhattan Study. Stroke 2006;37:13–19. [DOI] [PubMed] [Google Scholar]
- 20.Sacco RL, Boden-Albala B, Chen X, Lin IF, Kargman DE, Paik MC. Relationship of 6-month functional outcome and stroke severity: implications for acute stroke trials from the Northern Manhattan Stroke Study. Neurology 1998;50(suppl 4):A327. [Google Scholar]
- 21.Sacco RL, DeRosa JT, Haley EC Jr, et al. Glycine antagonist in neuroprotection for patients with acute stroke: GAIN Americas: a randomized controlled trial. JAMA 2001;285:1719–1728. [DOI] [PubMed] [Google Scholar]
- 22.Gan R, Sacco RL, Kargman DE, Roberts JK, Boden-Albala B, Gu Q. Testing the validity of the lacunar hypothesis: the Northern Manhattan Stroke Study experience. Neurology 1997;48:1204–1211. [DOI] [PubMed] [Google Scholar]
- 23.Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke 1988;19:547–554. [DOI] [PubMed] [Google Scholar]
- 24.Dapueto JJ, Francolino C, Servente L, et al. Evaluation of the Functional Assessment of Cancer Therapy-General (FACT-G) Spanish Version 4 in South America: classic psychometric and item response theory analyses. Health Qual Life Outcomes 2003;1:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinar D, Gross CR, Bronstein KS, et al. Reliability of the activities of daily living scale and its use in telephone interview. Arch Phys Med Rehabil 1987;68:723–728. [PubMed] [Google Scholar]
- 26.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry 1983;40:812. [DOI] [PubMed] [Google Scholar]
- 27.Long SK, Coughlin TA, Kendall SJ. Access to care among disabled adults on Medicaid. Health Care Financ Rev 2002;23:159–173. [PMC free article] [PubMed] [Google Scholar]
- 28.Calman NS, Golub M, Ruddock C, Le L, Hauser D. Separate and unequal care in New York City. J Health Care Law Policy 2006;9:105–120. [PubMed] [Google Scholar]
- 29.Nydevik I, Hulter-Asberg K. Sickness impact after stroke: a 3-year follow-up. Scand J Prim Health Care 1992;10:284–289. [DOI] [PubMed] [Google Scholar]
- 30.Doshi JA, Zhu J, Lee BY, Kimmel SE, Volpp KG. Impact of a prescription copayment increase on lipid-lowering medication adherence in veterans. Circulation 2009;119:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dufouil C, de Kersaint-Gilly A, Besancon V, et al. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology 2001;56:921–926. [DOI] [PubMed] [Google Scholar]
- 32.Das RR, Seshadri S, Beiser AS, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke 2008;39:2929–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhakaran S, Wright CB, Yoshita M, et al. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology 2008;70:425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 35.Koton S, Schwammenthal Y, Merzeliak O, et al. Cerebral leukoaraiosis in patients with stroke or TIA: clinical correlates and 1-year outcome. Eur J Neurol 2009;16:218–225. [DOI] [PubMed] [Google Scholar]
- 36.van den Bos GA, Smits JP, Westert GP, van Straten A. Socioeconomic variations in the course of stroke: unequal health outcomes, equal care? J Epidemiol Community Health 2002;56:943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel MD, McKevitt C, Lawrence E, Rudd AG, Wolfe CD. Clinical determinants of long-term quality of life after stroke. Age Ageing 2007;36:316–322. [DOI] [PubMed] [Google Scholar]
- 38.de Haan RJ, Limburg M, Van der Meulen JH, Jacobs HM, Aaronson NK. Quality of life after stroke: impact of stroke type and lesion location. Stroke 1995;26:402–408. [DOI] [PubMed] [Google Scholar]
- 39.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke 1999;30:2131–2140. [DOI] [PubMed] [Google Scholar]
- 40.Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke 1999;30:1362–1369. [DOI] [PubMed] [Google Scholar]


