Abstract
Objectives
This study assessed the extent and mechanism of complement activation in community-acquired sepsis at presentation to the emergency department (ED) and following 24 hours of quantitative resuscitation.
Methods
A prospective pilot study of patients with severe sepsis and healthy controls was conducted among individuals presenting to a tertiary care ED. Resuscitation, including antibiotics and therapies to normalize central venous and mean arterial pressure (MAP) and central venous oxygenation, was performed on all patients. Serum levels of Factor Bb (alternative pathway), C4d (classical and mannose-binding lectin [MBL] pathway), C3, C3a, and C5a were determined at presentation and 24 hours later among patients.
Results
Twenty patients and 10 healthy volunteer controls were enrolled. Compared to volunteers, all proteins measured were abnormally higher among septic patients (C4d 3.5-fold; Factor Bb 6.1-fold; C3 0.8-fold; C3a 11.6-fold; C5a 1.8-fold). Elevations in C5a were most strongly correlated with alternative pathway activation. Surprisingly, a slight but significant inverse relationship between illness severity (by sequential organ failure assessment [SOFA] score) and C5a levels at presentation was noted. Twenty-four hours of structured resuscitation did not, on average, affect any of the mediators studied.
Conclusions
Patients with community-acquired sepsis have extensive complement activation, particularly of the alternative pathway, at the time of presentation that was not significantly reversed by 24 hours of aggressive resuscitation.
Keywords: complement pathway, alternative, complement pathway, classical, complement C5a, Bayesian statistics
A key pathophysiologic feature of sepsis is the dysregulation of innate immunity, including the complement system. Activation of complement pathways during sepsis presumably takes place both by direct contact with bacterial pathogens and by unregulated activation on surfaces of injured host cells such as endothelial cells.1 There is extensive evidence in animal models that inappropriate complement activation worsens illness. The primary culprit is believed to be C5a, which serves as a neutrophil chemoattractant and activator and provokes deleterious responses in a number of tissues, including vascular endothelium and smooth muscle, the heart, and the brain.2,3 At least eight different strategies targeting either C5a or its primary cellular receptor have been patented and are at various stages of preclinical development.4
Most insight into complement and C5a in sepsis comes from animal models. Much less is known in humans. C5a levels during sepsis and life-threatening infection have been reported in fewer than 150 patients in the past 30 years. There are very few human data examining the relationship between C5a production and other features of acute illness. To our knowledge, C5a abnormalities at initial presentation and their changes following resuscitation have never been reported.
Our intent was twofold; first, we measured, in a cohort of emergency department (ED) patients with severe sepsis, the extent of C5a activation at presentation and at 24 hours after the delivery of a structured resuscitation protocol. Second, we evaluated the influ-ence of classical and mannose-binding lectin (MBL) pathway (marker C4d) and the alternative pathway (marker Bb) on C5a dysregulation. To place these find-ings in context with what is known of complement activation in human sepsis from previous intensive care unit (ICU)-based studies, we carried out a systemic review of the available published literature to quantify the current state of knowledge regarding C5a in human sepsis. Data from previous reports were formally and quantitatively incorporated into a Bayesian analysis of our experimental data.
METHODS
Study Design
This was a prospective pilot study of a cohort of critically ill patients and an accompanying set of healthy control individuals. This study was approved by the institutional review board. All subjects provided written consent.
Study Setting and Population
The study took place at a tertiary teaching facility with > 115,000 ED visits annually. Patients were enrolled if they had 1) suspected or confirmed infection; 2) any two of four criteria of systemic inflammatory response; and 3) systolic blood pressure (sBP) < 90 mm Hg or mean arterial pressure (MAP) < 65 after 20 mL / kg crystalloid (septic shock) or a whole blood lactate ≥ 4 mmol / L (severe sepsis). Exclusion criteria were age < 18 years, pregnancy, established prior ‘‘do not attempt resuscitation’’ orders, any primary diagnosis other than sepsis, requirement for immediate surgery, or need for cardiopulmonary resuscitation prior to enrollment.
Inclusion was not consecutive. Rather, patients were selected from the ranks of a larger ongoing study in this population. Given the sparse literature regarding the relationship between complement activation and illness severity, cases were selected to provide a wide range of initial sequential organ failure assessment (SOFA) scores.5 The available patients with the 10 highest and lowest initial scores were selected. Ten healthy volunteers (no acute illness in the prior 2 weeks, no current corticosteroids, or immunosuppressive medications) were also enrolled via a posted advertisement regarding the study. No formal sample size calculations were performed. Rather, prior findings in the published literature were chosen to justify the sample used.
Study Protocol
All sepsis subjects were treated in the ED using a quantitative resuscitation protocol that required normalization of the central venous pressure, MAP, and central venous oxygen saturation (ScvO2) using predefined interventions; this protocol is described in Jones et al.6 Patients meeting inclusion criteria were volume resuscitated to reach a central venous pressure of ≥ 8 mm Hg and were intubated and mechanically ventilated as needed to maintain oxygen saturation measurements ≥ 94%. Arterial pressure transducers were placed and either norepinephrine or dopamine was infused to reach an MAP target of 65 mm Hg. Once the MAP target was reached, patients received transfused packed red blood cells and dobutamine as needed to maintain ScvO2 ≥ 70%. At the time that subjects had their central venous catheter placed, and 24 hours later, blood was drawn into serum separation tubes (SST) clot tubes (BD Vacutainer, Franklin Lakes, NJ) and stored at −80°C.
Measurements
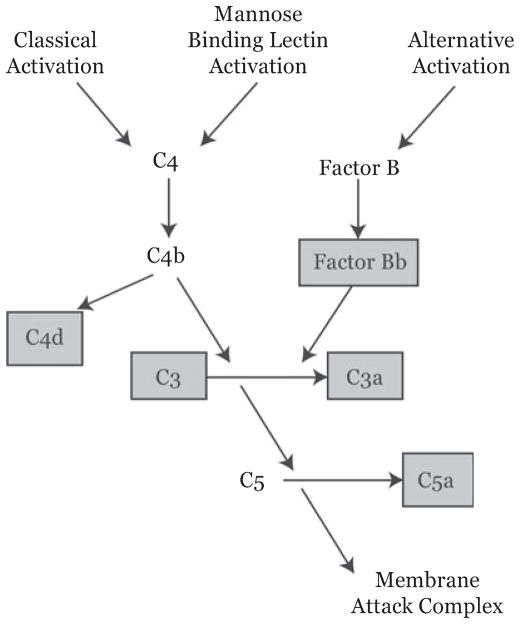
The complement proteins measured were chosen to consider the pathway of activation, the extent of global activation, and the specific production of C5a (Figure 1). C3 was measured with the Cobas Integra platform (Roche Diagnostics, Ltd., West Sussex, UK). Other proteins were measured using eOptEIA (BD Biosciences, San Jose, CA) for C3a and C5a and MicroVue Complement (Quidel, San Diego, CA) for Bb and C4d. Both C3a and C5a are quickly converted to the des-Arg forms by a number of serum carboxypeptidases; as these forms are indistinguishable by the assays we used, in this report we will refer to the parent proteins and their des-Arg forms collectively as C3a and C5a.
Figure 1.
Overview of the complement system components relevant to the current work. Components in gray boxes are those for which serum measurements were made. Assayed elements were chosen to highlight pathway of activation (i.e., C4d for the classical and MBL pathways, Factor Bb for the alternative pathway), for assessing the total amount of activation (C3, C3a), and for the production of the peptide felt to be most physiologically relevant in this context, C5a. MBL = mannose-binding lectin.
Literature Review to Develop Statistical Prior Probabilities
A formal literature review was undertaken to establish prior probabilities for Bayesian analysis of our prospective measurements. The search was conducted in six databases by a professional clinical librarian (GKR) in July 2008. Six discrete searches were conducted in each of the databases. No year or language limits were applied to any of the searches. Efforts were taken to be as inclusive as possible in the search retrieval. The librarian performed minor evaluation of the articles retrieved to ensure topical (or potentially topical) relevancy. Of over 800 reports initially identified, three provided data specifically related to extent of C5a activation in sepsis or septic shock compared to healthy controls, and two were suitable for inclusion in our analysis.7,8
Data Analysis
Because of the availability of previous ICU studies with similar study designs, we chose a Bayesian, rather than frequentist, approach. Patient data were analyzed using the Bayesian package WinBUGS 1.4 (MRC Biostatistics Unit, Cambridge, UK) with R2WinBUGS serving as the interface with R.9 Results are reported as means and 95% credible intervals. The Bayesian credible interval is distinct from the more commonly used frequentist con-fidence interval (CI). The former is the posterior probability, given prior expectations and new experimental data, that the value of a parameter of interest lays between two bracketing values. The latter is the range in which one would expect to find an estimate of a parameter of interest after many repeated, but not actually conducted, experiments. As our systematic literature review provided specific prior information about C5a in septic patients, we used Bayesian methods to take advantage of those data. In all cases, prior data (i.e., those data recovered from our literature review) were assumed to be normally distributed with mean μ, and variance σ2, where μ also was normally distributed and σ2 was inverse-gamma-distributed. For the prior probability of C5a among healthy and septic patients, values were taken from two reports in the literature. For Schreiber et al.,7 original data were very graciously provided by Dr. Markus Huber-Lang. For Weinberg et al.,8 the C5a values were manually extracted from a scatter plot in the printed manuscript. To combine the information from the two reports into prior probabilities, the pooled mean for healthy and septic C5a levels was calculated as the mean of the mean values from both reports weighted by their precision. The pooled standard deviation (SD) was calculated as the mean of the SDs weighted by their sample size. For protein analyses other than C5a, prior information was not available, and therefore vague prior probabilities (μ = 0, σ = 100) were used in the analysis.10
The WinBUGS Gibbs sampling method was used for estimation. Three Markov chains, each with 5,000 iterations, were used to generate posterior means and 95% credible values. A similar strategy, also with vague priors, was used for linear regression.
RESULTS
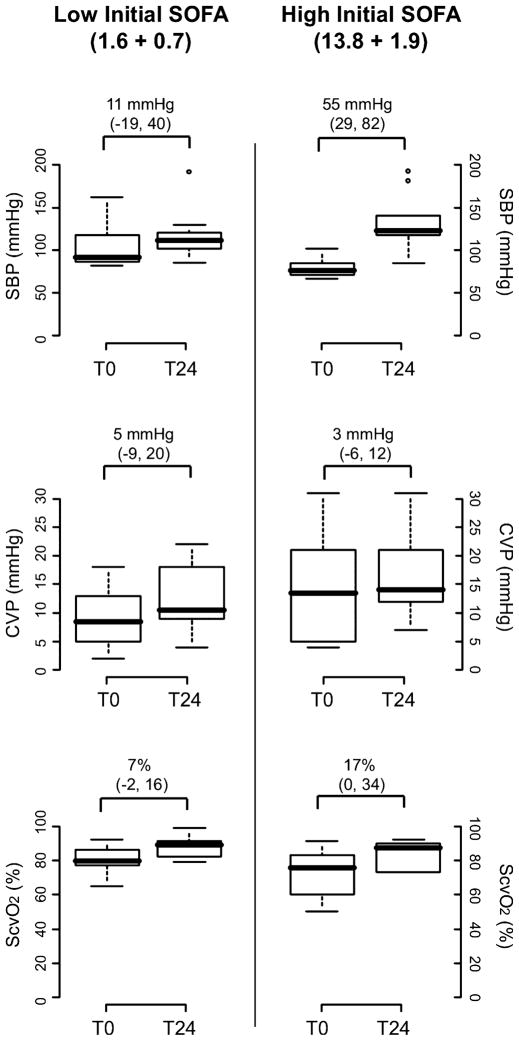
Clinical features of the 20 septic patients are shown in Table 1. In general, the subjects were representative of community-acquired sepsis. Controls were similar in age and in sex distribution, although all controls were of white race. Figure 2 shows the extent of physiologic abnormalities seen at the time of enrollment (namely, systolic and central venous pressure and central venous oxyhemoglobin saturation) and the effect of 24 hours of resuscitation directed at normalizing systemic and central venous pressures and ScvO2. Improvements were seen in both low- and high-SOFA score groups, although most strikingly in the latter.
Table 1.
Patient Demographics, Clinical Characteristics, and Physiologic Measurements
| Variable | Cases (n = 20) | Controls (n = 10) |
|---|---|---|
| Age, yr (mean ± SD) | 55 ± 18.2 | 49 ± 8* |
| Race, n (%) | ||
| Caucasian | 7 (35) | 10 (100) |
| Black or African American | 11 (55) | |
| Hispanic | 2 (10) | |
| Sex, n (%) | ||
| Male | 8 (40) | 3 (30) |
| Female | 12 (60) | 7 (70) |
| Comorbidities, n (%) | ||
| CAD | 4 (20) | |
| CVA | 1 (5) | |
| Diabetes mellitus | 3 (15) | |
| COPD | 3 (15) | |
| End-stage renal disease | 1 (5) | |
| Cancer | 6 (30) | |
| Indwelling vascular line | 1 (5) | |
| Nursing home resident | 1 (5) | |
| ED vital signs (mean ± sd) | ||
| sBP (mm Hg) | 96 ± 24 | |
| Pulse (beats / min) | 98 ± 21 | |
| RR (breaths / min) | 22 ± 4.7 | |
| Temp (°F) | 99 ± 3.7 | |
| O2 sat (%) | 97 ± 32.2 | |
| CVP (mm Hg) | 9 ± 8.0 | |
| ScvO2 (%) | 75 ± 22 | |
| ED SOFA score (mean ± SD) | 7.7 ± 6.4 | |
| Suspected source of infection, | n (%) | |
| Pulmonary | 7 (35) | |
| Urinary tract | 5 (25) | |
| Intraabdominal | 3 (15) | |
| Skin / soft tissue | 1 (5) | |
| Blood (bacteremia) | 1 (5) | |
| Unknown | 3 (15) | |
| Hospital outcomes | ||
| Hospital days | 8 ± 7.1 | |
| ICU days | 4 ± 4.6 | |
| Mortality | 3 (15) | |
CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident; CVP = central venous pressure; O2 sat = oxygen saturation; sBP = systolic blood pressure; RR = respiratory rate; ScvO2 = central venous oxygen saturation; SOFA = sequential organ failure assessment.
95% credible interval for difference: −4 to 15 years.
Figure 2.
Results of resuscitation protocol. Patients were included in the study on the basis of presenting SOFA score (see text for details). This figure shows the change in three key physiologic measures that both guided the intensity of resuscitation and reflected its success, the sBP, the central venous pressure, and the ScvO2. Summary measures are shown, divided by low-and high-SOFA groups, at presentation (t0) and as the best value measured over the first full day after resuscitation (t24). Box plots represent 5th, 25th, 50th, 75th, and 95th percentiles. Asterisks represent extreme values. Values over the box pairs represent the mean difference between groups and that difference’s posterior 95% credible interval. See text for details. CVP = central venous pressure; sBP = systolic blood pressure; ScvO2 = central venous oxygen saturation.
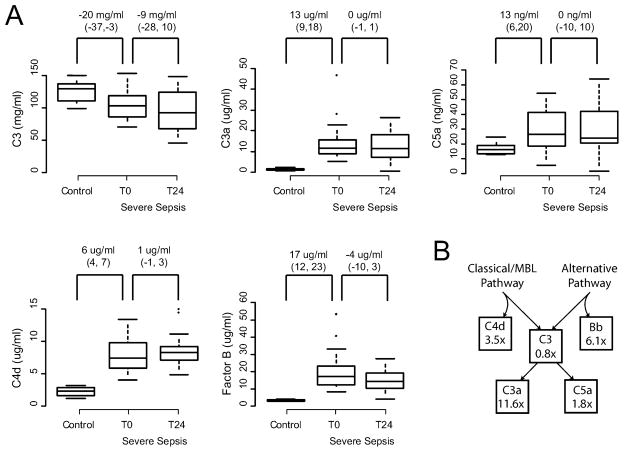
Figure 3 compares, between healthy and septic patients, as well as within septic patients at presentation and 24 hours after presentation, the concentrations of each of the complement proteins measured. As expected, there was significant consumption of C3, which was present on arrival and slightly worse, on average, the following day. Elevation was seen in all of the activation products, with the greatest fold change (11.3-fold, 95% CI = 10.1 to 13.1) seen in C3a concentration. Both the classical / MBL and the alternative pathways were activated, with the alternative pathway marker Factor Bb 6.1-fold (95% CI = 4.4 to 7.7) increased over healthy controls and the classical / MBL marker C4d slightly less increased at 3.5-fold (95% CI = 2.6 to 4.6). Only 3 of the 20 patients enrolled (15%) died of their illness, and as would be expected given our modest sample, no statistically significant differences were seen in any of the complement proteins in this group.
Figure 3.
Serum complement protein concentrations. 20 patients with severe sepsis and 10 healthy controls were studied. (A) Among septic patients, measurements were made at the onset of resuscitation (t0) and 24 hours later (t24). Box plots represent 5th, 25th, 50th, 75th, and 95th percentiles. Asterisks represent extreme outliers. Values over the box pairs represent the mean difference between groups and that difference’s posterior 95% credible interval. See text for details. C3 levels were significantly depressed at presentation and thereafter, and all other measured mediators were significantly elevated in patients over controls. No significant resolution of these abnormalities was detected 24 hours after the onset of standardized quantitative resuscitation. (B) The mean fold change over control values of each protein concentration in severe sepsis, by position in the complement pathway. Values here map to those reflected in the complement pathway shown in Figure 1. 95% credible intervals are reported in the text. MBL = mannose-binding lectin.
An unexpected inverse relationship between initial SOFA score and initial serum C5a concentration was noted (slope = −1.0 ng / ml / SOFA point, 95% credible interval = −1.7 to −0.3, r2 = 0.18). The alternative pathway appeared to explain more C5a variation (slope = 0.8 ng C5a / ng Bb, 95% credible interval = 0.3 to 1.3, r2 = 0.24) than did the classical / MBL pathway (slope = 2.1 ng C5a / ng C4d, 95% credible interval = 0.4 to 3.9, r2 = 0.14), although both contributed in a statistically significant way.
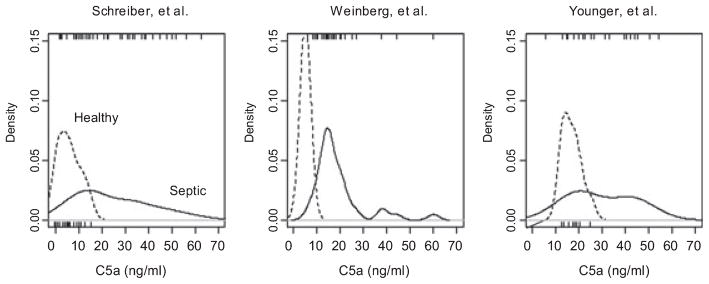
Figure 4 shows the estimated distributions of C5a concentrations in our series and in the two most comparable previously published reports. Data from all three sources suggest either skewed or possibly multimodal distributions of C5a in septic patients. Neither we nor the authors of the other two articles were able to statistically discern a relationship between worsening illness severity and the long upward tail of these distributions.
Figure 4.
Observed distributions of C5a concentration in previous reports by Schreiber et al.,7 Weinberg et al.,8 and in our data. Distributions are plotted using Gaussian smoothing. The vertical axis is scaled as to make the curves formal probability density functions (i.e., their areas equal 1). Individual data points from each source are plotted as rugs beneath (for control patients) or above (for septic patients) each graph with the exception of the Weinberg control data, for which the original report provided only a mean and standard deviation, not individual data points. All three data sets reflect a long rightward tail. The rightward shift in control values of approximately 10 ng / ml in our data may reflect the use of serum rather than plasma for protein determinations in the current work.
DISCUSSION
We report a detailed characterization of C5a dysregula-tion in patients with community-acquired severe sepsis. The magnitude of C5a production in these ED patients prior to treatment was comparable to that seen in previously reported ICU series when formally considered with Bayesian methods. Both the classical / MBL and the alternative pathways appear to contribute to cascade activation in vivo, with the latter having a stronger statistical association with serum C5a concentrations.
A structured resuscitation protocol failed, at 24 hours, to reverse any of the complement abnormalities seen. We did not detect significant improvement in any of the measured complement proteins 24 hours after the onset of initial treatment designed to quickly correct hypoper-fusion, ameliorate oxygen debt, and provide antibiotic coverage. In 2007, Rivers and colleagues11 reported the impact of structured ED resuscitation on temporal trends of several circulating mediators in human sepsis. Most exhibited clearance half-lives of approximately 12 hours, and in several instances clearance was hastened by structured resuscitation. In our modest sample, we did not detect a shift toward resolution in any of the complement components we measured at 24 hours. The reason for the difference between these two findings is unclear and may relate in part to the strategy used in the Rivers et al. report to subset patients based on illness severity. An alternative explanatory hypothesis is that all of the mediators reported by Rivers et al. were shed (intercellular adhesion molecule-1 [ICAM-1]) or secreted (TNF-α , IL-1ra, IL-8, caspase-3) proteins attributable to inflammatory cells.11 Therapies effective in quenching cellular inflammatory responses by correcting oxygen debt and acidosis may not be similarly effective in reducing the activation of the complement cascade, which once deployed into the circulation, can proceed in the complete absence of host cells.
Activation of, and exacerbated injury due to, the complement cascade is recognized in many infectious and ischemic conditions. In animals, uncontrolled activation of the system adversely affects cardiovascular, respiratory, renal, and coagulation function in sepsis and other types of shock.12–20 While there is considerable interest in C5a as a therapeutic target in human sepsis, our review of the literature indicates that clinical experience with this mediator is modest. In none of the studies identified (nor in the current report) are sample sizes sufficient to undertake a careful analysis of the clinical features associated with exuberant pathway activation. One previous report21 and our own data appear to indicate an inverse relationship between the extent of activation and illness severity. A much larger, prospectively gathered cohort would be helpful.
Regardless of sample size, the relationship between illness severity and circulating C5a levels is likely to be complex. C5a can be generated both by encountered microorganisms and by tissue injured in the inflamma-tory response to those microorganisms. Removal of C5a appears to be driven primarily by two cell surface receptors for this anaphylatoxin, C5aR and C5aL2. These receptors are present on myeloid and nonmyeloid cells and are intracellularly internalized upon ligand binding.22 The determination of C5a in any body fluid is therefore a measurement of one compartment of a dynamic system that includes uncertainties in the rate of production and the rate and extent of scavenging. Future efforts in untangling the role of C5a in human sepsis will benefit from study designs that incorporate measures not just of protein levels over time, but that also attempt to track the state of C5a receptors and the predominant cell types harboring these receptors over time. Such strategies will likely require flow cytometry of surface receptors from critically ill patients, a technique that poses substantial technical challenges when being conducted in emergent settings.23
Another important question that we are unable to address in the current work is the specificity of complement activation in sepsis compared to other critical illnesses. Existing evidence makes a weak case for the use of C5a as a biomarker in sepsis. Nevertheless, as complement activation is known to take place during several noninfectious serious illnesses, such as acute injury and nonseptic shock, it is hoped that future work can also examine this pathway’s presence, and possible role, in these illnesses as well.
LIMITATIONS
While the magnitude of complement abnormalities among septic patients compared to healthy individuals was so great that statistically meaningful differences were easily detected, our sample size was sufficiently small to prevent more detailed exploration of relationships between clinical features and the extent and timing of activation and resolution. There was a difference in race between cases and controls. Among cases, race was not related to extent of complement activation. However, a more complex interaction between race and activation cannot be excluded.
CONCLUSIONS
Complement activation at the time of initial hospital presentation is significant in patients diagnosed in the ED with severe sepsis. Although both the classical / mannose-binding lectin and the alternative pathways contribute, the latter appears to be the dominant mechanism. Unlike other mediators that have been studied in this context, complement activation did not significantly improve following 24 hours of structured resuscitation in the ED and intensive care unit.
Acknowledgments
This work was funded by National Institute of General Medical Sciences grants GM069438 (JGY) and GM076652 (AEJ) and by a medical student grant from the Emergency Medicine Foundation (HC).
The authors thank Barbara Smith for her invaluable assistance.
References
- 1.Gallin JI. Snyderman R Complement. In: Gallin JI, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. Philadelphia, PA: Lippincott, Williams, and Wilkins; 1999. [Google Scholar]
- 2.Riedemann NC, Guo RF, Neff TA, et al. Increased C5a receptor expression in sepsis. J Clin Invest. 2002;110:101–8. doi: 10.1172/JCI15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rittirsch D, Flierl M, Nadeau B, et al. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14:551–7. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo RF, Ward PA. C5a, a therapeutic target is sepsis. Recent Pat Antiinfect Drug Discov. 2006;1:57–65. doi: 10.2174/157489106775244091. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL. Organ dysfunction as an outcome measure: the Sofa score. Sepsis. 1997;1:53–4. [Google Scholar]
- 6.Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132:425–32. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreiber H, Rittirsch D, Flierl M, et al. Complement activation during sepsis in humans. In: Lambris J, editor. Current Topics in Complement. New York, NY: Springer Science+Business Media, LLC; 2006. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg P, Matthay M, Webster R, Roskos KV, Goldstein IM, Murray JF. Biologically active products of complement and acute lung injury in patients with the sepsis syndrome. Am Rev Respir Dis. 1984;130:791–6. doi: 10.1164/arrd.1984.130.5.791. [DOI] [PubMed] [Google Scholar]
- 9.Lunn D, Thomas A, Best N, et al. WinBUGS–a Bayesian modeling framework: concepts, structure, and extensibility. Stat Comp. 2000;10:325–37. [Google Scholar]
- 10.Gelman A, Carlin J, Stern H, et al. Bayesian Data Analysis. 2. Boca Raton, FL: Chapman & Hall / CRC Press; 2004. [Google Scholar]
- 11.Rivers E, Kruse J, Jacobsen G, et al. The influence of early hemodynamic optimization on biomarker patterns of severe sepsis and septic shock. Crit Care Med. 2007;35:2016–24. doi: 10.1097/01.ccm.0000281637.08984.6e. [DOI] [PubMed] [Google Scholar]
- 12.Czermak B, Sarma V, Pierson C, et al. Protective effects of C5a blockade in sepsis. Nat Med. 1999;5:788–92. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- 13.Guo R, Huber-Lang M, Wang X, et al. Protective effects of anti-C5a in sepsis-induced thymocyte apoptosis. J Clin Invest. 2000;106:1271–80. doi: 10.1172/JCI10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo R, Sun L, Gao H, et al. In vivo regulation of apoptosis by C5a during sepsis. J Leukoc Biol. 2006;80:1575–83. doi: 10.1189/jlb.0106065. [DOI] [PubMed] [Google Scholar]
- 15.Guo R, Ward P. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–52. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 16.Huber-Lang M, Sarma JV, Zetoune F, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–7. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda N, Nagasawa K, Horiuchi T, Tsuru T, Nishi-zaka H, Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb Haemost. 1997;77:394–8. [PubMed] [Google Scholar]
- 18.Laudes IJ, Chu JC, Sikranth S, et al. Anti-C5a ameliorates coagulation / fibrinolytic protein changes in a rat model of sepsis. Am J Pathol. 2002;160:1867–75. doi: 10.1016/S0002-9440(10)61133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younger JG, Sasaki N, Delgado J, et al. Systemic and lung physiological changes after intravascular activation of complement with cobra venom factor. J Appl Physiol. 2001;90:2289–95. doi: 10.1152/jappl.2001.90.6.2289. [DOI] [PubMed] [Google Scholar]
- 20.Younger JG, Sasaki N, Waite MD, et al. Detrimental effects of complement activation in hemorrhagic shock. J Appl Physiol. 2000;90:441–6. doi: 10.1152/jappl.2001.90.2.441. [DOI] [PubMed] [Google Scholar]
- 21.Nakae H, Shigeatsu E, Inada K, Yoshida M. Chronological changes in the complement system in sepsis. Surg Today. 1996;26:225–9. doi: 10.1007/BF00311579. [DOI] [PubMed] [Google Scholar]
- 22.Ward P. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4:133–42. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 23.Greineder CF, Nelson PW, Dressel AL, Erba HP, Younger JG. In vitro and in silico analysis of the utility of annexin V binding to lymphocytes as a bio-marker in ED studies of sepsis. Acad Emerg Med. 2007;14:763–71. doi: 10.1197/j.aem.2007.01.017. [DOI] [PubMed] [Google Scholar]