Abstract
Background
Compared with men, women have more evidence of myocardial ischemia with no obstructive CAD. While low endogenous estrogen levels are associated with endothelial dysfunction, the role of low dose hormone therapy has not been fully evaluated. We postulate a 12-week duration of low dose hormone replacement therapy is associated with myocardial ischemia and endothelial dysfunction.
Methods and Results
Using a multicenter, randomized, placebo-controlled design, subjects were randomized to receive either 1 mg norethindrone/10 mcg ethinyl estradiol (1/10 NA/EE) or placebo for twelve weeks. Chest pain and menopausal symptoms, cardiac magnetic resonance spectroscopy (MRS), brachial artery reactivity (BART), exercise stress testing, psychosocial questionnaires were evaluated at baseline and exit. Recruitment was closed prematurely due to failure to recruit following publication of the Women’s Health Initiative hormone trial. Of the 35 women who completed the study, there was less frequent chest pain in the treatment group compared to the placebo group (p=0.02) at exit. Women taking 1/10 NA/EE also had significantly fewer hot flashes/night sweats (p=0.003), less avoidance of intimacy (p=0.05), and borderline differences in sexual desire and vaginal dryness (p=0.06). There were no differences in MRS, BART, compliance or reported adverse events between the groups.
Conclusions
These data suggest that low dose hormone therapy improved chest pain symptoms, menopausal symptoms and quality of life, but did not improve ischemia or endothelial dysfunction. Given that it was not possible to enroll the pre-specified sample size, these results should not be considered definitive.
Compared to men, women have more evidence of myocardial ischemia in the setting of no obstructive coronary artery disease (CAD)1. Coronary endothelial dysfunction has been suggested as a mechanism that may contribute to signs and symptoms of ischemia in the absence of severe coronary obstruction2. Preliminary evidence suggests that low estrogen levels may contribute to endothelial dysfunction3–4, and that estrogen replacement abolishes this effect3,5–6. But this has not been evaluated in women with symptoms and signs of myocardial ischemia in the absence of severe obstructive CAD.
The goal of this Women’s Ischemia Syndrome Evaluation (WISE) ancillary study was to evaluate the effect of low dose hormone replacement therapy with 1 mg norethindrone/10 mcg ethinyl estradiol (1/10 NA/EE) in postmenopausal women with a history of chest discomfort, myocardial ischemia and no obstructive CAD on: 1) inducible myocardial ischemia measured by MRS, 2) endothelial dysfunction assessed by BART, 3) physical functional disability assessed by exercise testing, and 4) quality of life assessed by cardiac symptoms and psychological questionnaires.
METHODS
The WISE is a National Heart, Lung and Blood Institute (NHLBI) sponsored study to improve the diagnostic evaluation of ischemic heart disease in women. Institutional Review Boards at each site approved the overall protocol as well as this ancillary study protocol, which is in accordance with the Declaration of Helsinki. Written informed consent was obtained from each participant. Data were monitored by an independent Data and Safety Monitoring Committee. Details of the WISE protocol and design are previously published7. A subgroup of women from the Universities of Florida and Alabama sites was screened for this ancillary study with informed consent to participate in the additional testing, treatment and follow-up detailed below.
The ancillary study was a randomized, placebo-controlled, double-blind design among women and has been registered at ClinicalTrials.gov with an identifier number NCT00600106. The inclusion criteria included being postmenopausal by WISE criteria7, no use of hormone therapy in the last 6 weeks, with a history of chest discomfort and normal or only minimally diseased (<50% diameter stenosis) epicardial coronary arteries measured by the WISE Angiographic Core Laboratory8, and evidence of myocardial ischemia. Myocardial ischemia was defined for this trial as an abnormal result on any of the following qualifying tests: 1) abnormal P-31 gated MRS characterized by a fall in quantitative PCr/ATP ratio >20% from rest during handgrip exercise; 2) positive treadmill exercise stress test (>1.0 mm horizontal/ downsloping or >1.5 upsloping ST segment depression measured 0.08 msec after the J point); 3) reversible stress radionuclide perfusion defect > equivocal and not attributable to breast/imaging artifact; 4) coronary flow reserve <2.25 to intracoronary adenosine. Exclusion criteria included inability to withdraw vasoactive medication, contraindication to hormone therapy, or comorbid illness that precluded participation.
Subjects were randomized to receive either 1 mg norethindrone/10 mcg ethinyl estradiol (1/10 NA/EE) or placebo for twelve weeks, as well as to discontinue their vasoactive medications, except beta-blockers and sublingual nitrates, for at least 12 hours prior their baseline evaluation. Baseline assessments using previously published WISE methods including MRS, BART, exercise stress testing and coronary flow reserve, as well as WISE psychosocial questionnaire, SF-36, and blood lipid and hormone level. Because the MRS data were acquired using different instruments and techniques at the sites (Phillips MR and DRESS technique at University of Alabama, and GE MR and ISIS technique at University of Florida, respectively), spectra were read by site investigators blinded to subjects’ clinical information and treatment assignment. WISE blood lipid and hormone core laboratories used previously published methods9–10. Psychosocial and Quality of Life (QOL) questionnaires included Cook Medley Hostility Scores (CMH)11, Beck Depression Inventory (BDI)12, Autonomic Perception Questionnaire (APQ)13, SF-3614–15, and the Menopause-specific quality of life questionnaire that focuses on hot flashes, poor memory, change in sexual desire, vaginal dryness and avoiding intimacy16. Patients were contacted by telephone at 2, 6, and 10 weeks to enhance study compliance and assess status. At the exit visit following 12 weeks of drug treatment, all tests were repeated.
Statistical Analysis
Sample Size Considerations
The recruitment goal for this trial was 74 women, 37 assigned to each of two groups: placebo and 1/10 NA/EE. The sample size calculation was based on the primary measures of interest: 1) % change of PCr/ATP in myocardium by MRS at stress testing compared to at rest; 2) % change in ratio of brachial artery diameter at peak hyperemia after release of occlusion compared to before occlusion. Both measures were expressed as percent change and as continuous variables and standard deviations (SDs). With 74 participants the differences between the 1/10 NA/EE and placebo groups that can be detected as statistically significant for a two-sided test (α=0.05 and β=20%) are as follows: 7.5% (SD 10.9%) change in PCr/ATP and a 7.1% (SD 10.3%) change in brachial artery diameter.
Data analysis
Data were presented in tables as means and SDs for continuous variables and frequencies for categorical variables. Comparisons between the placebo and 1/10 NA/EE groups were done using Wilcoxon rank sum test for continuous measurement and Fisher’s Exact test for discrete data. The strategy of analysis was ‘intention to treat’ with comparing the study groups in term of the treatment to which they were randomly allocated. All tests were two-sided with p values <0.05.
RESULTS
A total of 1142 women were screened by chart review for possible entrance and most were not eligible due to obstructive coronary artery disease observed during coronary angiography. Of the 57 eligible women who entered the testing phase, 37 women went on to randomization. There was no difference in the type of qualifying test by treatment group (p=0.87). Among the 37 women, 30 (81%) had previously used hormone therapy, with a mean prior use of 10.7±9.5 years, and 14 (38%) had used hormone therapy in the prior 3 months and washed out to participate. There were no differences in prior or current hormone therapy use between two groups.
Baseline characteristics
Two groups were comparable in baseline characteristics, except more women in placebo group has a history of hypertension, higher heart rate (Table 1), and higher frequency of using antihypertensive angiotensin converting enzyme (ACE) inhibitors (47% vs. 11%, p=0.03) compared to 1/10 NA/EE group. At study exit, characteristics and medication use remained consistent with baseline findings, although medication dose changes were not collected. There were no differences in comparing two groups regarding chest pain frequency, CMH, BDI, or APQ results, as well as BART, exercise, or MRS (Table 2), menopause symptom, or SF-36 scales (Table 3). In exercise stressing testing, however, there is a trend in improved ST segment depression in 1/10 NA/EE group compared with placebo group (0.21±0.19 vs. −0.35±0.22, p=0.07).
Table 1.
Baseline Characteristics by Treatment Assignment
| Characteristic | Placebo (n=19) |
1/10 NA/EE (n=18) |
p value |
|---|---|---|---|
| Age in years (mean ± SD) | 59 ± 7 | 56 ± 9 | 0.43 |
| Race-nonwhite (%) | 16 | 17 | 0.99 |
| High School or less (%) | 68 | 56 | 0.42 |
| Current HT use-prior to entry (%) | 42 | 33 | 0.58 |
| History Hypertension (%) | 72 | 29 | 0.01 |
| History Diabetes (%) | 26 | 22 | 0.77 |
| History Dyslipidemia (%) | 39 | 61 | 0.18 |
| Current Smoking (%) | 11 | 11 | 0.99 |
| Total Cholesterol (mg/dl) | 205 ± 39 | 195 ± 47 | 0.44 |
| HDL-Cholesterol (mg/dl) | 54 ± 9 | 48 ± 16 | 0.08 |
| LDL-Cholesterol (mg/dl) | 124 ± 38 | 120 ± 51 | 0.96 |
| Triglycerides (mg/dl) | 134 ± 64 | 132 ± 92 | 0.62 |
| Systolic Blood Pressure (mmHg) | 136 ± 18 | 130 ± 18 | 0.47 |
| Diastolic Blood Pressure (mmHg) |
78 ± 9 | 76 ± 13 | 0.73 |
| Pulse (beats per minute) | 76 ± 11 | 68 ± 8 | 0.03 |
| Fasting Blood Sugar (mg/dl) | 94 ± 20 | 93 ± 17 | 0.96 |
| BMI (kg/m2) | 31 ± 7 | 31 ± 9 | 0.52 |
| Waist (cm.) | 93 ± 15 | 92 ± 17 | 0.77 |
BMI=body mass index; HDL=high density lipoprotein; HT=hormone therapy; LDL=low density lipoprotein; SD=standard deviation
Table 2.
Menopausal Symptoms and SF 36 scale at Baseline and Exit by Treatment
| Placebo (n=19) |
1/10 NA/EE (n=18) |
P | Placebo (n=18) |
1/10 NA/EE (n=17) |
P | |
|---|---|---|---|---|---|---|
| Baseline | Exit | |||||
| Menopause symptoms (%) | ||||||
| Hot flushes or flashing | 68 | 89 | 0.23 | 89 | 41 | 0.003 |
| Poor Memory | 53 | 76 | 0.14 | 78 | 59 | 0.23 |
| Change in Sexual Desire | 37 | 50 | 0.42 | 67 | 35 | 0.06 |
| Vaginal Dryness | 58 | 44 | 0.41 | 67 | 35 | 0.06 |
| Avoiding Intimacy | 37 | 39 | 0.90 | 56 | 24 | 0.05 |
| SF-36 Scale (mean±SD) | ||||||
| Physical Functioning | 43.8 ± 27.4 | 60.8± 27.5 | 0.08 | 44.4± 29.5 | 59.4± 23.5 | 0.09 |
| Role-Physical | 37.3± 40.2 | 54.2± 41.3 | 0.19 | 25.0± 33.2 | 58.8± 37.4 | 0.01 |
| Role- Emotional | 66.7± 41.6 | 70.3± 42.6 | 0.72 | 66.7± 39.6 | 76.5± 34.9 | 0.42 |
| Bodily Pain | 42.1± 17.4 | 53.3± 21.4 | 0.09 | 41.5± 21.6 | 54.5± 23.6 | 0.08 |
| General Health | 57.4± 15.1 | 55.4± 19.4 | 0.67 | 57.2± 15.8 | 55.2± 19.7 | 0.67 |
| Mental Health | 65.7± 19.9 | 66.7± 20.6 | 0.88 | 69.3± 18.8 | 66.4± 19.6 | 0.78 |
| Vitality | 42.9± 20.4 | 35.6± 23.1 | 0.31 | 41.2± 18.0 | 35.6± 24.5 | 0.40 |
| Social Functioning | 53.2± 24.3 | 59.4± 14.7 | 0.48 | 56.1± 20.6 | 59.4± 16.0 | 0.71 |
Table 3.
Menopausal Symptoms and SF 36 scale at Baseline and Exit by Treatment
| Placebo (n=19) |
1/10 NA/EE (n=18) |
P | Placebo (n=18) |
1/10 NA/EE (n=17) |
P | |
|---|---|---|---|---|---|---|
| Baseline | Exit | |||||
| Menopause symptoms (%) | ||||||
| Hot flushes or flashing | 68 | 89 | 0.23 | 89 | 41 | 0.003 |
| Poor Memory | 53 | 76 | 0.14 | 78 | 59 | 0.23 |
| Change in Sexual Desire | 37 | 50 | 0.42 | 67 | 35 | 0.06 |
| Vaginal Dryness | 58 | 44 | 0.41 | 67 | 35 | 0.06 |
| Avoiding Intimacy | 37 | 39 | 0.90 | 56 | 24 | 0.05 |
| SF-36 Scale (mean±SD) | ||||||
| Physical Functioning | 43.8 ± 27.4 | 60.8± 27.5 | 0.08 | 44.4± 29.5 | 59.4± 23.5 | 0.09 |
| Role-Physical | 37.3± 40.2 | 54.2± 41.3 | 0.19 | 25.0± 33.2 | 58.8± 37.4 | 0.01 |
| Role- Emotional | 66.7± 41.6 | 70.3± 42.6 | 0.72 | 66.7± 39.6 | 76.5± 34.9 | 0.42 |
| Bodily Pain | 42.1± 17.4 | 53.3± 21.4 | 0.09 | 41.5± 21.6 | 54.5± 23.6 | 0.08 |
| General Health | 57.4± 15.1 | 55.4± 19.4 | 0.67 | 57.2± 15.8 | 55.2± 19.7 | 0.67 |
| Mental Health | 65.7± 19.9 | 66.7± 20.6 | 0.88 | 69.3± 18.8 | 66.4± 19.6 | 0.78 |
| Vitality | 42.9± 20.4 | 35.6± 23.1 | 0.31 | 41.2± 18.0 | 35.6± 24.5 | 0.40 |
| Social Functioning | 53.2± 24.3 | 59.4± 14.7 | 0.48 | 56.1± 20.6 | 59.4± 16.0 | 0.71 |
Response to Intervention –Exercise Stress Testing, Endothelial Function, and Myocardial Ischemia
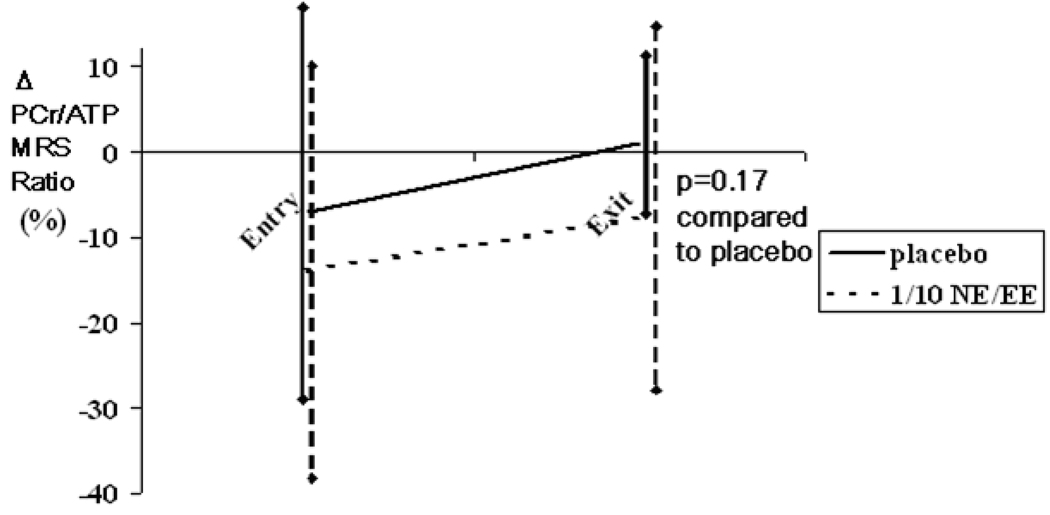
Notably, 7/35 (20%) of the women could not perform the exercise stress test within the time frame at the exit visit due to orthopedic injury or surgery. Among those women able to exercise, there were trends toward improvements among the 1/10 NA/EE group in functional capacity, which was measured as metabolic equivalents (METs), exercise duration and exercise-induced chest pain that did not reach statistical significance (Table 2). There was no difference for the BART (Table 3). Among the 35 women, 28 baseline and 26 exit MRS results were technically adequate for spectral analyses with rest, handgrip exercise and recovery spectra. There was no difference in the cardiac MRS between groups (Figure 1), with both groups demonstrating similar trends toward improvement with repeat testing. Further analyses with regard to hypertension, baseline use of ACE-inhibitors, or change in use of ACE-inhibitors did not alter these cardiac MRS or BART results.
Figure 1.
P-31 gated magnetic resonance cardiac spectroscopy (MRS) reported as change (Δ) in PCr/ATP ratio, with isometric submaximal handgrip stress at study baseline (n=28) and exit (n=26) by treatment in placebo and 1/10 NA/EE groups. Δ PCr/ATP ratio defined as: (stress- [average of rest and recovery periods])/average of rest and recovery periods X 100, and expressed as % mean±SD. For this trial, myocardial ischemia was pre-specified as a fall in quantitative PCr/ATP ratio >20% from rest, and a lower value is considered indicative of greater ischemia.
Response to Intervention – Chest Pain, Menopausal Symptoms and Quality of Life
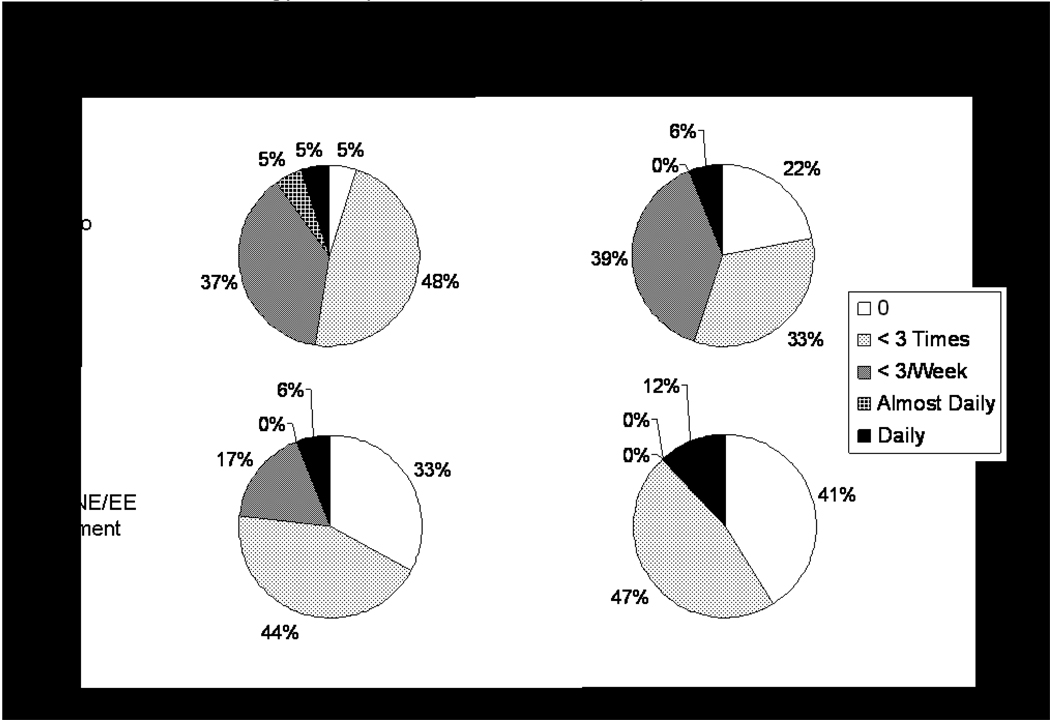
While there is no difference in chest pain frequency at baseline, at study exit women in the 1/10 NA/EE group reported less frequent chest pain than those in the placebo group (p=0.02)(Figure 2). While both groups reported a high frequency of hot flashes/night sweats, poor memory, and vaginal dryness at baseline, at the exit, women assigned 1/10 NA/EE reported significantly fewer hot flashes/night sweats (p=0.003), less avoidance of intimacy (p=0.05), and trends were observed (p=0.06) for less change in sexual desire and vaginal dryness (Table 2) compared with those assigned placebo.
Figure 2.
Chest pain frequency in the last 6 weeks measured at study baseline (n=37) and exit (n=35) according to treatment.
There was no difference in the CMH questionnaire, BDI or APQ results for either group (data not shown). Women in the 1/10 NA/EE group had higher scores in the physical role function in SF-36 than those in the placebo group (p=0.01), indicating better functioning at work and other daily activities with less limitation in performing in activities (Table 2).
Study Recruitment, Compliance and Adverse Events
In the year following publication of the Women’s Health Initiative (WHI) hormone trial results17, women eligible for participation regularly declined enrollment so recruitment was closed prior to reaching the planned sample size. Of the 37 women randomly assigned to treatment, 35 completed the study and 2 withdrew. One woman withdrew due to time constraints, and the other woman withdrew following the publication of the WHI findings.
There were no differences in study medication compliance assessment, in which excellent compliance was defined as >85% by pill count, by randomization group (100% and 78% in placebo and 1/10 NA/EE groups, respectively, p=0.23). One woman in the 1/10 NA/EE group had a hospitalization for angina and a stroke, and one woman in the 1/10 NA/EE was hospitalized for biliary colic (p=0.23).
DISCUSSION
These results suggest that while low dose hormone therapy reduces chest pain, menopausal symptoms and improves quality of life in symptomatic women with evidence of myocardial ischemia but no obstructive CAD, there was no significant improvement in the variables assessing endothelial dysfunction, exercise testing and ischemia measured by cardiac MRS. These results suggest that hormone therapy may be helpful in postmenopausal women with myocardial ischemia and no obstructive CAD for symptom management, however 12-week treatment period does not appear to have a prominent effect on either measures of myocardial ischemia or endothelial dysfunction. Given that it was not possible to enroll the pre-specified sample size, the results should be interpreted with caution.
Our findings are consistent with prior reports. Prior WISE study findings indicate that a history of hormone therapy was linked with less menopausal symptoms and better QOL in symptomatic women with or without obstructive CAD18. These findings confirm and extend prior published trial reports in women with chest pain, abnormal stress testing and no obstructive CAD19. Previous study has suggested that estrogen hormone therapy is associated with improved exercise-induced angina and exercise capacity20. While our data suggest a trend for improved exercise stress testing parameters at exit for 1/10 NA/EE group, the absence of statistical significance may be due to low statistical power, precluding a definitive result. Given the current data trend, a total of 100 women would have been required to detect the observed exit differences in ST segment depression and chest pain occurrence. Our current study findings extend prior findings to include improvement in daily life angina. Notably, others have suggested that while estrogen-testosterone hormone therapy was associated with improved emotional well-being in postmenopausal women with angina and “normal” angiograms21, it was not associated with less chest pain occurrence and/or its threshold during exercise22. Comparing their data with ours suggests that estrogen without testosterone may be more beneficial for chest pain and related symptoms.
Prior reports evaluating coronary endothelial function using high pharmacological doses of estrogen acutely suggested improvement3. But subsequent reports evaluating chronic and more physiological hormone therapy and peripherally measured endothelial function have mixed results with regard to a beneficial effect23–25. Additionally, a number of hormone therapy trials now consistently document a risk of increased cardiovascular disease events in postmenopausal women17, 26–28, indicating that any apparent beneficial effect on indices of endothelial function may be outweighed by adverse vascular effects leading to clinical outcomes. The relevance of this to our cohort is unclear, because women in our and prior cohorts2 are substantially younger than most of these prior hormone trials. More recent studies suggest a lower incidence of cardiovascular events among women who initiated hormone therapy at younger age29–30. A study of myocardial blood flow has suggested that hormone therapy may normalize flow only among women without risk factors31, although prospective testing is needed. While the one cardiovascular adverse event experienced in the current study was in a woman randomized to the hormone therapy, we were clearly underpowered and exposure was too brief to evaluate clinical events in present study.
No prior studies have employed cardiac MRS to assess high energy phosphate metabolism and this is considered as a biochemical reference for myocardial ischemia. We have previously demonstrated that patient with myocardial ischemia measured by cardiac MRS has a higher risk for adverse cardiovascular events, including death, myocardial infarction, and stroke, re-hospitalization and repeat coronary angiography32. However, our cardiac MRS results showed a relatively wide variability in PCr/ATP change with isometric handgrip stress at baseline. Compared with our findings in two other subgroups32–33 reported from the WISE, the SD was approximately 2 fold greater in this ancillary study, suggesting that our limited sample size again may have precluded our ability to detect an effect of the intervention. The trend toward improvement in both groups is suggestive of a training effect; however these results could be due to changes in other anti-ischemia therapy dosing, spontaneous biological and measurement variability, or the measurement phenomenon of regression to the mean. Exploration of this finding in relation to the baseline differences in hypertension, or use of ACE-inhibitors did not provide further understanding of the results.
The approach to management of myocardial ischemia in patients with no obstructive CAD remains unclear. A majority of these patients are women2, and both short34 and intermediate-term29, 32 follow-up indicate a more adverse prognosis35–37, as well as a high cost for care38. Persistent chest pain39, evidence of coronary endothelial dysfunction40, and evidence of ischemia32 in women with no obstructive CAD portend an adverse cardiovascular prognosis that is comparable to that of obstructive CAD. While a number of strategies, including beta blockers but not calcium antagonists41, L-arginine42, imipramine43, and exercise44 may be associated with symptom relief, no clinical trials to determine the effectiveness of treatment on adverse outcomes have been conducted in this population.
Conclusions
Among postmenopausal women with myocardial ischemia and no obstructive CAD, hormone therapy with 1/10 NA/EE is associated with reduced chest pain symptoms, menopausal symptoms and improved quality of life with trends for improved exercise performance, but not improvement in myocardial ischemia or endothelial function measures. Given that it was not possible to enroll the pre-specified sample size, the results should be interpreted with caution. The efficacy and safety of this type of hormone therapy for symptomatic women in this selected cohort requires prospective trial testing. The current trial results can be used for future sample size estimation.
Acknowledgments
Source of Funding. This work was supported by Parke-Davis/Pfizer, and contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, a GCRC grant MO1-RR00425 from the National Center for Research Resources, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Denville, New Jersey, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, California, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, Pennsylvania, and QMED, Inc., Laurence Harbor, New Jersey.
The authors are solely responsible for the design and conduct of this study; all study analyses, the drafting and editing of the paper and its final contents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Panza JA. Myocardial ischemia and the pains of the heart. N Engl J Med. 2002;346:1934–1935. doi: 10.1056/NEJMp020047. [DOI] [PubMed] [Google Scholar]
- 2.Cannon RO., 3rd Does coronary endothelial dysfunction cause myocardial ischemia in the absence of obstructive coronary artery disease? Circulation. 1997;96:3251–3254. [PubMed] [Google Scholar]
- 3.Williams JK, Adams MR, Klopfenstein HS. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990;81:1680–1687. doi: 10.1161/01.cir.81.5.1680. [DOI] [PubMed] [Google Scholar]
- 4.Williams JK, Shively CA, Clarkson TB. Determinants of coronary artery reactivity in premenopausal female cynomolgus monkeys with diet-induced atherosclerosis. Circulation. 1994;90:983–987. doi: 10.1161/01.cir.90.2.983. [DOI] [PubMed] [Google Scholar]
- 5.Gilligan DM, Quyyumi AA, Cannon RO., 3rd Effects of physiological levels of estrogen on coronary vasomotor function in postmenopausal women. Circulation. 1994;89:2545–2551. doi: 10.1161/01.cir.89.6.2545. [DOI] [PubMed] [Google Scholar]
- 6.Reis SE, Gloth ST, Blumenthal RS, et al. Ethinyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in postmenopausal women. Circulation. 1994;89:52–60. doi: 10.1161/01.cir.89.1.52. [DOI] [PubMed] [Google Scholar]
- 7.Merz CN, Kelsey SF, Pepine CJ, et al. The Women's Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453–1461. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 8.Sharaf BL, Pepine CJ, Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory) Am J Cardiol. 2001;87:937–941. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 9.The Manual of Laboratory Operations 1. Lipid and Lipoprotein Analysis. Bethesda: Lipid Research Clinics Program. National Institutes of Health. DHEW Publication No. 75; 1974:628.
- 10.Barrett-Connor E, Goodman-Gruen D. Prospective study of endogenous sex hormones and fatal cardiovascular disease in postmenopausal women. BMJ. 1995;311:1193–1196. doi: 10.1136/bmj.311.7014.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmer DC, Ragland DR, Syme SL. Hostility and coronary artery disease. Am J Epidemiol. 1991;133:112–122. doi: 10.1093/oxfordjournals.aje.a115850. [DOI] [PubMed] [Google Scholar]
- 12.Naughton MJ, Shumaker SA, Anderson RT, et al. Psychological aspects of health-related quality of life measurement: Tests and Scales. Philadelphia: Lippincott-Raven Publishers; 1996. [Google Scholar]
- 13.Mandler G, Mandler JM, Uviller ET. Autonomic feedback: the perception of autonomic activity. J Abnorm Psychol. 1958;56:367–373. doi: 10.1037/h0048083. [DOI] [PubMed] [Google Scholar]
- 14.Ware JE, Kosinski M, Gandek B. SF-36 Health Survey. Manual and interpretation guide. Lincoln: Quality Metric Inc.; 1993. [Google Scholar]
- 15.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 16.Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas. 1996;24:161–175. doi: 10.1016/s0378-5122(96)82006-8. [DOI] [PubMed] [Google Scholar]
- 17.Rossouw JE, Anderson FL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 18.Rosano GM, Sarrel PM, Poole-Wilson PA, et al. Beneficial effect of oestrogen on exercise-induced myocardial ischaemia in women with coronary artery disease. Lancet. 1993;342:133–136. doi: 10.1016/0140-6736(93)91343-k. [DOI] [PubMed] [Google Scholar]
- 19.Olson MB, Kelsey, Matthews K, et al. Symptoms, myocardial ischaemia and quality of life in women: results from the NHLBI-sponsored WISE Study. Eur Heart J. 2003;24:1506–1514. doi: 10.1016/s0195-668x(03)00279-3. [DOI] [PubMed] [Google Scholar]
- 20.Albertsson PA, Emanuelsson H, Milsom I. Beneficial effect of treatment with transdermal estradiol-17-beta on exercise-induced angina and ST segment depression in syndrome X. Int J Cardiol. 1996;54:13–20. doi: 10.1016/0167-5273(96)02560-0. [DOI] [PubMed] [Google Scholar]
- 21.Adamson DL, Webb CM, Collins P. Esterified estrogens combined with methyltestosterone improve emotional well-being in postmenopausal women with chest pain and normal coronary angiograms. Menopause. 2001;8:233–238. doi: 10.1097/00042192-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Rosano GM, Peters NS, Lefroy D, et al. 17-beta-Estradiol therapy lessens angina in postmenopausal women with syndrome X. J Am Coll Cardiol. 1996;28:1500–1505. doi: 10.1016/s0735-1097(96)00348-8. [DOI] [PubMed] [Google Scholar]
- 23.Gerhard M, Walsh BW, Tawakol A, et al. Estradiol therapy combined with progesterone and endothelium-dependent vasodilation in postmenopausal women. Circulation. 1998;98:1158–1163. doi: 10.1161/01.cir.98.12.1158. [DOI] [PubMed] [Google Scholar]
- 24.Herrington DM, Werbel BL, Riley WA, et al. Individual and combined effects of estrogen/progestin therapy and lovastatin on lipids and flow-mediated vasodilation in postmenopausal women with coronary artery disease. J Am Coll Cardiol. 1999;33:2030–2037. doi: 10.1016/s0735-1097(99)00128-x. [DOI] [PubMed] [Google Scholar]
- 25.Koh KK, Cardillo C, Bui MN, et al. Vascular effects of estrogen and cholesterol-lowering therapies in hypercholesterolemic postmenopausal women. Circulation. 1999;99:354–360. doi: 10.1161/01.cir.99.3.354. [DOI] [PubMed] [Google Scholar]
- 26.Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522–529. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- 27.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 28.Waters DD, Alderman EL, Hsia J, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288:2432–2440. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 29.Lobo RA. Evaluation of cardiovascular event rates with hormone therapy in healthy, early postmenopausal women: results from 2 large clinical trials. Arch Intern Med. 2004;164:482–484. doi: 10.1001/archinte.164.5.482. [DOI] [PubMed] [Google Scholar]
- 30.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 31.Campisi R, Nathan L, Pampaloni MH, et al. Noninvasive assessment of coronary microcirculatory function in postmenopausal women and effects of short-term and long-term estrogen administration. Circulation. 2002;105:425–430. doi: 10.1161/hc0402.102860. [DOI] [PubMed] [Google Scholar]
- 32.Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:2993–2999. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 33.Buchthal SD, den Hollander JA, Merz CN, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342:829–835. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 34.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 35.Kaski JC, Rosano GM, Collins P, et al. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995;25:807–814. doi: 10.1016/0735-1097(94)00507-M. [DOI] [PubMed] [Google Scholar]
- 36.Kemp HG, Kronmal RA, Vlietstra RE, et al. Seven year survival of patients with normal or near normal coronary arteriograms: a CASS registry study. J Am Coll Cardiol. 1986;7:479–483. doi: 10.1016/s0735-1097(86)80456-9. [DOI] [PubMed] [Google Scholar]
- 37.Lichtlen PR, Bargheer K, Wenzlaff P. Long-term prognosis of patients with anginalike chest pain and normal coronary angiographic findings. J Am Coll Cardiol. 1995;25:1013–1018. doi: 10.1016/0735-1097(94)00519-v. [DOI] [PubMed] [Google Scholar]
- 38.Shaw LJ, Merz CN, Pepine CJ, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health--National Heart, Lung, and Blood Institute--sponsored Women's Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- 39.Johnson BD, Shaw LJ, Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women's Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27:1408–1415. doi: 10.1093/eurheartj/ehl040. [DOI] [PubMed] [Google Scholar]
- 40.von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 41.Lanza GA, Colonna G, Pasceri V, et al. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am J Cardiol. 1999;84:854–856. doi: 10.1016/s0002-9149(99)00450-6. A8. [DOI] [PubMed] [Google Scholar]
- 42.Lerman A, Burnett JC, Jr, Higano ST, et al. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123–2128. doi: 10.1161/01.cir.97.21.2123. [DOI] [PubMed] [Google Scholar]
- 43.Cannon RO, 3rd, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med. 1994;330:1411–1417. doi: 10.1056/NEJM199405193302003. [DOI] [PubMed] [Google Scholar]
- 44.Eriksson BE, Tyni-Lenne R, Svedenhag J, et al. Physical training in Syndrome X: physical training counteracts deconditioning and pain in Syndrome X. J Am Coll Cardiol. 2000;36:1619–1625. doi: 10.1016/s0735-1097(00)00931-1. [DOI] [PubMed] [Google Scholar]