Abstract
This study examines how variations in the duty cycle (the duration of applied loading) of deformational loading can influence the mechanical properties of tissue engineered cartilage constructs over one month in bioreactor culture. Dynamic loading was carried out with three different duty cycles: 1 h on/1 h off for a total of 3 h loading/day, 3 h continuous loading, or 6 h of continuous loading per day, with all loading performed 5 days/week. All loaded groups showed significant increases in Young’s modulus after one month (vs. free swelling controls), but only loading for a continuous 3 and 6 h showed significant increases in dynamic modulus by this time point. Histological analysis showed that dynamic loading can increase cartilage oligomeric matrix protein (COMP) and collagen types II and IX, as well as prevent the formation of a fibrous capsule around the construct. Type II and IX collagen deposition increased with increased with duration of applied loading. These results point to the efficacy of dynamic deformational loading in the mechanical preconditioning of engineered articular cartilage constructs. Furthermore, these results highlight the ability to dictate mechanical properties with variations in mechanical input parameters, and the possible importance of other cartilage matrix molecules, such as COMP, in establishing the functional material properties of engineered constructs.
Keywords: Functional tissue engineering, Cartilage oligomeric matrix protein, Cartilage, Agarose, Bioreactor, Dynamic modulus, Mechanical properties, Collagen, Chondrocyte
INTRODUCTION
Articular cartilage lines the bony surfaces of diarthrodial joints and functions to transmit the high stresses associated with joint loading. The ability of this tissue to carry out this function is dependent on its unique material properties and dense extracellular matrix (made up mostly of proteoglycans and type II collagen).3 While the tissue can function well over a lifetime, traumatic injury or the degenerative changes associated with osteoarthritis (OA) can result in significant erosion of the articular layer, leading to joint pain and instability.24 The poor healing capacity of the tissue, coupled with continual increases in life expectancy and the limited number of effective clinical remedies, have motivated a search for an engineered articular cartilage replacement. To this end, a number of studies have investigated the growth of engineered articular cartilage, both in vitro and in vivo, in a variety of three-dimensional hydrogels and/or fibrous scaffolds and bioreactor systems (e.g.,6,9,12,13,15–17,41).
A number of in vivo animal5,25,26,30,55 and in vitro explant19,31,51,52,58 studies suggest that the duration, magnitude, and nature of the mechanical input can influence the mechanical properties, biosynthetic activity, and biochemical constituents of the native tissue. These findings suggest that a mechanical stimulus for functional tissue engineering purposes may be modulated to optimize construct material properties. Indeed, functional tissue engineering approaches using deformational loading,23,29,43 or hydrostatic pressure9 have employed a variety of duty cycles with varying degrees of success. While providing a different physical loading regimen to the chondrocytes and thereby affecting mechanotransduction, variation of duty cycle may further influence construct development by modulating the loading-induced convective transport of solutes.1,40,46,48
Using the well-established, chondrocyte-seeded, agarose model4,7 with immature bovine chondrocytes, our laboratory has shown in the past that deformational loading at 10% platen-to-platen deformation at 1 Hz for three, one hour load/rest cycles applied five days/week in culture media with 20% fetal bovine serum results in robust enhancement of construct properties, with tissues exhibiting a Young’s modulus that is ¾ of native values and a dynamic modulus that is ¼ of native values when compared to immature bovine cartilage after 42 days in culture.43 In light of the above, it is hypothesized in this study that continuous (rather than intermittent) applied deformational loading over a 28-day culture period will lead to further improvements in the mechanical properties of the resulting tissue and that this improvement will be further modulated by the duration of loading.
METHODS
Tissue Isolation and Cell Culture
Cell-seeded agarose hydrogels were prepared as previously described.43 Briefly, cartilage was isolated from the carpometacarpal (CMC) joint of a minimum of five young (4- to 6-month-old) calves and digested in Dulbecco’s Modified Eagle’s Medium (DMEM, 5 mL per gram of tissue) with 2.5 mg/mL pronase (Calbiochem, San Diego, CA) for 1 h at 37 °C with stirring, followed by 0.5 mg/mL collagenase type II (Sigma Chemicals, St. Louis, MO) for 4 h at 37 °C with stirring. Cell suspensions were filtered, centrifuged at 1000×g for 10 min, and then resuspended in fresh DMEM supplemented as described previously.43 Cell suspensions were mixed in equal parts with 4% agarose (Type VII, low gelling temperature) in phosphate buffered saline (PBS) to produce final cell concentration of 60 million cells/mL in 2% agarose. After gelling at room temperature for 20 min between parallel plates, disks (Ø 4.76 × 2.3 mm) were cored, and cultured in 100 mm petri dishes (20–25 disks per plate) with 30 mL of high glucose DMEM with 50 µg/mL fresh ascorbic acid at 37 °C and 5% CO2 for two days to allow cells to adjust to their new culture environment. After this period, media with 20% fetal bovine serum (FBS) and fresh ascorbate were changed daily. Cylindrical articular cartilage samples (Ø 4 mm) were also harvested from the same joints for analysis of mechanical and biochemical properties.
Loading Protocols
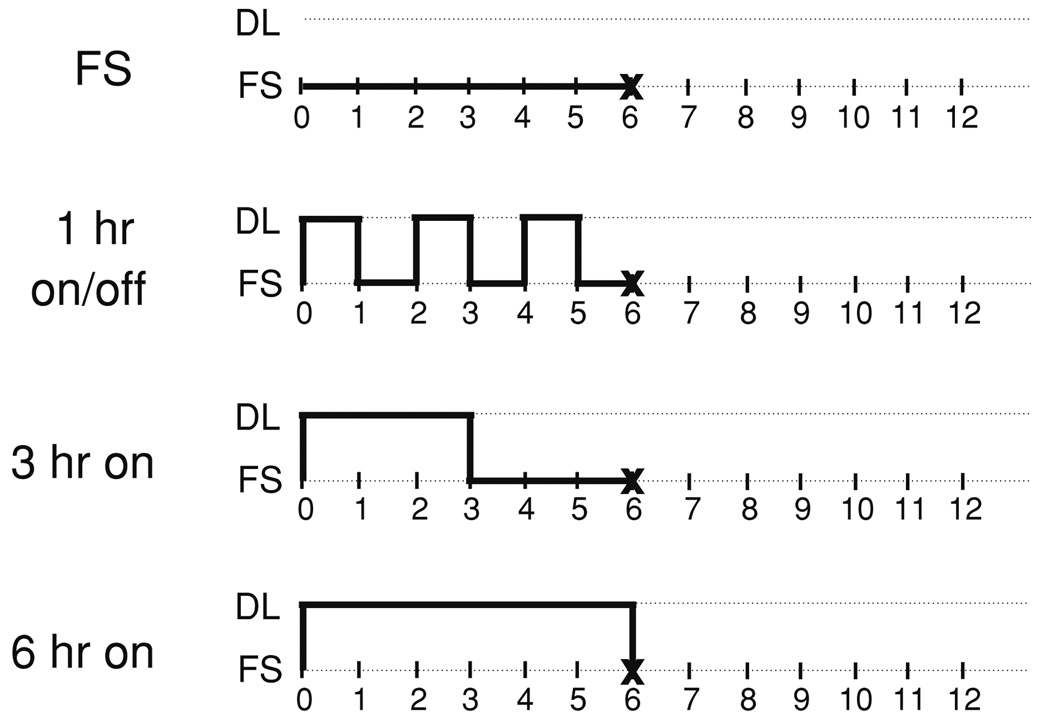
Dynamic loading (DL) was carried out in a custom deformational loading bioreactor41 in a volume of 5 mL DMEM. Loading at 1 Hz with an applied strain of 10% (strain-controlled) was carried out with three different duty cycles (Fig. 1): 1 h on/1 h off for a total of 3 h loading/day, 3 h continuous loading, or 6 h of continuous loading per day, with all loading performed 5 days/week. Free swelling (FS) controls were maintained in the same amount of media adjacent to the loading device for the 6 h loading period. After loading, disks were cultured in free swelling conditions with 30 mL of medium for overnight culture. Every two weeks, 3–4 disks were removed for analysis over a four-week period. The strain magnitude, frequency, and duration of deformational loading were chosen from values in the literature to approximate certain aspects of the “normal” physiologic mechanical environment,2,14,35 while ensuring that the hydrogel did not undergo permanent deformation/damage or experience lift-off of the platen from the construct surface during deformational loading.41
FIGURE 1.
Loading regimes employed to investigate the effect of a variation in duty cycle on construct growth. FS indicates period of free swelling culture, DL indicates dynamic deformational loading (10% peak-to-peak deformation at 1 Hz). The “X” indicates the time at which constructs were transferred from loading chambers to overnight free swelling culture.
Mechanical Testing
Mechanical testing was carried out in unconfined compression between impermeable platens in a custom mechanical testing device.56 For testing, constructs were first equilibrated in creep under a tare load of ~0.02 N, followed by stress relaxation tests with a ramp displacement of 1 µm/s to 10% strain (based on the measured post-creep thickness). After equilibrium was reached (~2000 s), a sinusoidal displacement of 40 µm magnitude was applied at a frequency of 1 Hz. The compressive Young’s modulus (EY) and unconfined dynamic modulus was calculated from the specimen geometry (surface area and thickness) and the measured load and deformation. Articular cartilage specimens from which the cells were isolated (with underlying bone removed) were tested similarly, with a creep load of ~0.1 N, and a dynamic displacement of 20 µm amplitude.
Histology
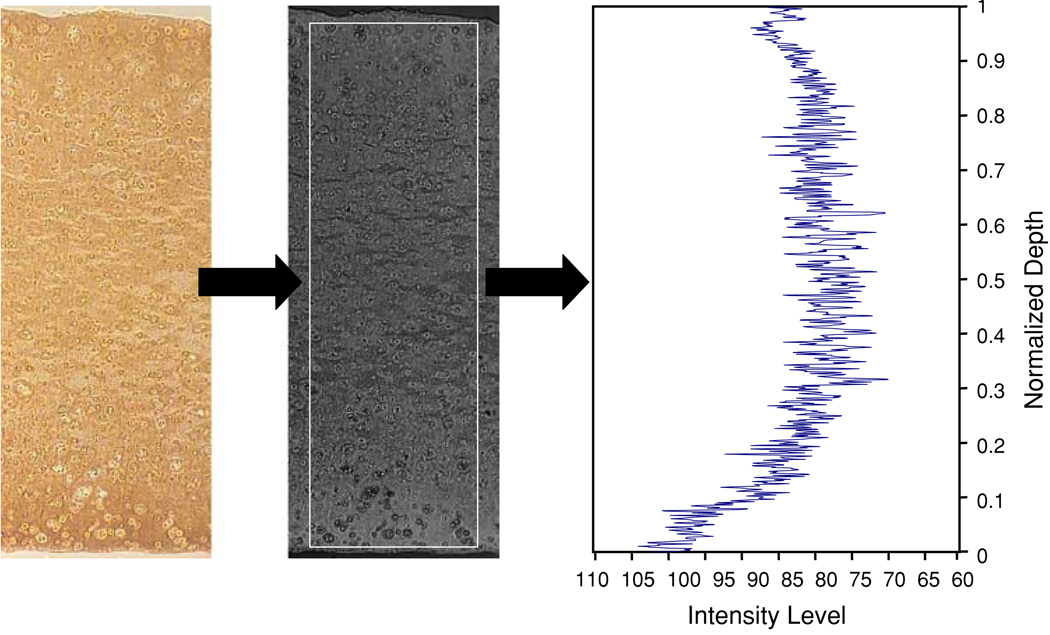
Samples for histology were fixed overnight at 4 °C in acid–formalin–ethanol,34 dehydrated in a graded series of ethanol, embedded in paraffin, then sectioned to 8 µm and affixed to microscope slides. Slides were stained with Safranin O for glycosaminoglycan (GAG) content/distribution and Picrosirius Red for total collagen content/distribution as described previously.43 Collagen type II and type IX were visualized via immunohistochemistry as previously described.28 Cartilage oligomeric matrix protein (COMP) protein staining was performed using Vectastain Universal Elite ABC Kit (Vector) with diaminobenzidine as the chromogen and in-house purified F8 COMP antibody.10,21 All histology images were captured using the same microscope, digital camera, and lighting conditions. Images were converted to grayscale, inverted, and then analyzed for differences in the average staining intensity via ImageJ (NIH) (Fig. 2), with absence of staining showing as a 0 staining intensity. Similar techniques have been used to quantify biochemical content from histological staining by previous researchers.38
FIGURE 2.
Images taken of histologically stained sample sections were semi-quantitatively analyzed by first performing a background subtraction, then converting the images to grayscale and then analyzing the intensity of the histological stains across a rectangular area of the specimen section.
Statistics
Statistics were performed using ANOVA with Young’s modulus, dynamic modulus, and semi-quantitative staining intensity as the dependent variables and loading group and culture duration as the independent variables. Fisher’s LSD post-hoc tests, with α = 0.05, were performed to determine significant differences. All data are reported as the mean ±standard deviation of 3–4 samples. Multiple regression analyses were carried out on pooled and individual data sets to determine the dependence of the measured mechanical properties on the loading duty cycle.42
RESULTS
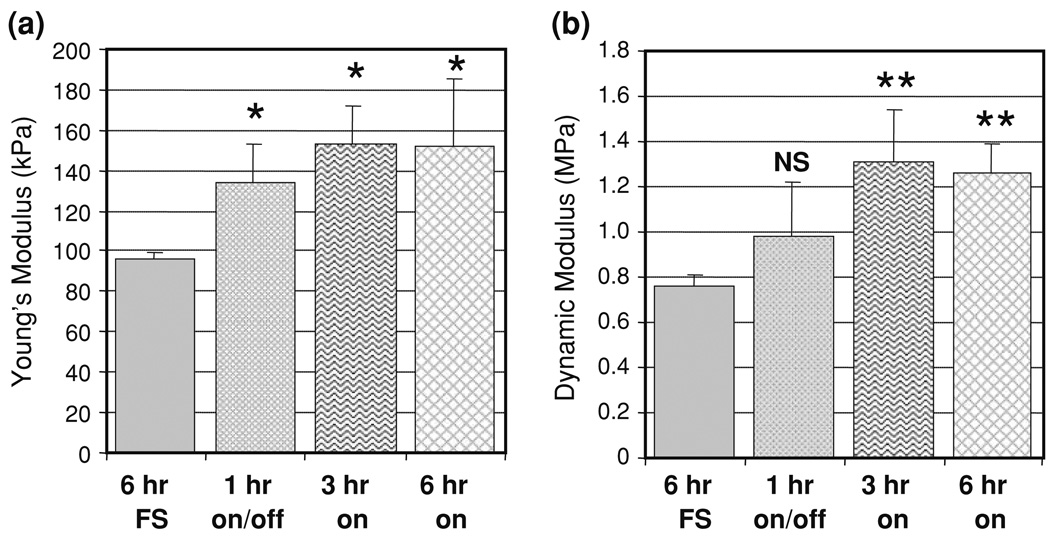
By day 28, dynamic deformational loading at duty cycles of 1 h on/1 h off (p < 0.01), 3 h on (p < 0.001), and 6 h on (p < 0.001) all led to increases in the Young’s modulus (Fig. 3a) vs. free swelling controls. The increases due to loading were not statistically different among the various loading regimes (p > 0.16). At this time point, dynamic loading for 3 and 6 h resulted in significant increases in the dynamic modulus (at 1 Hz testing frequency) of the engineered cartilage constructs vs. free-swelling controls (p < 0.001) and intermittently loaded constructs (p < 0.0025) (Fig. 3b). The dynamic modulus of constructs maintained in free-swelling was not different from the 1 h on/off group (p > 0.10). Tests on full-thickness, immature bovine CMC cartilage yielded a Young’s modulus of 277 ± 83 kPa (n = 5) and a dynamic modulus at 1 Hz of 7.0 ± 0.7 MPa.
FIGURE 3.
Effect of a variation in duty cycle on (a) Young’s modulus and (b) 1 Hz dynamic modulus on day 28. Data presented as the mean and standard deviation of 3–4 samples. NS indicates no significant difference (p > 0.05) from the free-swelling group, * indicates significant difference (p < 0.05) from free swelling condition, ** indicates significant difference (p < 0.05) from both free-swelling and 1 h on/1 h off loading conditions.
Statistical analysis to correlate the various input loading parameter and the resulting mechanical properties found that the resultant EY of constructs depended on the presence of loading (p < 0.0025) and time in culture (p < 0.001), with a significant interaction of these stimuli (p < 0.025). There was no significant correlation found with duration of loading and the resultant EY. The dynamic modulus, however, was found to depend on loading duration in addition to the time in culture and the frequency at which the dynamic modulus was measured (p < 0.001).
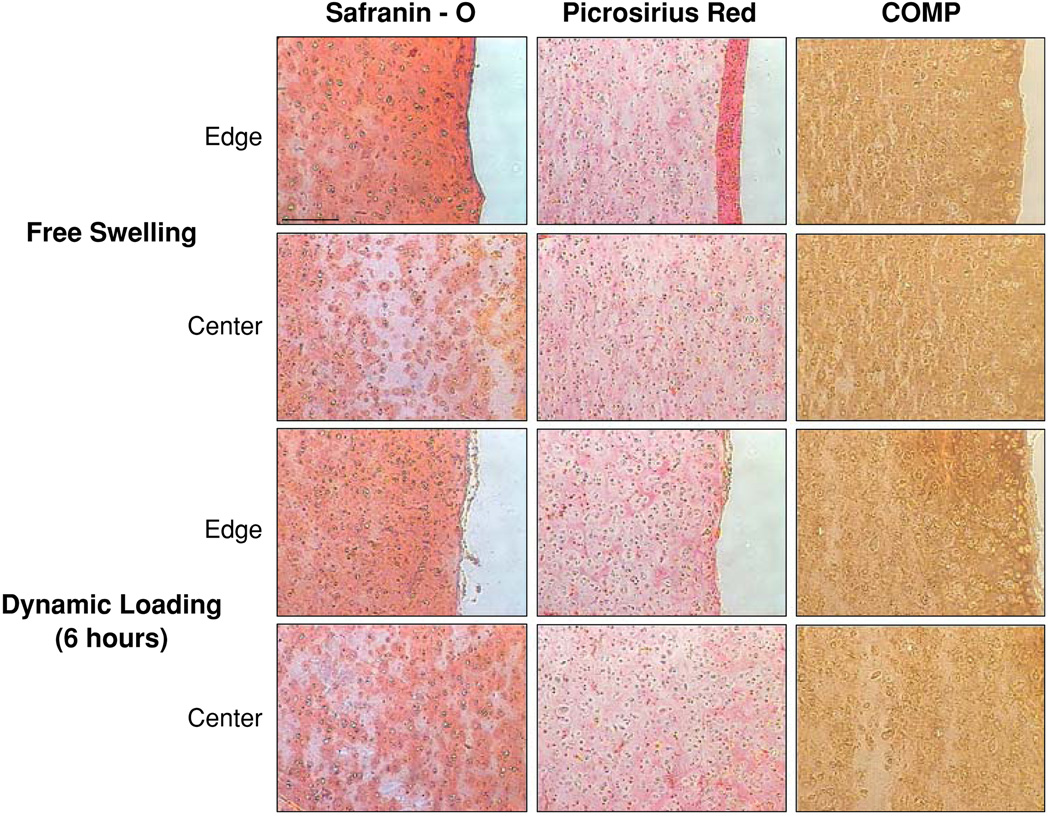
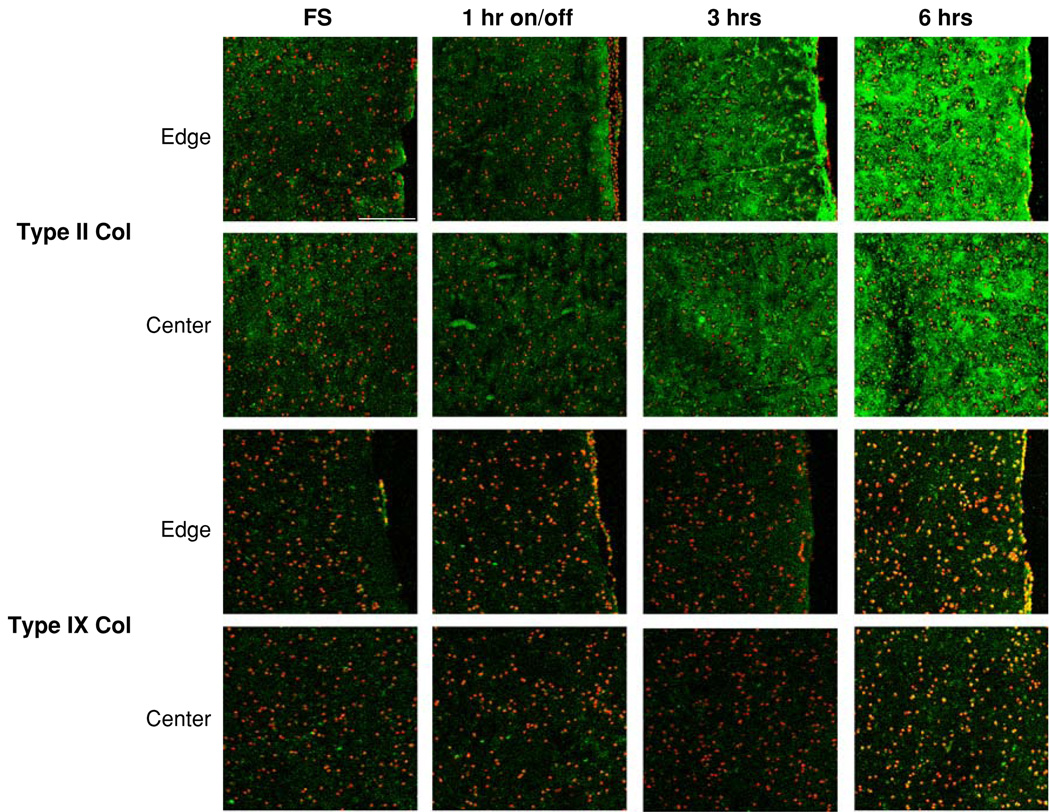
Semi-quantitative histological analysis revealed increased matrix presence in all constructs after 28 days in culture vs. day 0 constructs (not shown). Free-swelling samples possessed a highly cellular, matrix-rich outer “skin” comprising ~10% of construct thickness that possessed ~50% more glycosaminoglycan (GAG) and ~100% more collagen than central regions of the free-swelling constructs (Fig. 4). With this outer region, free-swelling samples possessed the same average staining intensity as that of dynamically loaded samples (Table 1). However, exclusion of the peripheral region resulted in greater average staining intensity in dynamic loading constructs vs. free-swelling (Table 1). Dynamically loaded constructs showed no intensity differences between loading durations for GAG and collagen histological stains (Table 1). Type II and IX collagens and COMP were present in all constructs (Figs. 4, 5). Dynamic loading for 3 and 6 h continuously resulted in significantly increased type II collagen staining (p ≤ 0.05, Table 2), especially along the periphery of the 6 h loading group (Fig. 5). The presence of COMP protein was significantly elevated only in the 6 h loading group (Table 2), with increased COMP deposition found along the periphery of the constructs (Fig. 4).
FIGURE 4.
Safranin O, Picrosirius Red, and COMP histological staining of edge and center of free-swelling and 6-h dynamically loaded constructs. No differences were observed in staining between loaded constructs. Scale bar = 200 µm.
TABLE 1.
Average staining intensity for GAG (Safranin O) and collagen (Picrosirius Red) histological stains for day 28 constructs cultured in free swelling (FS) conditions or under dynamic loading for 1 h on/off (DL 1 h on/off), 3 h continuously (DL 3 h), and 6 h continuously (DL 6 h).
| Day 28 histology average staining intensity | |||||
|---|---|---|---|---|---|
| FS | |||||
| + Fibrous layer | − Fibrous layer | DL 1 h on/off | DL 3 h | DL 6 h | |
| GAG (Safranin O) | 91.776 ± 22.232 | 71.105 ± 15.727* | 110.297 ± 11.337 | 113.534 ± 16.571 | 103.787 ± 13.282 |
| Collagen (P. Red) | 72.774 ± 15.190 | 47.446 ± 10.598* | 70.012 ± 16.243 | 69.741 ± 17.167 | 69.087 ± 15.559 |
p < 0.05 vs. all other groups.
FIGURE 5.
Type II and type IX collagen immunohistological staining of edge and center of free-swelling and dynamically loaded constructs. Scale bar = 200 µm.
TABLE 2.
Average staining intensity for type II collagen, type IX collagen, and COMP immunohistochemistry for constructs cultured for 28 days in free swelling (FS) conditions or under dynamic loading for 1 h on/off (DL 1 h on/off), 3 h continuously (DL 3 h), and 6 h continuously (DL 6 h).
| Day 28 immunohistochemistry average staining intensity | ||||||||
|---|---|---|---|---|---|---|---|---|
| FS | DL 1 h on/off | DL 3 h | DL 6 h | |||||
| Edge | Center | Edge | Center | Edge | Center | Edge | Center | |
| Type II collagen | 43.000 ± 5.196 | 42.197 ± 4.477 | 41.557 ± 4.822 | 42.103 ± 4.490 | 83.948 ± 10.074† | 70.907 ± 9.411† | 142.611 ± 18.568*† | 102.001 ± 12.061† |
| Type IX collagen | 29.342 ± 4.192 | 27.678 ± 3.202 | 29.582 ± 3.356 | 27.647 ± 3.186 | 29.479 ± 3.741 | 30.641 ± 4.084 | 40.675 ± 5.581† | 35.998 ± 3.936† |
| COMP | 81.825 ± 11.591 | 54.360 ± 7.412 | 83.914 ± 9.060 | 56.366 ± 6.448 | 96.823 ± 14.080 | 74.061 ± 10.075 | 109.150 ± 12.917*,† | 64.920 ± 8.736 |
p < 0.05 vs. center,
p < 0.05 vs. FS of respective location.
DISCUSSION
In this study, continuous deformational loading for periods ranging of 3 and 6 h resulted in constructs with improved mechanical properties and distribution of matrix molecules relative to those experiencing an intermittent mechanical stimulus (1 h on/off), particularly with regard to the dynamic modulus. Overall, the resulting tissue possessed a Young’s modulus that is ~1/2 that of the native tissue and a dynamic modulus in unconfined compression that is ~1/5 that of the native tissue. The finding that even a few variations of the duty cycle of deformational loading can modulate the mechanical properties of constructs over a one-month culture period suggests that loading conditions can be optimized to expedite the production of a mechanically viable construct over long-term culture. Given these promising results, further experiments are planned to optimize the loading duty cycle in serum-free culture conditions32,44 that are more desirable for clinical translation.
There can be several mechanisms behind the changes in biochemical composition in the loaded constructs. Prior to significant matrix formation, applied compressive loading to a chondrocyte-seeded scaffold will lead to deformation of the seeded cells and translate into changes in matrix synthesis.11,29 However, after pericellular matrix formation, which has been found to occur within 2–4 days, the chondrocytes are no longer proportionally strained with applied scaffold compression.6 This implies that chondrocyte response to cellular compression cannot fully explain the results observed in the present study over the 28 day culture period. Dynamic compressive loading of the scaffold generates a hydrostatic pressure within the construct, but this is also unlikely to be a major mechanism as the dynamic modulus of the chondrocyte-seeded agarose constructs would indicate that the pressures generated are unlikely to be in the physiologic range where cell responses have been previously observed.20 Indeed, preliminary experiments applying dynamic hydrostatic pressure alone at a magnitude of 3 MPa did not elicit significant changes in chondrocyte-seeded agarose hydrogels relative to free-swelling controls.39 These findings lead us to believe that hydrostatic pressure also cannot explain the compressive loading results found in this study.
Another aspect of deformational loading is the strain and frequency-dependent fluid flow and the resulting convective transport of molecules. Experimental studies have shown that deformational loading can affect an increase in the rate of influx and equilibrium level of large molecules in cartilage explants5,46 and agarose hydrogel constructs.1 Our laboratory has developed theoretical models describing loading-enhanced solute transport in porous permeable gels that support these observations.40 Using an applied deformational loading protocol (similar to those employed in this study) as an input parameter in this model, it was found that for large molecules like growth factors and transport molecules found in serum,8 the characteristic time for transport is ~3–4 h under loading, while for free swelling conditions the characteristic time is ~50 h.40 This theoretical characteristic time for loading agrees with the increased tissue properties observed in the experimental results of the 3 and 6 h experimental groups. Loading-induced convective transport may also directly affect the formation of larger matrix structures. In a free-swelling agarose hydrogel system, Quinn et al.49 reported (using a radionucleotide tracer technique) that up to 14 days are necessary for formed proteoglycan molecules to come in contact with one another. The movement and aggregation of these large molecules may be accelerated by deformational loading, decreasing the time necessary for molecular interactions and resulting in differences in matrix formation. These loading-induced transport mechanisms explain the increased cellular biosynthesis and more evenly distributed GAG and collagen staining in dynamically loaded samples compared to the concentrated peripheral staining apparent in the free-swelling counterparts observed in this study.
The above fluid-flow-based mechanisms, however, do not fully explain the observed similar total biochemical content (GAG, collagen) but differing mechanical properties between DL and FS samples obtained in previous studies by our laboratory and others at high seeding density and long culture periods.28,43,53 To better address this issue, we refined our analyses to include examination of the structural molecules COMP and type IX collagen that may hold relevance as quantitative indicators of mechanically functional matrix development. The increases in cartilage oligomeric matrix protein (COMP), type II collagen, and type IX collagen due to mechanical loading continuously for 3 h and/or 6 h observed in this study and in others18,28,61 implies a functional shift in the synthetic activity of the chondrocytes vs. free-swelling controls.
The loading duration-dependent increases in type II collagen may explain the concomitant increases in the dynamic modulus given the known relationship between collagen and the dynamic mechanical response of articular cartilage.59,60 In addition, the increased presence of type II collagen with loading over free-swelling constructs is important since type II collagen is the predominant collagen of healthy hyaline articular cartilage as opposed to the fibro-cartilage that develops in response to cartilage injury in vivo.36,37 Both COMP and type IX collagen are known to interact cooperatively with type II collagen for proper formation and cross-linking of the extracellular matrix10,22,50,57,62 and may be relevant indicators of the quality of matrix structure and overall tissue behavior. Taken together with our findings of loading mediated structural changes in agarose constructs,27 these results suggest that the loading-induced improvements in mechanical properties may ultimately be due to function-structure changes in matrix formation.
The results presented in this paper demonstrated that the increases in GAG and collagen content and mechanical properties in engineered cartilage imparted by applied mechanical loading can be further enhanced by changes in the loading regimen. In addition, mechanical loading continuously for 3 or 6 h was found to increase type IX collagen and COMP synthesis, matrix molecules that may play important structural and mechanical roles in the tissue. The accompanying stimuli that arise from dynamic loading (e.g., fluid flow, pressure, radial strain, etc.33,47) make it difficult to isolate the precise mechanisms that direct the various aspects of neo-cartilage tissue development. As cells within the scaffold material produce a functional extracellular matrix, their response to a particular mechanical loading regime differs (e.g.,6) and the applied loading regimes may need to be further adjusted over time in culture, perhaps increasing the loading duration as tissue permeability declines due to matrix elaboration. However, there are limitations in extending the duration of loading as preliminary data by our group has shown no benefits of loading constructs continuously for 12 h. Studies by Hunter et al.23 that employed a continuous 24 h loading cycle in a fibrin gel resulted in a decrease in proteoglycan synthesis. The negative response of chondrocytes under these long duration loading conditions is suggestive of a joint overuse condition that may be a mechanism of OA progression in vivo. Future studies will attempt to more rigorously separate the mechanical, biochemical, and structural ramifications of these physical stimuli on chondrocytes and cartilage as a whole. This is important not only for modeling the behavior of cartilage and chondrocytes in a mechanical environment but also to develop a clinically relevant model for an optimized tissue engineered cartilage that replicates both the macro- and micro-scale mechanical and biological behavior of the native tissue (e.g.,45,54).
ACKNOWLEDGMENT
This study was supported by Grants from the National Institutes of Health (R01 AR46532, AR46568; R03 AR053668) and a pre-doctoral fellowship from the Whitaker Foundation. Special thanks to Ashby Thomas, Qiqi Cheng, and Nicole Gabriel for their technical assistance throughout this study.
REFERENCES
- 1.Albro MB, Chahine NO, Li R, Yeager K, Hung CT, Ateshian GA. Dynamic loading of deformable porous media can induce active solute transport. J. Biomech. 2008;41:3152–3157. doi: 10.1016/j.jbiomech.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong CG, Bahrani AS, Gardner DL. In vitro measurement of articular cartilage deformations in the intact human hip joint under load. J. Bone Joint Surg. Am. 1979;61:744–755. [PubMed] [Google Scholar]
- 3.Ateshian GA, Hung CT. Functional properties of native articular cartilage. In: Guilak F, Mooney D, Butler D, Goldstein SA, editors. Functional Tissue Engineering. New York: Springer-Verlag; 2003. [Google Scholar]
- 4.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 5.Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J. Orthop. Res. 2001;19:11–17. doi: 10.1016/S0736-0266(00)00004-8. [DOI] [PubMed] [Google Scholar]
- 6.Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J. Cell Sci. 1995;108(Pt 4):1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 7.Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J. Orthop. Res. 1992;10:745–758. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 8.Cartwright T, Shah GP. Culture media. In: Davis JM, editor. Basic Cell Culture: A Practical Approach. Oxford: Oxford University; 1996. pp. 57–91. [Google Scholar]
- 9.Carver SE, Heath CA. Increasing extracellular matrix production in regenerating cartilage with intermittent physiological pressure. Biotechnol. Bioeng. 1999;62:166–174. [PubMed] [Google Scholar]
- 10.Chen H, Deere M, Hecht JT, Lawler J. Cartilage oligomeric matrix protein is a calcium-binding protein, and a mutation in its type 3 repeats causes conformational changes. J. Biol. Chem. 2000;275:26538–26544. doi: 10.1074/jbc.M909780199. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury TT, Bader DL, Shelton JC, Lee DA. Temporal regulation of chondrocyte metabolism in agarose constructs subjected to dynamic compression. Arch. Biochem. Biophys. 2003;417:105–111. doi: 10.1016/s0003-9861(03)00340-0. [DOI] [PubMed] [Google Scholar]
- 12.Cook JL, Kreeger JM, Payne JT, Tomlinson JL. Three-dimensional culture of canine articular chondrocytes on multiple transplantable substrates. Am. J. Vet. Res. 1997;58:419–424. [PubMed] [Google Scholar]
- 13.Cook JL, Williams N, Kreeger JM, Peacock JT, Tomlinson JL. Biocompatibility of three-dimensional chondrocyte grafts in large tibial defects of rabbits. Am. J. Vet. Res. 2003;64:12–20. doi: 10.2460/ajvr.2003.64.12. [DOI] [PubMed] [Google Scholar]
- 14.Dillman CJ. Kinematic analyses of running. Exerc. Sport Sci. Rev. 1975;3:193–218. [PubMed] [Google Scholar]
- 15.Dunkelman NS, Zimber MP, Lebaron RG, Pavelec R, Kwan M, Purchio AF. Cartilage production by rabbit articular chondrocytes on polyglycolic acid scaffolds in a closed bioreactor system. Biotechnol. Bioeng. 1995;46:299–305. doi: 10.1002/bit.260460402. [DOI] [PubMed] [Google Scholar]
- 16.Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R. Biodegradable polymer scaffolds for tissue engineering. Biotechnology (NY) 1994;12:689–693. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 17.Freed LE, Vunjak-Novakovic G, Langer R. Cultivation of cell-polymer cartilage implants in bioreactors. J. Cell. Biochem. 1993;51:257–264. doi: 10.1002/jcb.240510304. [DOI] [PubMed] [Google Scholar]
- 18.Giannoni P, Siegrist M, Hunziker EB, Wong M. The mechanosensitivity of cartilage oligomeric matrix protein (COMP) Biorheology. 2003;40:101–109. [PubMed] [Google Scholar]
- 19.Gray ML, Pizzanelli AM, Lee RC, Grodzinsky AJ, Swann DA. Kinetics of the chondrocyte biosynthetic response to compressive load and release. Biochim. Biophys. Acta. 1989;991:415–425. doi: 10.1016/0304-4165(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 20.Hall AC, Urban JP, Gehl KA. The effects of hydrostatic pressure on matrix synthesis in articular cartilage. J. Orthop. Res. 1991;9:1–10. doi: 10.1002/jor.1100090102. [DOI] [PubMed] [Google Scholar]
- 21.Hecht JT, Deere M, Putnam E, Cole W, Vertel B, Chen H, Lawler J. Characterization of cartilage oligomeric matrix protein (COMP) in human normal and pseudoachondroplasia musculoskeletal tissues. Matrix Biol. 1998;17:269–278. doi: 10.1016/s0945-053x(98)90080-4. [DOI] [PubMed] [Google Scholar]
- 22.Holden P, Meadows RS, Chapman KL, Grant ME, Kadler KE, Briggs MD. Cartilage oligomeric matrix protein interacts with type IX collagen, and disruptions to these interactions identify a pathogenetic mechanism in a bone dysplasia family. J. Biol. Chem. 2001;276:6046–6055. doi: 10.1074/jbc.M009507200. [DOI] [PubMed] [Google Scholar]
- 23.Hunter CJ, Mouw JK, Levenston ME. Dynamic compression of chondrocyte-seeded fibrin gels: effects on matrix accumulation and mechanical stiffness. Osteoarthr. Cartilage. 2004;12:117–130. doi: 10.1016/j.joca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Hunziker EB. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthr. Cartilage. 1999;7:15–28. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- 25.Jurvelin J, Kiviranta I, Saamanen AM, Tammi M, Helminen HJ. Indentation stiffness of young canine knee articular cartilage—influence of strenuous joint loading. J. Biomech. 1990;23:1239–1246. doi: 10.1016/0021-9290(90)90381-c. [DOI] [PubMed] [Google Scholar]
- 26.Jurvelin J, Kiviranta I, Tammi M, Helminen HJ. Effect of physical exercise on indentation stiffness of articular cartilage in the canine knee. Int. J. Sports Med. 1986;7:106–110. doi: 10.1055/s-2008-1025743. [DOI] [PubMed] [Google Scholar]
- 27.Kelly TA, Ng KW, Wang CC, Ateshian GA, Hung CT. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J. Biomech. 2006;39:1489–1497. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 28.Kelly TA, Wang CC, Mauck RL, Ateshian GA, Hung CT. Role of cell-associated matrix in the development of free-swelling and dynamically loaded chondrocyte-seeded agarose gels. Biorheology. 2004;41:223–237. [PubMed] [Google Scholar]
- 29.Kisiday JD, Jin M, DiMicco MA, Kurz B, Grodzinsky AJ. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J. Biomech. 2004;37:595–604. doi: 10.1016/j.jbiomech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Kiviranta I, Tammi M, Jurvelin J, Saamanen AM, Helminen HJ. Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the knee joint of young beagle dogs. J. Orthop. Res. 1988;6:188–195. doi: 10.1002/jor.1100060205. [DOI] [PubMed] [Google Scholar]
- 31.Lee RC, Frank EH, Grodzinsky AJ, Roylance DK. Oscillatory compressional behavior of articular cartilage and its associated electromechanical properties. J. Biomech. Eng. 1981;103:280–292. doi: 10.1115/1.3138294. [DOI] [PubMed] [Google Scholar]
- 32.Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthr. Cartilage. 2007;15:1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lima EG, Mauck RL, Han SH, Park S, Ng KW, Ateshian GA, Hung CT. Functional tissue engineering of chondral and osteochondral constructs. Biorheology. 2004;41:577–590. [PubMed] [Google Scholar]
- 34.Lin W, Shuster S, Maibach HI, Stern R. Patterns of hyaluronan staining are modified by fixation techniques. J. Histochem. Cytochem. 1997;45:1157–1163. doi: 10.1177/002215549704500813. [DOI] [PubMed] [Google Scholar]
- 35.Macirowski T, Tepic S, Mann RW. Cartilage stresses in the human hip joint. J. Biomech. Eng. 1994;116:10–18. doi: 10.1115/1.2895693. [DOI] [PubMed] [Google Scholar]
- 36.Mankin HJ. The response of articular cartilage to mechanical injury. J. Bone Joint Surg. Am. 1982;64:460–466. [PubMed] [Google Scholar]
- 37.Mankin HJ, Mow VC, Buckwalter JA, Iannotti JP, Ratcliffe A. Articular cartilage structure, composition, and function. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic Basic Science. Biology and Biomechanics of the Musculoskeletal System. Rosemont: American Academy of Orthopaedic Surgeons; 2000. pp. 443–470. [Google Scholar]
- 38.Martin I, Obradovic B, Freed LE, Vunjak-Novakovic G. Method for quantitative analysis of glycosaminoglycan distribution in cultured natural and engineered cartilage. Ann. Biomed. Eng. 1999;27:656–662. doi: 10.1114/1.205. [DOI] [PubMed] [Google Scholar]
- 39.Mauck RL, Ho MM, Hung CT, Ateshian GA. Growth factor supplementation and dynamic hydrostatic pressurization for articular cartilage tissue engineering. Adv. Bioeng. 2003 Paper 0283. [Google Scholar]
- 40.Mauck RL, Hung CT, Ateshian GA. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J. Biomech. Eng. 2003;125:602–614. doi: 10.1115/1.1611512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 42.Mauck RL, Seyhan SL, Ateshian GA, Hung CT. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann. Biomed. Eng. 2002;30:1046–1056. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 43.Mauck RL, Wang CC, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthr. Cartilage. 2003;11:879–890. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Ng KW, DeFrancis JG, Kugler LE, Kelly TA, Ho MM, O’Conor CJ, Ateshian GA, Hung CT. Amino acids supply in culture media is not a limiting factor in the matrix synthesis of engineered cartilage tissue. Amino Acids. 2008;35:433–438. doi: 10.1007/s00726-007-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng KW, Wang CC, Mauck RL, Kelly TA, Chahine NO, Costa KD, Ateshian GA, Hung CT. A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs. J. Ort-hop. Res. 2005;23:134–141. doi: 10.1016/j.orthres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 46.O’Hara BP, Urban JP, Maroudas A. Influence of cyclic loading on the nutrition of articular cartilage. Ann. Rheum. Dis. 1990;49:536–539. doi: 10.1136/ard.49.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park S, Hung CT, Ateshian GA. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthr. Cartilage. 2004;12:65–73. doi: 10.1016/j.joca.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Quinn TM, Morel V, Meister JJ. Static compression of articular cartilage can reduce solute diffusivity and partitioning: implications for the chondrocyte biological response. J. Biomech. 2001;34:1463–1469. doi: 10.1016/s0021-9290(01)00112-9. [DOI] [PubMed] [Google Scholar]
- 49.Quinn TM, Schmid P, Hunziker EB, Grodzinsky AJ. Proteoglycan deposition around chondrocytes in agarose culture: construction of a physical and biological interface for mechanotransduction in cartilage. Biorheology. 2002;39:27–37. [PubMed] [Google Scholar]
- 50.Rosenberg K, Olsson H, Morgelin M, Heinegard D. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J. Biol. Chem. 1998;273:20397–20403. doi: 10.1074/jbc.273.32.20397. [DOI] [PubMed] [Google Scholar]
- 51.Sah RL, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Effects of compression on the loss of newly synthesized proteoglycans and proteins from cartilage explants. Arch. Biochem. Biophys. 1991;286:20–29. doi: 10.1016/0003-9861(91)90004-3. [DOI] [PubMed] [Google Scholar]
- 52.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J. Orthop. Res. 1989;7:619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 53.Seidel JO, Pei M, Gray ML, Langer R, Freed LE, Vunjak-Novakovic G. Long-term culture of tissue engineered cartilage in a perfused chamber with mechanical stimulation. Biorheology. 2004;41:445–458. [PubMed] [Google Scholar]
- 54.Selmi TA, Verdonk P, Chambat P, Dubrana F, Potel JF, Barnouin L, Neyret P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J. Bone Joint Surg. Br. 2008;90:597–604. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 55.Setton LA, Mow VC, Muller FJ, Pita JC, Howell DS. Mechanical behavior and biochemical composition of canine knee cartilage following periods of joint disuse and disuse with remobilization. Osteoarthr. Cartilage. 1997;5:1–16. doi: 10.1016/s1063-4584(97)80027-1. [DOI] [PubMed] [Google Scholar]
- 56.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J. Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 57.Thur J, Rosenberg K, Nitsche DP, Pihlajamaa T, Ala-Kokko L, Heinegard D, Paulsson M, Maurer P. Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J. Biol. Chem. 2001;276:6083–6092. doi: 10.1074/jbc.M009512200. [DOI] [PubMed] [Google Scholar]
- 58.Torzilli PA, Grigiene R, Huang C, Friedman SM, Doty SB, Boskey AL, Lust G. Characterization of cartilage metabolic response to static and dynamic stress using a mechanical explant test system. J. Biomech. 1997;30:1–9. doi: 10.1016/s0021-9290(96)00117-0. [DOI] [PubMed] [Google Scholar]
- 59.Williamson AK, Chen AC, Masuda K, Thonar EJ, Sah RL. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J. Orthop. Res. 2003;21:872–880. doi: 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 60.Wong M, Ponticiello M, Kovanen V, Jurvelin JS. Volumetric changes of articular cartilage during stress relaxation in unconfined compression. J. Biomech. 2000;33:1049–1054. doi: 10.1016/s0021-9290(00)00084-1. [DOI] [PubMed] [Google Scholar]
- 61.Wong M, Siegrist M, Cao X. Cyclic compression of articular cartilage explants is associated with progressive consolidation and altered expression pattern of extracellular matrix proteins. Matrix Biol. 1999;18:391–399. doi: 10.1016/s0945-053x(99)00029-3. [DOI] [PubMed] [Google Scholar]
- 62.Wu JJ, Woods PE, Eyre DR. Identification of cross-linking sites in bovine cartilage type IX collagen reveals an antiparallel type II–type IX molecular relationship and type IX to type IX bonding. J. Biol. Chem. 1992;267:23007–23014. [PubMed] [Google Scholar]