Abstract
Context
Goal-directed resuscitation for severe sepsis and septic shock has been reported to reduce mortality when applied in the emergency department.
Objective
To test the hypothesis of noninferiority between lactate clearance and central venous oxygen saturation (ScvO2) as goals of early sepsis resuscitation.
Design, Setting, and Patients
Multicenter randomized, noninferiority trial involving patients with severe sepsis and evidence of hypoperfusion or septic shock who were admitted to the emergency department from January 2007 to January 2009 at 1 of 3 participating US urban hospitals.
Interventions
We randomly assigned patients to 1 of 2 resuscitation protocols. The ScvO2 group was resuscitated to normalize central venous pressure, mean arterial pressure, and ScvO2 of at least 70%; and the lactate clearance group was resuscitated to normalize central venous pressure, mean arterial pressure, and lactate clearance of at least 10%. The study protocol was continued until all goals were achieved or for up to 6 hours. Clinicians who subsequently assumed the care of the patients were blinded to the treatment assignment.
Main Outcome Measure
The primary outcome was absolute in-hospital mortality rate; the noninferiority threshold was set at Δ equal to −10%.
Results
Of the 300 patients enrolled, 150 were assigned to each group and patients were well matched by demographic, comorbidities, and physiological features. There were no differences in treatments administered during the initial 72 hours of hospitalization. Thirty-four patients (23%) in the ScvO2 group died while in the hospital (95% confidence interval [CI], 17%–30%) compared with 25 (17%; 95% CI, 11%–24%) in the lactate clearance group. This observed difference between mortality rates did not reach the predefined −10% threshold (intent-to-treat analysis: 95% CI for the 6% difference, −3% to 15%). There were no differences in treatment-related adverse events between the groups.
Conclusion
Among patients with septic shock who were treated to normalize central venous and mean arterial pressure, additional management to normalize lactate clearance compared with management to normalize ScvO2 did not result in significantly different in-hospital mortality.
The rate of severe sepsis hospitalizations has doubled during the last decade with estimates indicating that at least 750 000 persons are affected annually in the United States.1–3 Approximately, 500 000 patients with severe sepsis in the United States annually are initially treated in emergency departments.4 The Surviving Sepsis Campaign international consensus guidelines recommend protocol-driven treatment that uses quantitative resuscitation for emergency department patients with severe sepsis and septic shock.5
Quantitative resuscitation refers to the use of an explicit protocol that targets predefined physiological or laboratory goals to be achieved within the first several hours. This concept was pioneered by Shoemaker et al6 to treat high-risk surgical patients. Results of a recent meta-analysis indicated a survival benefit associated with the use of an early and quantitative resuscitation strategy applied to heterogeneous populations of patients with sepsis.7
The optimal goals for quantitative resuscitation of sepsis remain uncertain. It is generally accepted that hemodynamic targets should include some measure of the adequacy of cardiac pre-load, such as central venous pressure, and perfusion pressure, such as mean arterial pressure.8 A more controversial issue is the method of determining tissue oxygen delivery. Citing a single-center study, the Surviving Sepsis Campaign guidelines recommend the use of central venous oxygen saturation (ScvO2) or mixed venous oxygen saturation to assess the balance of tissue oxygen delivery and consumption9; however, since its publication in 2001 a substantial amount of controversy about this single-center study has been generated in the scientific community.10–12 Additionally, recently published practice surveys have indicated that the time, expertise, and specialized equipment required to measure ScvO2 collectively pose a major barrier to the implementation of protocol-driven quantitative resuscitation programs.13,14 In contrast, lactate clearance, derived from calculating the change in lactate concentration from 2 blood specimens drawn at different times, potentially represents a more accessible method to assess tissue oxygen delivery.15,16
To address the potential utility of lactate clearance as a substitute for ScvO2, we conducted a multicenter, randomized trial among patients presenting to the emergency department with severe sepsis and septic shock, with the primary hypothesis that early resuscitation targeting lactate clearance as the marker of adequacy of oxygen delivery was noninferior to the currently recommended ScvO2 monitoring for the outcome of in-hospital mortality.
METHODS
Study Design
This study was a prospective randomized, parallel group, nonblinded, clinical trial designed to assess the noninferiority of lactate clearance vs ScvO2 as the protocol goal that evaluated adequacy of oxygen delivery during early quantitative resuscitation of severe sepsis and septic shock. The trial took place from January 2007 to January 2009 in the emergency departments of 3 large urban medical centers in the United States. The research protocol was approved by the local institutional review boards and performed in accordance with Good Clinical Practice guidelines.
Participants
Patients with severe sepsis or septic shock were assessed for inclusion, which required that patients be older than 17 years with confirmed or presumed infection, have 2 or more systemic inflammatory response criteria,17 and have hypoperfusion evidenced by either a systolic blood pressure lower than 90 mm Hg after a minimum of 20 mL/kg rapid volume challenge or a blood lactate concentration of at least 36 mg/dL (4 mmol/L). The criteria for exclusion from the study were pregnancy, any primary diagnosis other than sepsis, suspected requirement for immediate surgery within 6 hours of diagnosis, an absolute contraindication to chest or neck central venous catheterization, cardiopulmonary resuscitation, transfer from another institution with a sepsis-specific resuscitative therapy underway, and advanced directive orders that would restrict the study procedure. Using a 24-hour day, 7-day-week method that was previously established for the routine clinical care of sepsis patients at each of the participating institutions, an alert was sent to inform clinical care resources when patients were identified as candidates for early aggressive resuscitation. This alert was also received by study staff who responded and screened the patients for study enrollment. Each enrolled patient or the patient’s legally authorized next of kin provided written informed consent prior to collection of data. Patients or family members self-identified their race.
Treatment Assignment
Patients were randomly assigned to 1 of 2 groups (eFigure, available at http://www.jama.com). Each group received structured quantitative resuscitation while in the emergency department. The ScvO2 group was resuscitated by sequentially providing therapy needed to meet thresholds of central venous pressure, followed by mean arterial pressure, and then ScvO2. The lactate clearance group had similarly targeted thresholds in central venous pressure, followed by mean arterial pressure, and then lactate clearance instead of ScvO2. Standard measures were used to ensure appropriate concealment of group assignment until after informed consent was obtained. The group assignment sequence was generated by an independent statistician using a parallel design, balanced randomization schedule (1:1 ratio of cases and controls), using the PROC PLAN function in SAS incorporating a sample size of 300, block size equal to 10, with a seed of 6 457 149 (SAS Institute Inc, Cary, North Carolina). After written informed consent was obtained, study staff opened an opaque sealed envelope containing the randomization assignment. Study staff then enforced the study protocol. By design, the clinical staff in the emergency departments could not be blinded to group assignment; however, the clinical staff (physicians and nurses) who assumed subsequent care of the patients in the intensive care units (ICUs) were unaware of group assignment. Prior to entry in the study, patients were cared for by emergency physicians, who provided basic care processes according to participating institutional standards.
Treatment Interventions
Appropriate specimens were taken for culture, and antibiotics were administered as soon as practical. Blood pressure was monitored by either noninvasive automated cuff sphygmomanometer or arterial catheter according to the clinical team’s preference. All patients received chest or neck central venous catheter capable of measuring continuous ScvO2 (PreSep, Edwards Lifesciences, Irvine, California).
Patients randomized to the ScvO2 group had their central venous catheters connected to a computerized spectrophotometer (Edwards Lifesciences) that displayed continuous ScvO2 readings. The patients were then cared for according to the prespecified treatment plan. First, isotonic crystalloid was administered in boluses to achieve a central venous pressure of 8 mm Hg or higher. Second, the mean arterial pressure goal of 65 mm Hg or higher, if not achieved with fluid administration, was targeted by initiating and titrating vasopressors (dopamine or norepinephrine) to achieve this desired blood pressure goal. Finally, the ScvO2 goal of 70% or higher was targeted after central venous and mean arterial pressure goals were met. If the ScvO2 was lower than 70% and the hematocrit was lower than 30%, packed red blood cells were transfused to achieve a hematocrit of at least 30%. If the ScvO2 remained lower than 70% after the hematocrit was 30% or higher, dobutamine was initiated and titrated in attempts to achieve an ScvO2 of at least 70%.
Patients randomized to the lactate clearance group received an identical central venous catheter capable of measuring continuous ScvO2. The primary intervention consisted of the act of not connecting the catheter to the computerized spectrophotometer thus preventing display of ScvO2 at any time in the emergency department. These patients were cared for according to an identical prespecified treatment plan for central venous and mean arterial pressure targets as outlined for the ScvO2 group. However, in the lactate clearance group, clinicians used lactate clearance instead of ScvO2 as the last resuscitation goal in the protocol and targeted a lactate clearance of at least 10%.15,16 The lactate clearance was defined by the equation [(lactateinitial − lactatedelayed)/lactateinitial] × 100%, for which lactate initial was the measurement at the start of the resuscitation and lactate delayed was another measurement after a minimum of 2 hours after resuscitation was initiated. If the lactate clearance was not at least 10% at the first delayed measurement and the hematocrit was less than 30%, packed red blood cells were transfused to achieve a hematocrit of at least 30%. If the lactate clearance remained lower than 10% after the hematocrit was at least 30%, dobutamine was initiated and titrated in attempts to achieve a lactate clearance of at least 10%. When treatment was continued due to lactate clearance less than 10%, subsequent lactate measurements were performed at a minimum of 1-hour intervals and repeat lactate clearance calculated. Lactate measurements were performed using venous whole blood samples using US Food and Drug Administration–approved devices performed either at the point of care or in the central hospital laboratory, according to participating institutional standards.18 The lactate clearance goal was met by a lactate clearance of at least 10% or if both the initial and delayed lactate concentrations were not elevated (≤18 mg/dL [2 mmol/L]).
Study patients were treated in the emergency department during the entire study treatment period, from randomization to either of the 2 study termination criteria: all treatment goals were achieved or 6 hours had elapsed. Patients were then transferred to an ICU where the critical care physicians, unencumbered by the study protocol in any way, assumed the care of all patients. The study investigators did not provide care for the patients or influence their care in the ICU.
As a safety measure, clinical physicians could elect study group crossover. For patients assigned to the ScvO2 group, clinicians could order a second lactate concentration to calculate the lactate clearance. In the lactate clearance group, the clinician could request to connect the central venous catheter to monitor ScvO2. To execute 1 of these options, the clinician was required to indicate clinical deterioration based on 1 of the following criteria: (1) falling systolic blood pressure or inadequate urine output (<0.5 mL/kg per hour); (2) worsening ventilatory status based on either clinical (respiratory rate, oxygen saturation, or oxygen requirement), arterial blood gas, or mechanical ventilator parameters; or (3) worsened mental status. Patients for whom the clinicians enacted the study group crossover methods were assessed using an intent-to-treat analysis of the original group assignment.
Assessments and Outcome Measures
During the study resuscitation treatment period, the patient’s physiological parameters were measured routinely. All data needed to calculate the Simplified Acute Physiology Score (SAPS) II,19 Sequential Organ Failure Assessment (SOFA) score,20 and the Mortality in Emergency Department Sepsis21 were collected. After ICU admission, patients were assessed daily for 72 hours and detailed data were collected. Patients were followed up until hospital discharge or death.
The primary end point was absolute in-hospital mortality rate. Secondary end points were ICU length of stay, hospital length of stay, ventilator-free days, and new onset multiple organ failure. Other end points assessed were the number of resuscitative goals achieved, administered treatments, and predefined protocol-related serious adverse events.
Statistical Analysis
The sample size calculation and primary analytical plan centered on the hypothesis of noninferiority of lactate clearance vs ScvO2. For the sample size estimate, previously published data suggested a 25% primary outcome event rate for the ScvO2 group; we predicted no difference between the groups for the primary outcome event rate, and we set the noninferiority margin (Δ) at −10%.22–24 The −10% margin was chosen because it represented a two-thirds proportion of the active comparator’s (ScvO2 group) established superiority in a similar clinical trial9 and large databases indicate that the mortality rate for severe sepsis can be expected to vary up to 10% between both state-of-the art medical facilities and regions of the United States.25 Using a 1-sided test of noninferiority, assuming a control group mortality rate of 25% and α= .05, a sample size of 150 per group gave 71% power to determine the intervention did not increase mortality by more than 10%.
When appropriate, categorical data were compared using a χ2 test or Fisher exact test and continuous variables were compared with a Mann-Whitney U test or unpaired t test, 2-sided with P<.05 considered significant (Stats Direct v 2.7.7, Cheshire, England). Two pre-planned blinded interim safety analyses were performed after one-third and two-thirds of the participants were enrolled, and these results were reviewed by an independent safety monitor who had the authority to terminate the study for safety concerns. No sample size adjustments were planned during the interim analyses. We planned both intent-to-treat and per-protocol analyses after study completion.
RESULTS
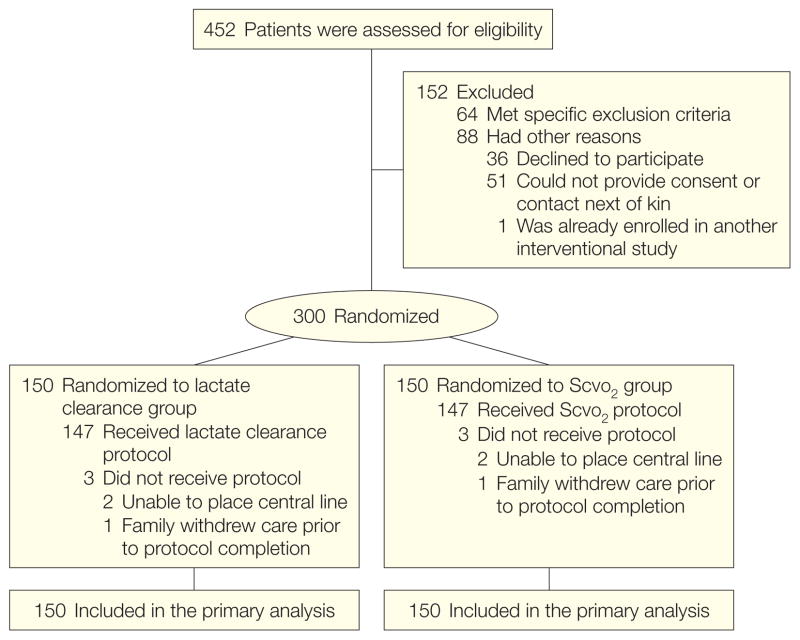
Of 452 patients who underwent screening for eligibility, 300 underwent randomization (Figure). Of these 300 patients, the same pattern of protocol noncompletion was observed in each group: 2 because a chest or neck central venous catheter could not be placed and 1 because family members had decided to withdraw support before the research procedure was completed. One patient in the lactate clearance group had the crossover enacted by a physician after all study goals had been met. The ScvO2 value in this patient was normal, so no treatment changes were made. Because the study treatment period had ended, this patient was analyzed in the lactate clearance group for both the intent-to-treat and per-protocol analyses. None were lost to follow-up or voluntarily withdrew from the study, leaving 150 patients in each group for the intent-to-treat analysis and 147 in each group for the per-protocol analysis.
Figure.
Study Flow Diagram
ScvO2 indicates central venous oxygen saturation.
The baseline characteristics of the 2 groups are shown in Table 1 and in Table 2. There were no significant differences between the groups in demographics, comorbid conditions, severity of illness scores, or suspected site of infection. The lungs were the most common source of infection, and 38% of patients had a blood specimen that yielded growth of bacteria, whereas 84% had at least 1 culture specimen that was positive. The median time from emergency department triage to eligibility was 111 minutes (interquartile range [IQR], 56–192 minutes) in the lactate clearance group and 105 minutes (IQR, 60–175 minutes) in the ScvO2 group (P=.67); the median time from eligibility to study entry was 14 minutes (IQR, 1–48 minutes) in the lactate clearance group and 13 minutes (IQR, 1–55 minutes) in the ScvO2 group (P =.72). Prior to enrollment in the study, the mean (SD) amount of intravenous fluid administered was 2.3 L(1.4 L) in the lactate clearance group and 2.4 L (1.4 L) in the ScvO2 group (P =.37).
Table 1.
Patient Demographics and Clinical Characteristicsa
| Variable | No. (%) of Patients | |
|---|---|---|
| Lactate Clearance Group (n = 150) | ScvO2 Group (n = 150) | |
| Age, mean (SD), y | 59.8 (17.6) | 61.6 (17.6) |
| Race White |
88 (59) |
77 (51) |
| Black | 47 (31) | 56 (37) |
| Sex Men |
83 (55) |
80 (53) |
| Women | 67 (45) | 70 (47) |
| Comorbidities Diabetes mellitus |
45 (30) |
57 (38) |
| Chronic obstructive pulmonary disease | 25 (17) | 25 (17) |
| Human immunodeficiency virus infection | 12 (8) | 13 (9) |
| End-stage renal disease | 15 (10) | 14 (9) |
| Active malignancy | 42 (28) | 32 (21) |
| Organ transplant | 5 (3) | 4 (6) |
| Indwelling vascular line | 6 (4) | 10 (7) |
| Nursing home resident | 28 (19) | 28 (19) |
| Disease severity SAPS II score |
44.8 (18.4) |
44.1 (17.3) |
| SOFA score | 6.7 (3.6) | 6.6 (3.5) |
| MEDS score | 10.9 (3.9) | 10.6 (3.4) |
| Suspected source of infection Pulmonary |
48 (32) |
54 (36) |
| Urinary tract | 40 (27) | 39 (26) |
| Intra-abdominal | 34 (23) | 24 (16) |
| Skin/soft tissue | 19 (13) | 23 (15) |
| Blood | 8 (5) | 9 (6) |
| Unknown | 13 (9) | 9 (6) |
| Features of sepsis Lactate ≥4 |
61 (41) |
56 (37) |
| Shockb | 121 (81) | 123 (82) |
| Culture positive | 123 (82) | 127 (85) |
| Blood culture positive | 62 (41) | 53 (35) |
| Gram positive | 33 (22) | 36 (24) |
| Gram negative | 29 (19) | 17 (11) |
Abbreviations: MEDS, mortality in emergency department sepsis; SAPS, Simplified Acute Physiology Score; ScvO2, central venous oxygen saturation; SOFA, Sequential Organ Failure Assessment.
Continuous data are compared using an unpaired t test; categorical data, using the χ2 test.
Shock is defined as a systolic blood pressure of 90 mm Hg or less after receiving a 20 mL/kg-fluid bolus.
Table 2.
Systemic Inflammatory Response Criteria and Dysfunctional Organ Systems
| Variable | No. (%) of Patients | |
|---|---|---|
| Lactate Clearance Group (n = 150) | ScvO2 Group (n = 150) | |
| SIRS criteria Abnormal white blood cell count |
117 (78) |
104 (69) |
| Elevated heart rate | 100 (67) | 108 (72) |
| Elevated respiratory rate | 96 (64) | 89 (59) |
| Abnormal body temperature | 61 (41) | 64 (43) |
| Organ dysfunction Respiratory |
85 (57) |
86 (57) |
| Liver | 43 (29) | 43 (29) |
| Neurological | 47 (31) | 58 (39) |
| Coagulation | 51 (34) | 39 (26) |
| Cardiovascular | 132 (88) | 128 (85) |
| Renal | 110 (73) | 109 (73) |
Abbreviations: ScvO2, central venous oxygen saturation; SIRS, systemic inflammatory response syndrome.
Table 3 shows the physiological and severity of illness variables during the first 72 hours of hospitalization. During the first 24 hours, both groups of patients tended to show a trend toward slightly worsening severity of illness in the form of lower systolic blood pressures and higher SOFA scores. The mean initial lactate concentrations were 35.1 mg/dL (3.9 mmol/L) in the lactate clearance group and 37.8 mg/dL (4.2 mmol/L) in the ScvO2 group (P=.39). The mean (SD) lactate concentration measured at 2 hours in the lactate clearance group was 23.4 (23.3) mg/dL (2.6 [2.59] mmol/L) and the median lactate clearance at 2 hours was 40% (IQR, 18%–64%). After the initial 24 hours, survivors in both groups manifested improvements in their physiological and severity of illness scores. We observed that lactate measurements did not worsen over the initial 72 hours (Table 3), suggesting that the initial lactate levels were often the most abnormal.
Table 3.
Physiological and Severity of Illness Measurements
| Variable by Study Time Point, ha | Lactate Clearance Group (n = 150) | ScvO2 Group (n = 150) | P Valueb |
|---|---|---|---|
| Systolic blood pressure, mm Hg 0 |
91 (24.6) |
92 (21.0) |
.62 |
| 24 | 73 (20.8) | 79 (15.3) | .01 |
| 48 | 94 (22.1) | 95 (21.3) | .91 |
| 72 | 103 (19.9) | 103 (19.1) | .87 |
| Heart rate, beats/min 0 |
103 (23.6) |
106 (24.4) |
.36 |
| 24 | 117 (23.6) | 119 (21.9) | .37 |
| 48 | 106 (19.7) | 107 (20.5) | .51 |
| 72 | 105 (22.1) | 103 (20.1) | .56 |
| Central venous pressure, mm Hg 0 |
11 (6.5) |
11 (6.2) |
.55 |
| 24 | 16 (7.8) | 15 (6.6) | .47 |
| 48 | 13 (6.4) | 14 (6.5) | .45 |
| 72 | 12 (6.5) | 14 (8.3) | .14 |
| Central venous oxygen saturation, % 0 |
74 (12.3) |
||
| 24 | 65 (19.9) | 64 (13.1) | .71 |
| 48 | 70 (16.6) | 68 (14.8) | .53 |
| 72 | |||
| Lactate level, mg/dLc 0 |
35.1 (28.1) |
37.8 (27.7) |
.39 |
| 24 | |||
| 48 | |||
| 72 | 35.1 (30.3) | 36.9 (29.6) | .67 |
| SOFA score, median (IQR) 0 |
6 (4–9) |
6 (4–9) |
.71 |
| 24 | 8 (5–11) | 7 (5–11) | .98 |
| 48 | 4 (2–7) | 5 (2–7) | .90 |
| 72 | 3 (1–6) | 3 (1–6) | .62 |
| SAPS II score 0 |
44.8 (18.4) |
44.1 (17.3) |
.69 |
| 24 | |||
| 48 | |||
| 72 | 33.4 (14.1) | 34.6 (17.2) | .54 |
| MEDS score 0 |
10.9 (3.9) |
10.6 (3.4) |
.46 |
| 24 | |||
| 48 | |||
| 72 | 8.4 (4.2) | 8.4 (4.5) | .93 |
| Glasgow coma scale 0 |
13 (4.1) |
13 (3.7) |
.67 |
| 24 | 12 (4.3) | 12 (3.9) | .68 |
| 48 | 13 (3.7) | 13 (3.5) | .91 |
| 72 | 15 (3.1) | 14 (4.0) | .04 |
Abbreviations: IQR, interquartile range; MEDS, Mortality in Emergency Department Sepsis; SAPS, Simplified Acute Physiology Score; ScvO2, central venous oxygen saturation; SOFA, Sequential Organ Failure Assessment.
SI conversion factor: to convert lactate concentration from mg/dL to mmol/L, multiply by 0.111.
Values represent the mean (SD) measurements at enrollment (0) and the most abnormal values at each hour of measurement, except for the SOFA score. Lactate values and SAPS and MEDS scores were not recorded at 24 and 48 hours.
Continuous data are compared using an unpaired t test; categorical variables, using the χ2 test.
Lactate levels at 72 hours represent worst value over the initial 72 hours of hospitalization.
There were no differences in the administered treatments through the initial 72 hours of hospitalization as shown in Table 4. During the emergency department–based 6-hour resuscitation period, patients received approximately 4.5 L of crystalloid, 221 patients (74%; 95% confidence interval [CI], 68%–79%) required vasopressors for hypotension, and 79 patients (26%; 95% CI, 21%–32%) required mechanical ventilation. Notably, only 29 patients (10%; 95% CI, 7%–14%) required either dobutamine infusion or packed red blood cell transfusion during the initial 6 hours of treatment. Activated protein C administration was only administered in 5 patients (2%; 95% CI, 1%–4%).
Table 4.
Administered Treatments and Resuscitation Goals
| Intervention, h | No. (%) of Patients | P Valuea | |
|---|---|---|---|
| Lactate Clearance Group (n = 150) | ScvO2 Group (n = 150) | ||
| Crystalloid volume, mean (SD), L 0-<6 |
4.5 (2.36) |
4.3 (2.21) |
.55 |
| 6–72 | 12.4 (6.15) | 11.8 (6.41) | .44 |
| Vasopressor administration 0-<6 |
108 (72) |
113 (75) |
.60 |
| 6–72 | 100 (67) | 108 (72) | .45 |
| Dobutamine administration 0-<6 |
5 (3) |
8 (5) |
.57 |
| 6–72 | 10 (7) | 13 (9) | .66 |
| PRBC transfusion 0-<6 |
11 (7) |
5 (3) |
.20 |
| 6–72 | 35 (23) | 31 (21) | .78 |
| Mechanical ventilation 0-<6 |
40 (27) |
39 (26) |
.99 |
| 6–72 | 69 (46) | 75 (50) | .56 |
| Activated protein C 0-<6 |
0 |
0 |
|
| 6–72 | 3 (2) | 2 (1) | .68 |
| Parenteral corticosteroids 0-<6 |
18 (12) |
26 (17) |
.25 |
| 6–72 | 59 (39) | 51 (34) | .40 |
Abbreviations: PRBC, packed red blood cell; ScvO2, central venous oxygen saturation.
Continuous variables are compared using unpaired t test; categorical variables, using χ2 test except activated protein C which was analyzed using Fisher exact test.
Among the 294 (147 per group) patients included in the per-protocol analysis, the central venous pressure goal was achieved in 133 patients (91%; 95% CI, 85%–95%) in the lactate clearance group and 133 (91%; 95% CI, 85%–95%) in the ScvO2 group (P =.99); the mean arterial pressure goal was achieved in 142 patients (97%; 95% CI, 92%–99%) in the lactate clearance group and 142 (97%; 95% CI, 92%–99%) in the ScvO2 group (P=.99); and the lactate clearance goal was met in 139 patients (95%; 95% CI, 90%–98%) in the lactate clearance group and the ScvO2 goal was met in 136 (93%; 95% CI, 87%–96%) patients in the ScvO2 group (P=.67). The median time from patient triage in the emergency department to first antibiotic administration was 115 (IQR, 62–180) minutes in the lactate clearance group and 115 (IQR, 66–170) minutes in the ScvO2 group(P =.98).
The primary and secondary study outcome analysis is outlined in Table 5. In the intent-to-treat analysis the inhospital mortality rate was 17% (25 of 150 [95% CI, 11%–24%]) in the lactate clearance group compared with 23% (34 of 150 [95% CI, 17%–30%]) in the ScvO2 group. The difference in these mortality rates was 6% (95% CI, −3% to 15%). The lower limit of this CI is well above the −10% predefined non-inferiority threshold, confirming the primary hypothesis of noninferiority between the lactate clearance and ScvO2 groups for in-hospital mortality. These results did not change substantially in the per-protocol analysis.
Table 5.
Hospital Mortality and Length of Stay
| Variable | Lactate Clearance Group (n = 150) | ScvO2 Group (n = 150) | Proportion Difference (95% Confidence Interval) | P Valueb |
|---|---|---|---|---|
| In-hospital mortality, No. (%)a Intent to treat |
25 (17) |
34 (23) |
6 (−3 to 15) |
|
| Per protocol | 25 (17) | 33 (22) | 5 (−3 to 14) | |
| Length of stay, mean (SD), d ICU |
5.9 (8.46) |
5.6 (7.39) |
.75 |
|
| Hospital | 11.4 (10.89) | 12.1 (11.68) | .60 | |
| Hospital complications Ventilator-free days, mean (SD) |
9.3 (10.31) |
9.9 (11.09) |
.67 |
|
| Multiple organ failure, No. (%) | 37 (25) | 33 (22) | .68 | |
| Care withdrawn, No. (%) | 14 (9) | 23 (15) | .15 |
Abbreviations: ICU, intensive care unit; ScvO2, central venous oxygen saturation.
Primary study end point.
Continuous data are compared using an unpaired t test; categorical variables, using the χ2 test.
There were no differences in the observed rates of predefined protocol-related serious adverse events between the lactate clearance (9 of 150 [6%; 95% CI, 3%–11%]) and ScvO2 (11 of 150 [7%; 95% CI, 4%–13%]) groups (P=.81).
COMMENT
The results of this large multicenter randomized controlled trial of 2 resuscitation protocols for early sepsis resuscitation indicate that a protocol targeting lactate clearance of at least 10% as evidence of adequate tissue oxygen delivery produces a similar short-term survival rate as a protocol using ScvO2 monitoring. Patients in the group resuscitated to a lactate clearance of 10% or higher had 6% lower in-hospital mortality than those resuscitated to an ScvO2 of at least 70% (95% CI for this difference, −3% to 15%) exceeding the −10% predefined noninferiority threshold. These data support the substitution of lactate measurements in peripheral venous blood as a safe and efficacious alternative to a computerized spectrophotometric catheter in the resuscitation of sepsis. To our knowledge, this is the largest randomized trial of emergency department–based early quantitative resuscitation for sepsis conducted to date and the first such trial to investigate the relative value of different goals of early, emergency department–resuscitation strategies.
The physiological basis for lactate clearance presumes that circulatory shock causes inadequate oxygen delivery, resulting in mitochondrial hypoxia. Under hypoxic conditions, mitochondrial oxidative phosphorylation fails, and energy metabolism becomes dependent on anaerobic glycolysis.26 Anaerobic glycolysis sharply increases the production of cellular lactate, which diffuses into the blood during prolonged cell hypoxia. In patients with a clinical picture of severe infection, the blood lactate concentration varies in proportion to the ongoing deficit in tissue oxygenation, and the ability of the patient to reduce the blood lactate concentration indicates restoration of oxygen delivery with resuscitation.27 Previous work has found that a lactate clearance of 10% or more predicts survival from septic shock, providing the rationale for this goal.15,16 In addition, we constructed the protocol to consider 2 normal lactate levels (≤18 mg/dL [2 mmol/L]) at least 2 hours apart as evidence of ongoing adequate tissue oxygenation. The rationale for including this criterion was that clinically it would make no sense to attempt to clear a value that is already normal and that 2 normal values provide a reasonable clinical signal that effective resuscitation has prevented worsening of tissue oxygenation and anaerobic metabolism.
We have previously documented that many clinicians perceive a significant degree of technical difficulty associated with the use of computerized spectrophotometric catheters to monitor ScvO2.28 These devices require equipment and expertise that are not available in many tertiary care emergency departments.13 Use of ScvO2 monitoring catheters requires preplanned training and real-time calibration and troubleshooting that can divert attention from the patient.28 We thus submit that the need exists for a simpler and more generalizable method to monitor the adequacy of tissue oxygen delivery as a research imperative in the treatment of patients with severe infection. Our results address this un-met need by providing data that justify the use of lactate clearance instead of continuous ScvO2 monitoring.
The sample size assumed a mortality rate of 25% in the active comparator (ScvO2) group. The observed mortality was 23% in the ScvO2 group, indicating that we maintained approximately 80% power to detect a true difference. Although our observed overall mortality rate (20%) is lower than the 37% overall rate reported in the sentinel emergency department–based resuscitation study,9 it is nearly identical to mortality rates that both individual investigators from our group22–24 and others29 have previously reported in effectiveness studies conducted in more heterogeneous populations. We believe that that the mortality rate represents a contemporaneous and accurate estimate of the true mortality rate for patients with severe sepsis and septic shock treated with an early quantitative resuscitation protocol in the emergency department.
Several limitations of our study warrant discussion. By its design, the groups could not be blinded, allowing for possible treatment bias. Our protocol was designed with safeguards to minimize this potential effect. For example, every participant received identical central venous catheters so that group assignment would not be easily identifiable. Also, investigators involved with the study were not allowed to provide care for the participants or influence their care in the ICU. Second, we did not have a method to assess whether an indicated therapeutic action was performed in response to a parameter below the intended goal (eg, if central venous pressure was 4 mm Hg, we did not record whether a fluid bolus was given). Rather, we only assessed for compliance with individual treatment goals during the study treatment period. Third, this study was conducted at 3 institutions that had established emergency department–based quantitative resuscitation programs for sepsis prior to initiation of the study. Therefore, our results may not be generalizable to centers that do not routinely perform early quantitative resuscitation. Fourth, if other influences on care were ignored, it could be suggested that the potential difference in protocol actions directly attributable to using lactate clearance vs ScvO2 was small, because only 10% of patients went on to receive dobutamine or packed red blood cell transfusion. Fifth, we did not have a mechanism to query ICU admission for potentially missed cases; thus, we may have missed patients who met criteria because a clinical alert was not activated. Finally, it is possible that knowledge of the study prompted clinical care providers to have a heightened awareness and provide differential treatment patterns (ie, a Hawthorne-like effect).
In conclusion, in this randomized trial, we found no difference in mortality for patients with severe sepsis and septic shock resuscitated with a protocol that used lactate clearance compared with a protocol that used ScvO2 as the method of measuring total body oxygen metabolism.
Acknowledgments
Funding/Support: This project was supported by grant GM76652 from the National Institutes of Health (NIH), National Institute of General Medical Sciences (Dr Jones). Dr Trzeciak was supported by grant GM83211 from the NIH. Dr Shapiro was supported by grants HL091757 and GM076659 from the NIH.
Role of the Sponsors: The sponsor of this study reviewed and approved the study design but had no role in the conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the article.
Footnotes
Caring for the Critically Ill Patient Section Editor: Derek C. Angus, MD, MPH, Contributing Editor, JAMA (angusdc@upmc.edu).
Trial Registration clinicaltrials.gov Identifier: NCT00372502
Online-Only Material: The eFigure is available at http://www.jama.com.
Additional Contributions: We thank J. Lee Garvey, MD, who functioned as the study monitor; Jackeline Hernandez, MD, and Nikita Young, BS (all from Carolinas Medical Center, Charlotte, North Carolina), and David Lundy, MD (Cooper University Hospital, Camden, New Jersey), for their invaluable assistance with monitoring the study and enrolling patients; Charlie Johnson, BS (paid consultant from Studymaker LLC, Newton, Massachusetts) for assistance with database management. We also thank R. Phillip Dellinger, MD (Cooper University Hospital, Camden, New Jersey), for his thoughtful critique of this report and who received no remuneration. We are indebted to the emergency department and ICU nurses and physicians at Carolinas Medical Center, Beth Israel Deaconess Medical Center, and Cooper University Hospital who willingly assisted in the care of the study patients. Finally, we humbly express our deepest gratitude to the patients who so willingly participated in this study.
Author Contributions: Dr Jones had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Jones, Shapiro, Trzeciak, Kline.
Acquisition of data: Jones, Shapiro, Trzeciak, Arnold, Claremont, Kline.
Analysis and interpretation of data: Jones, Shapiro, Trzeciak, Kline.
Drafting of the manuscript: Jones, Kline.
Critical revision of the manuscript for important intellectual content: Jones, Shapiro, Trzeciak, Arnold, Claremont, Kline.
Statistical analysis: Jones, Kline.
Obtained funding: Jones, Kline.
Administrative, technical, or material support: Jones, Shapiro, Arnold, Claremont, Kline.
Study supervision: Jones, Kline.
Financial Disclosures: Dr Jones reports receiving research support from Critical Biologics Corp and Hutchinson Technology and having served on an advisory board for Brahms and Siemens in 2009. Dr Trzeciak reports that he receives research support from Ikaria, Novo Nordisk, serves as a consultant to Spectral Diagnostics, and has received 3 honoraria from Edwards Lifesciences prior to 2005, but has not received any personal remuneration from any commercial interest since 2005. Dr Shapiro reports that he has received research support from Biosite, Hutchinson Technology, and Eli Lilly. Dr Kline reported that he is the inventor on US patent 7,083,574, a breath-based device that monitors patient response to resuscitation. Dr Arnold and Ms Claremont reported no financial conflicts.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 4.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007;35(8):1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Carlet JM, et al. International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses; American College of Chest Physicians; American College of Emergency Physicians; Canadian Critical Care Society; European Society of Clinical Microbiology and Infectious Diseases; European Society of Intensive Care Medicine; European Respiratory Society; International Sepsis Forum; Japanese Association for Acute Medicine; Japanese Society of Intensive Care Medicine; Society of Critical Care Medicine; Society of Hospital Medicine; Surgical Infection Society; World Federation of Societies of Intensive and Critical Care Medicine. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 6.Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94(6):1176–1186. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- 7.Jones AE, Brown MD, Trzeciak S, et al. Emergency Medicine Shock Research Network Investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med. 2008;36(10):2734–2739. doi: 10.1097/CCM.0b013e318186f839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollenberg SM, Ahrens TS, Annane D, et al. Practice parameter for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med. 2004;32(9):1928–1948. doi: 10.1097/01.ccm.0000139761.05492.d6. [DOI] [PubMed] [Google Scholar]
- 9.Rivers E, Nguyen B, Havstad S, et al. Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 10.Peake S, Webb S, Delaney A. Early goal-directed therapy of septic shock: we honestly remain skeptical. Crit Care Med. 2007;35(3):994–995. doi: 10.1097/01.ccm.0000257481.37623.3b. [DOI] [PubMed] [Google Scholar]
- 11.Ho BC, Bellomo R, McGain F, et al. The incidence and outcome of septic shock patients in the absence of early-goal directed therapy. Crit Care. 2006;10(3):R80. doi: 10.1186/cc4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perel A. Bench-to-bedside review: the initial hemodynamic resuscitation of the septic patient according to Surviving Sepsis Campaign guidelines—does one size fit all? Crit Care. 2008;12(5):223. doi: 10.1186/cc6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones AE, Kline JA. Use of goal-directed therapy for severe sepsis and septic shock in academic emergency departments. Crit Care Med. 2005;33(8):1888–1889. doi: 10.1097/01.ccm.0000166872.78449.b1. [DOI] [PubMed] [Google Scholar]
- 14.Carlbom DJ, Rubenfeld GD. Barriers to implementing protocol-based sepsis resuscitation in the emergency department—results of a national survey. Crit Care Med. 2007;35(11):2525–2532. doi: 10.1097/01.ccm.0000298122.49245.d7. [DOI] [PubMed] [Google Scholar]
- 15.Arnold RC, Shapiro NI, Jones AE, et al. Multi-center study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock. 2009;32(1):35–39. doi: 10.1097/shk.0b013e3181971d47. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen HB, Rivers EP, Knoblich BP, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32(8):1637–1642. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 17.Bone RC, Balk RA, Cerra FB, et al. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro NI, Fisher C, Donnino M, et al. The feasibility and accuracy of point-of-care lactate measurement in emergency department patients with suspected infection [published online ahead of print September 1, 2009] J Emerg Med. doi: 10.1016/j.jemermed.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeGall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 20.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro NI, Wolfe RE, Moore RB, Smith E, Burdick E, Bates DW. Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31(3):670–675. doi: 10.1097/01.CCM.0000054867.01688.D1. [DOI] [PubMed] [Google Scholar]
- 22.Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132(2):425–432. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trzeciak S, Dellinger RP, Abata NL, et al. Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest. 2006;129(2):225–232. doi: 10.1378/chest.129.2.225. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro NI, Howell MD, Talmor D, et al. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med. 2006;34(4):1025–1032. doi: 10.1097/01.CCM.0000206104.18647.A8. [DOI] [PubMed] [Google Scholar]
- 25.Agency for Healthcare Research and Quality Healthcare. [Accessed December 30, 2009];Cost and Utilization Project National Emergency Department Sample. 2006 http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=CA7BFB8F2750623A&Form=SelCROSSTAB&JS=Y&Action=%3E%3ENext%3E%3E&_Oneway=Yes.
- 26.Watts JA, Kline JA. Bench to bedside: the role of mitochondrial medicine in the pathogenesis and treatment of cellular injury. Acad Emerg Med. 2003;10(9):985–997. doi: 10.1111/j.1553-2712.2003.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 27.Weil MA, Afifi AA. Experimental and clinical studies on lactate and pyruvate as indicators of the severity of acute circulatory failure (shock) Circulation. 1970;41(6):989–1001. doi: 10.1161/01.cir.41.6.989. [DOI] [PubMed] [Google Scholar]
- 28.Jones AE, Shapiro NI, Roshon M. Implementing early goal-directed therapy in the emergency setting: the challenges and experiences of translating research innovations into clinical reality in academic and community settings. Acad Emerg Med. 2007;14 (11):1072–1078. doi: 10.1197/j.aem.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. 2007;35(4):1105–1112. doi: 10.1097/01.CCM.0000259463.33848.3D. [DOI] [PubMed] [Google Scholar]