Abstract
Objective
To identify effective chemotherapy regimens against uterine-serous-papillary-carcinoma (USPC).
Study Design
Six UPSC, half of which overexpress HER-2/neu at 3+ level, were evaluated for growth-rate and in-vitro-sensitivity to fourteen single-agent chemotherapies and five combinations by ChemoFx (Precision Therapeutics, Pittsburgh, PA).
Results
Cell lines overexpressing HER-2/neu showed higher proliferation when compared to low HER-2/neu-expressing cell lines and a lower half-maximum inhibitory concentration (IC50) when exposed to the majority of single-agent chemotherapies. High HER-2/neu-expressors were more sensitive to platinum compounds, manifesting a 5.22-fold decrease in carboplatin-IC50 (P=0.005) and a 5.37-fold decrease in cisplatin-IC50 (P=0.02). When all cell lines were analyzed as a group, chemotherapy agents tested demonstrated lower IC50s when used in combination than as individual agents.
Conclusions
USPC overexpressing HER-2/neu display greater in-vitro-sensitivity to platinum-compounds when compared to low HER-2/neu-expressors. Higher proliferative capability rather than increased drug resistance may be responsible for the adverse prognosis associated with HER-2/neu overexpression in USPC.
Keywords: Endometrial neoplasms, uterine serous tumors, HER2/neu, chemosensitivity, chemoresistance
Introduction
Endometrial cancer is the most common female genital tract malignancy in the United States, with an incidence of 42,160 new cases and 7,780 deaths annually1. Two types of endometrial carcinoma, namely Type I and Type II tumors, have been described, based on both clinical and histopathological variables2. Type I endometrial cancers, which account for the majority (about 80%) of cases, are usually well or moderately-differentiated and endometrioid in histology. These neoplasms are frequently diagnosed in younger women, are associated with a history of hyperestrogenism as the main risk factor, and typically have a favorable prognosis with appropriate therapy. In contrast, Type II endometrial cancers are poorly differentiated tumors, or tumors with serous papillary or clear cell histology. Although Type II tumors account for only a minority of endometrial cancers, most recurrences and deaths occur in this group of patients.
Uterine serous papillary adenocarcinoma (USPC), which accounts for about 10% of endometrial cancers, has a propensity for early intraabdominal and lymphatic spread even at presentation and is characterized by a highly aggressive biologic behavior3. Unlike the histologically similar high-grade ovarian cancer, USPC is a chemoresistant disease from onset, with in vivo responses to combined cisplatin-based chemotherapy in the order of 20% and of short duration4–6. Our group has recently reported HER-2/neu overexpression by IHC and amplification of the c-erbB2 gene by FISH in a large percentage of patients harboring USPC7–9. These findings, recently confirmed by other groups10 including the Gynecologic Oncology Group (GOG) in a cooperative multicentric study11, have identified HER-2/neu overexpression in USPC as an independent variable associated with poor outcome, and one that occurs more frequently in African-American women than in Caucasian women7–9.
Although HER-2/neu overexpression has been previously associated with resistance to chemotherapy and poor survival in multiple human malignancies including breast12, ovarian13 and endometrial carcinoma14, to our knowledge, no study has carefully evaluated the in vitro chemo-sensitivity/resistance of this highly aggressive variant of endometrial cancer. To fill this gap in knowledge, we used the ChemoFx (Precision Therapeutics, Inc., Pittsburgh, PA) to analyze the in vitro sensitivity of six primary UPSC cell lines recently established and characterized in our laboratory15 to fourteen standard single-agent chemotherapies and five combination chemotherapies16. Half the cell lines selected overexpress HER-2/neu at 3+ levels and harbor amplification of the c-erbB2 oncogene by FISH. Surprisingly, although in vivo HER-2/neu overexpression is correlated with a more aggressive disease in patients with UPSC8,9, growth-inhibition data suggest that these tumors display greater in vitro chemosensitivity to platinum compounds and higher proliferative capability when compared to HER-2/neu-negative tumors.
Materials and Methods
Establishment and HER-2/neu expression of USPC Cell Lines
Primary USPC tumor cell lines from six patients with invasive USPC were obtained from fresh tumor biopsies collected at the time of surgery, under approval of the Institutional Review Board. Tumors were staged according to the International Federation of Gynecologists and Obstetricians operative staging system. Six primary USPC cell lines (USPC ARK-1, USPC ARK-2, USPC ARK-3, USPC ARK-4, USPC ARK-5, and USPC ARK-6) were established after sterile processing of the tumor samples from surgical biopsies as described previously15. Source-patient characteristics of these six USPC cell lines are described in Table 1. The amplification of the c-erbB2 gene by FISH, expression levels of HER-2/neu receptor by immunohistochemistry (IHC) and mRNA expression levels by quantitative RT-PCR for these primary USPC cell lines have been recently reported15 and are presented in Table 2.
Table 1.
Patient characteristics from which the six USPC cell lines were established
| Patient | Age (years) | Race* | FIGO^ Stage |
USPC Histopathology |
Year of Diagnosis |
|---|---|---|---|---|---|
| USPC ARK-1 | 62 | AA | IVA | Pure | 1997 |
| USPC ARK-2 | 63 | AA | IVB | Pure | 1998 |
| USPC ARK-3 | 59 | AA | IVB | Mixed | 2006 |
| USPC ARK-4 | 73 | C | IVB | Pure | 2004 |
| USPC ARK-5 | 73 | AA | IIIC | Pure | 2006 |
| USPC ARK-6 | 62 | C | IB | Mixed | 2005 |
AA, African American; C, Caucasian;
FIGO, International Federation of Gynecology and Obstetrics
Table 2.
HER-2/neu expression in primary USPC cell lines
| Sample | RT-PCR° | ||
|---|---|---|---|
| IHC* | FISH^ | mRNA Copy Number | |
| Control | 1 | ||
| USPC ARK-1 | 3+ | 2.5 | 373 |
| USPC ARK-2 | 3+ | 5.2 | 607 |
| USPC ARK-3 | 3+ | 4.7 | 677 |
| USPC ARK-4 | 0 | 1.6 | 7 |
| USPC ARK-5 | 0 | 1.4 | 13 |
| USPC ARK-6 | 1+ | 0.9 | 6 |
IHC, Immunohistochemistry;
FISH, Fluorescent In-situ Hybridization;
RT-PCR, Real-time Polymerase Chain Reaction.
Primary UPSC growth rate analysis
The growth rate of each of the UPSC cell lines was determined by counting the number of live cells 24, 48, 72 and 96 hours after plating. In brief, cell lines were cultured in RPMI 1640 (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (Hyclone, Logan, Utah). When cultures had grown to approximately 80% confluence, cells were harvested from the flask using 0.25% Trypsin EDTA (Invitrogen, Carlsbad, CA), then counted on a hemacytometer chamber and assessed for viability via trypan blue exclusion. Cell density was adjusted to a concentration of 8,000 cells/mL, then cells were seeded into a 384-well microtiter plate (Corning Life Sciences, Lowell, MA) at a density of 320 cells per well. Twenty-two replicate wells were plated per cell line per time point. Cell plates were incubated at 37°C with 5% CO2 for 24, 48, 72, and 96 hours, at which time they were removed from the incubator, fixed with 95% ethanol (FisherScientific, Pittsburgh, PA) then stained with DAPI (Molecular Probes). When DAPI staining was complete, cell plates were scanned on an automated fluorescent imaging system, and the number of cells in each well was counted.
Chemoresponse assay
The chemoresponse assay was performed as previously described16,17. The chemotherapy agents, concentrations and combination used in the assays are described in Table 3. Briefly, cell lines were cultured in RPMI 1640 (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (Hyclone, Logan, Utah). When cultures had grown to approximately 80% confluence, cells were harvested from the flask using 0.25% Trypsin EDTA (Invitrogen, Carlsbad, CA), then counted on a hemacytometer chamber and assessed for viability via trypan blue exclusion. Cell density was adjusted to a concentration of 8,000 cells/mL. Then cells were seeded into a 384-well microtiter plate (Corning Life Sciences, Lowell, MA) at a density of 320 cells per well. Cell plates were incubated at 37°C with 5% CO2 overnight. On the next day, serial dilutions of each anticancer agent or combination of two agents were prepared in growth medium to create 8, 9 or 10 distinct testing concentrations. In total, fourteen single agents were tested as well as five combinations of two agents. Due to the limited amounts of cells available for testing for USPC-ARK-5 primary cell line, six of the fourteen single agents were not tested (i.e., cyclophosphamide, fluorouracil, ifosfamide, vinblastine, vincristine, and vinorelbine) nor any of the combinations. Three replicates of each dose of agent or combination were applied to each cell line. The cell plates were incubated for an additional 72 hours after treatment, at which time they were removed from the incubator, fixed with 95% ethanol (FisherScientific, Pittsburgh, PA) then stained with DAPI (Molecular Probes). When DAPI staining was complete, cell plates were scanned on an automated fluorescent imaging system, and the number of surviving cells in each well was counted, and compared to a control population not exposed to any agent.
Table 3.
Doses of chemotherapy agents
| Chemotherapy | Lowest Dose |
Highest Dose |
Units | Number of Concentrations |
|---|---|---|---|---|
| Carboplatin | 0.98 | 500 | µM | 10 |
| Cisplatin | 0.2 | 50 | µM | 8 |
| Cyclophosphamide | 0.33 | 14 | µM | 9 |
| Docetaxel | 0.1 | 25 | nM | 9 |
| Doxorubicin | 2.34 | 1200 | nM | 9 |
| Etoposide | 2.867 | 200 | µM | 9 |
| Fluorouracil | 0.2 | 50 | µM | 8 |
| Gemcitabine | 0.73 | 50 | nM | 9 |
| Ifosfamide | 0.2 | 100 | µM | 9 |
| Paclitaxel | 0.2 | 100 | nM | 9 |
| Topotecan | 0.78 | 200 | nM | 8 |
| Vinblastine | 0.15 | 10 | nM | 9 |
| Vincristine | 0.98 | 125 | nM | 8 |
| Vinorelbine | 0.42 | 29.41 | nM | 9 |
|
Carboplatin & Docetaxel |
0.98 | 500 | µM | 9 |
| 0.1 | 25 | nM | ||
|
Carboplatin & Doxorubicin |
0.98 | 500 | µM | 9 |
| 2.34 | 1200 | nM | ||
|
Carboplatin & Gemcitabine |
0.98 | 125 | µM | 8 |
| 0.73 | 19.53 | nM | ||
|
Carboplatin & Paclitaxel |
0.98 | 250 | µM | 9 |
| 0.2 | 50 | nM | ||
|
Carboplatin & Topotecan |
0.98 | 250 | µM | 9 |
| 0.39 | 100 | nM |
Statistical Analysis
For each cell line, the IC50 for a given drug was determined from the dose-response curve fit to the cell-count data using XLFit program (ID Business Solutions, Parsippany, NJ, USA). The Lavenburg-Marquardt algorithm was used to obtain and calculate the IC50 and IC50 curves for each drug and drug combination in each cell line using a non-linear regression-curve fit. To facilitate statistical inference on IC50 fold changes, IC50s were transformed to log10 units. Differences in log10(IC50)s between high and low HER-2/neu expressors were assessed for significance via unequal-variance t-test, while cell-line differences in log10(IC50)s for agents used singly versus in combination were assessed for significance via paired t-test. Fold changes in IC50s were calculated as 10 raised to the power of the differences in log10(IC50)s. Differences were considered significant at p values < 0.05. All statistical analysis was conducted using Excel 2007 (Microsoft corporation, Redmond, WA).
Results
Growth Curve
The doubling time of all 6 primary USPC cell lines was evaluated by counting the number of live cells 24, 48, 72, and 96 hours after plating as described in the methods section. As shown in Table 4, population doubling times for UPSC ARK-1, UPSC ARK-2, UPSC ARK-3 (all high HER-2/neu expressors), and UPSC ARK-4 (a low HER-2/neu expressor) were similar, with the USPC cell lines exhibiting doubling times of 22.3 hours, 21.9 hrs, 22.1 hours and 21.1 hours, respectively. In contrast, a lower growth rate was detected in UPSC ARK-5 and UPSC ARK-6 cells (both low HER-2/neu expressors), with doubling times of 72 hours and 45.7 hours, respectively (Table 4).
Table 4.
Growth rate of primary USPC cell lines
| Cell Line | Mean Cell Count | ||||
|---|---|---|---|---|---|
| Time 0 | 24 Hours | 48 Hours | 72 Hours | 96 Hours | |
| UPSC ARK-1 | 320 | 402.64 | 1357.14 | 3735.36 | 6269.05 |
| UPSC ARK-2 | 320 | 490.14 | 1802.86 | 4038.86 | 6561.27 |
| UPSC ARK-3 | 320 | 635.09 | 1968.32 | 3956.36 | 6330.41 |
| UPSC ARK-4 | 320 | 594.41 | 2072.14 | 5200.27 | 7399.00 |
| UPSC ARK-5 | 320 | 359.61 | 398.33 | 485.62 | 812.45 |
| UPSC ARK-6 | 320 | 337.14 | 520.23 | 564.64 | 1367.23 |
Chemoresponse assay
Single agent therapy
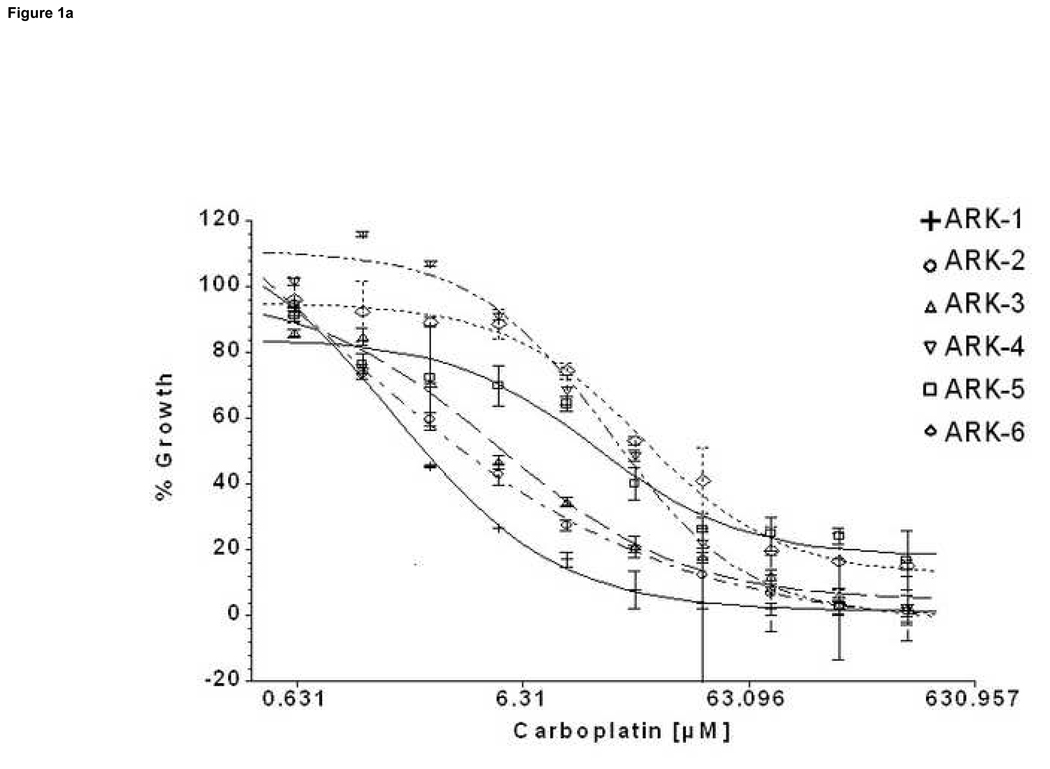
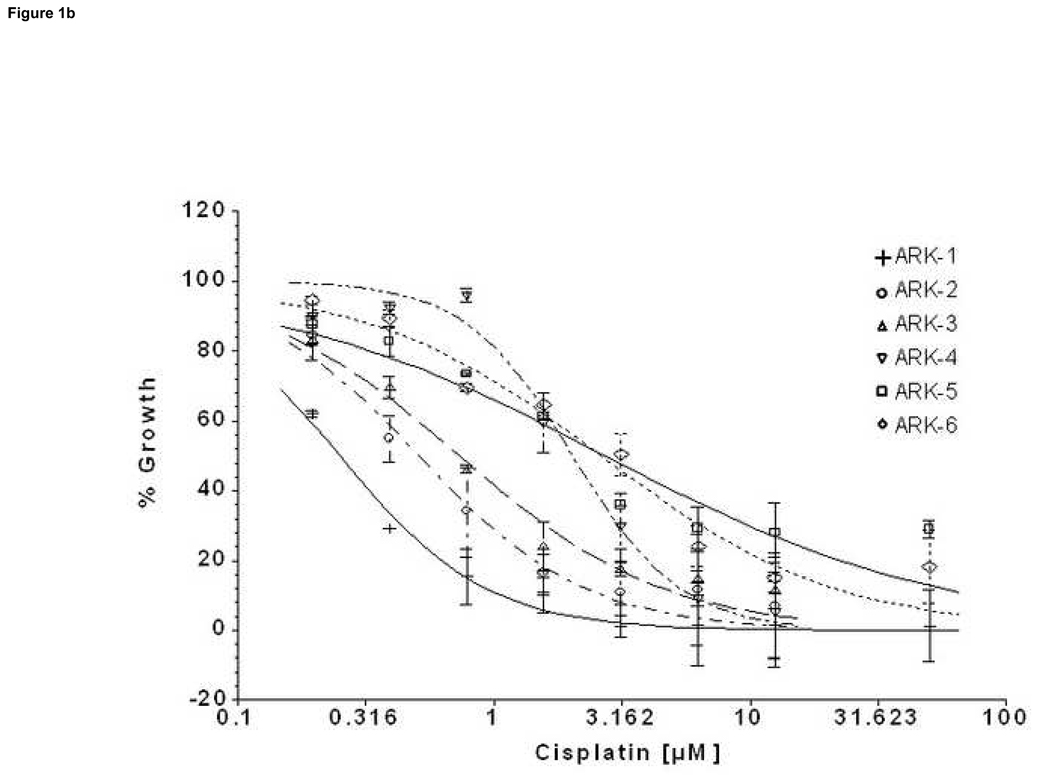
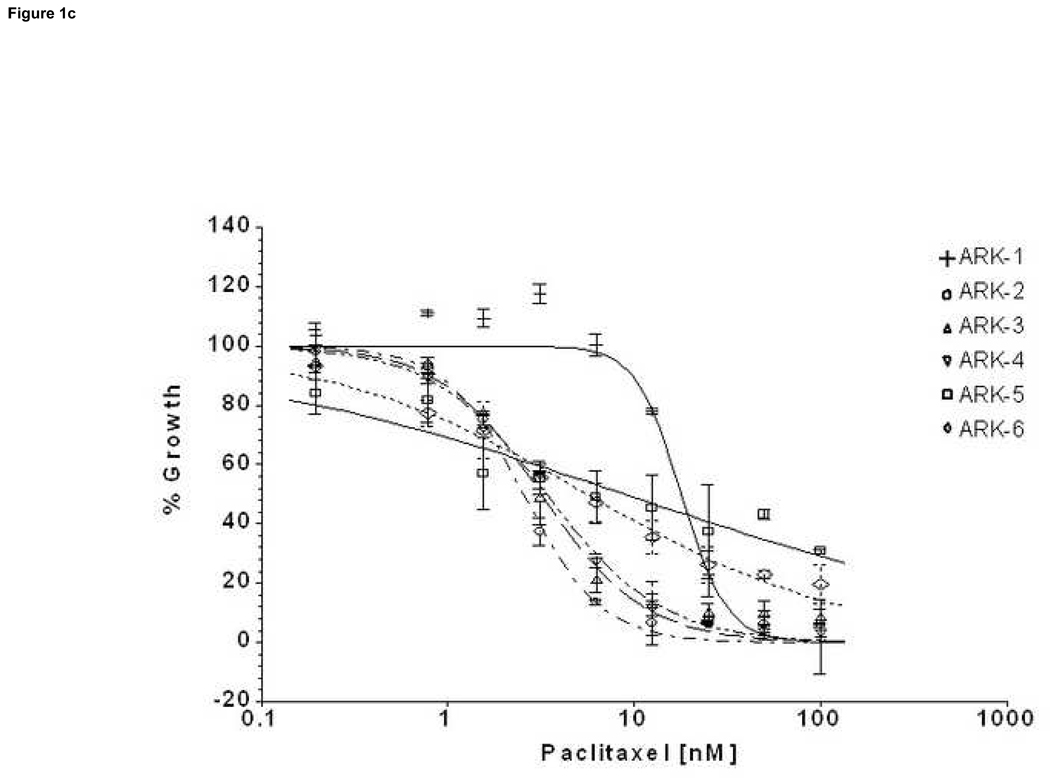
We compared by ChemoFx the effects of 14 single-agent chemotherapy drugs against 6 primary USPC cell lines expressing different levels of HER-2/neu using serial dilutions of each anticancer agent in growth medium to create 8, 9 or 10 distinct testing concentrations, as described in the methods section. A significant difference was observed in the IC50 of the high versus low HER-2/neu expressor USPC cell lines when treated with both carboplatin and cisplatin (Table 5, Figure 1a and 1b). Carboplatin log10(IC50)s showed an average ± standard deviation (SD) of 0.738 ± 0.173 among the HER-2/neu overexpressors (UPSC ARK-1, UPSC ARK-2 and UPSC ARK-3) versus 1.456 ± 0.120 among the low expressors (UPSC ARK-4, UPSC ARK-5 and UPSC ARK-6); the difference was significant (P=0.0059) and equivalent to a 5.22-fold decrease in carboplatin IC50 among high compared to low expressors. Similarly, cisplatin log10(IC50)s showed an average ± SD of only −0.345 ± 0.238 among overexpressors, compared to 0.385 ± 0.068 among low expressors, equivalent to a 5.37-fold decrease in cisplatin IC50 with overexpression (P=0.026). Importantly, the low HER-2/neu expressors, regardless of their fast or slow doubling times, were found to be highly resistant to both carboplatin and cisplatin (Figure 1a and 1b). When the sensitivity to other single agent drug was analyzed in the two groups of primary USPC cell lines, we found the average IC50s for the HER-2/neu overexpressors to be consistently less than those of the low HER-2/neu expressors for the majority of tested drugs, although this difference did not reach statistical significance (Table 5). Of interest, when paclitaxel was tested as single agent against the high and low HER-2/neu expressors, USPC-ARK-1 stood out as a highly resistant primary tumor cell line to the exposure to this agent (Figure 1c) as well as to multiple other microtubular inhibitor drug tested including docetaxel, vincristine and vinorelbine (data not shown).
Table 5.
Log10(IC50) values for HER-2/neu overexpressors versus low expressors treated with single-agent chemotherapies.
| Chemotherapy | mean±SD, log10(IC50) values | P† | Fold decrease‡ in IC50 |
|
|---|---|---|---|---|
| HER-2/neu negative | HER-2/neu positive | |||
| Carboplatin (µM) | 1.456±0.120 | 0.738±0.173 | 0.0059 | 5.22 |
| Cisplatin (µM) | 0.385±0.068 | −0.345±0.238 | 0.0262 | 5.37 |
| Cyclophosphamide (µM) | 0.852±0.417 | 0.257±0.041 | 0.2912 | 3.94 |
| Docetaxel (nM) | 0.631±0.384 | 0.460±0.093 | 0.5240 | 1.48 |
| Doxorubicin (nM) | 2.064±0.752 | 1.132±0.069 | 0.1639 | 8.55 |
| Etoposide (µM) | 0.025±0.478 | −0.479±0.098 | 0.2053 | 3.19 |
| Fluorouracil (µM) | 1.428±0.384 | 0.853±0.412 | 0.2309 | 3.76 |
| Gemcitabine (nM) | 1.291±0.490 | 0.602±0.062 | 0.1330 | 4.89 |
| Ifosfamide (µM) | 1.229±0.395 | 0.962±0.210 | 0.5061 | 1.85 |
| Paclitaxel (nM) | 0.741±0.209 | 0.721±0.464 | 0.9506 | 1.05 |
| Topotecan (nM) | 1.917±0.547 | 1.025±0.017 | 0.1056 | 7.80 |
| Vinblastine (nM) | 0.353±0.481 | 0.224±0.064 | 0.7692 | 1.35 |
| Vincristine (nM) | 1.191±1.281 | 0.699±0.333 | 0.6826 | 3.10 |
| Vinorelbine (nM) | 0.816±0.367 | 0.810±0.236 | 0.9858 | 1.01 |
P value, unequal-variance t-test.
Fold decrease (positive compared to negative) in IC50 (original units as shown), calculated as 10 to the power (difference in mean log10(IC50)s between groups).
Figure 1. Representative dose-response curves following exposure to carboplatin (a), cisplatin (b) and paclitaxel (c) of USPC primary cells with High versus Low HER-2/neu expression.
Survival was assessed by ChemoFx. Each point on the cell line graph represents the mean of 8 to 10 estimations ± SE. Average IC50 values, for each the cell lines are reported in Table 5.
Drug combinations
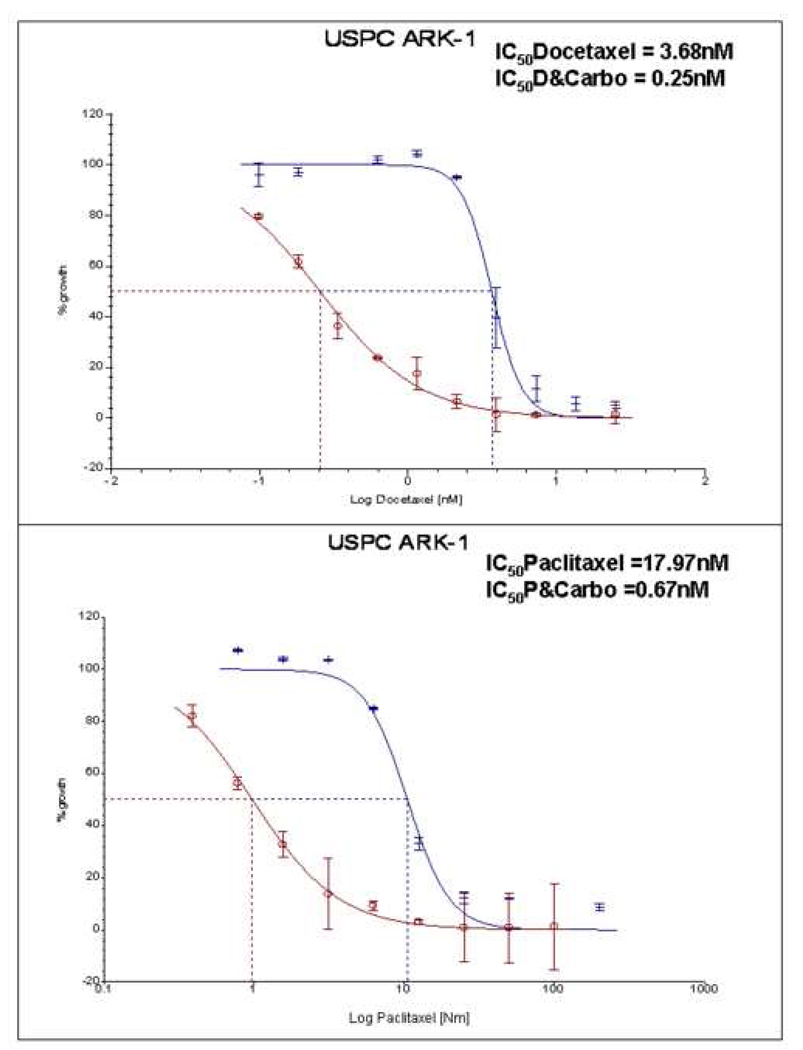
Combinations of carboplatin with paclitaxel, doxorubicin, gemcitabine, topotecan or docetaxel were evaluated in five USPC cell lines. With all USPC cell lines considered as a group, the chemotherapy agents demonstrated lower IC50s when used in combination than as individual agents (Table 6 a,b,c,d,e). The change in IC50 reached statistical significance for each of these agents when combined with carboplatin (Table 6 a,b,c,d,e). In all combinations used, the IC50 of carboplatin changed less when compared to carboplatin as a single agent, suggesting that carboplatin’s effect on the combination IC50 was greater than that of each of the other agents with which it was combined (Table 6 a,b,c,d,e). Importantly, as representatively shown for USPC ARK-1 cell line (Figure 2), a tumor overexpressing HER-2/neu found sensitive to platinum compounds but highly resistant to paclitaxel (Figure 1c) as well as to multiple microtubular inhibitors when used as single agents, the combination of carboplatin with paclitaxel, docetaxel, gemcitabine or topotecan, yielded a significantly lower IC50 for each of these drugs when used in combination with carboplatin (Figure 2).
Table 6.
Log10(IC50) values for USPC primary cell lines treated with drug combinations.
| A: Decreases in Log10 IC50 for Carboplatin and Docetaxel when used in combination | ||||||
|---|---|---|---|---|---|---|
| Carboplatin | Docetaxel | Combination | Difference | P† | Fold Decrease in IC50‡ |
|
| mean±SD* | 1.048±0.444 | 0.923± 0.405 | 0.125±0.128 | 0.0934 | 1.33 | |
| 0.493±0.263 | −0.178±0.359 | 0.672±0.346 | 0.0122 | 4.70 | ||
| B: Decreases in Log10 IC50 for Carboplatin and Doxorubicin when used in combination | ||||||
| Carboplatin | Doxorubicin | Combination | Difference | P† |
Fold Decrease in IC50 ‡ |
|
| mean±SD* | 1.048±0.444 | 0.675± 0.516 | 0.373±0.371 | 0.0878 | 2.36 | |
| 1.439±0.649 | 1.055±0.516 | 0.384±0.138 | 0.0034 | 2.42 | ||
| C: Decreases in Log10 IC50 for Carboplatin and Gemcitabine when used in combination | ||||||
| Carboplatin | Gemcitabine | Combination | Difference | P† |
Fold Decrease in IC50 ‡ |
|
| mean±SD* | 1.048±0.444 | 0.842± 0.491 | 0.206±0.166 | 0.0505 | 1.61 | |
| 0.851±0.480 | 0.440±0.333 | 0.411±0.210 | 0.0119 | 2.58 | ||
| D: Decreases in Log10 IC50 for Carboplatin and Paclitaxel when used in combination | ||||||
| Carboplatin | Paclitaxel | Combination | Difference | P† |
Fold Decrease in IC50 ‡ |
|
| mean±SD* | 1.048±0.444 | 0.771± 0.201 | 0.277±0.270 | 0.0835 | 1.89 | |
| 0.688±0.340 | 0.072±0.201 | 0.616±0.455 | 0.0389 | 4.13 | ||
| E: Decreases in Log10 IC50 for Carboplatin and Topotecan when used in combination | ||||||
| Carboplatin | Topotecan | Combination | Difference | P† |
Fold Decrease in IC50‡ |
|
| mean±SD* | 1.048±0.444 | 0.770± 0.359 | 0.278±0.179 | 0.0254 | 1.90 | |
| 0.334±0.553 | 0.372±0.359 | 0.961±0.251 | 0.0010 | 9.15 | ||
mean±SD of log10(IC50)s of agents used singly or in combination as shown, calculated for five USPC cell lines (ARK1, ARK2, ARK3, ARK4, and ARK6). Cell line ARK5 was excluded because it was not exposed to combinations.
P value, paired t-test.
Fold decrease in IC50 of agents in combination compared to when used as single agents. Fold decrease is calculated as 10 to the power of the mean difference in log10(IC50)s.
Figure 2. Representative dose-response curves following exposure to docetaxel and carboplatin + docetaxel (upper panel) versus paclitaxel and carboplatin + paclitaxel (lower panel) in USPC-ARK1 primary cell line.
Survival was assessed by ChemoFx. Each point on the cell line graph represents the mean of 8 to 10 estimations ± SE. Average IC50 values, for each of the cell lines when treated with the single agent or the combination are reported in Table 6.
Discussion
The treatment of advanced, metastatic and/or recurrent USPC no longer amenable to control with surgery or radiation therapy has not improved significantly with the advent of modern chemotherapy. Although the majority of USPC patients, similar to high grade ovarian cancer patients, are treated with multiple chemotherapy agents, the clinical responses to combined cisplatinum-based regimens remain low and of short duration4–6. A greater understanding of the chemosensitivity/chemoresistance profile of USPC and a better comprehension of the relationship between activation of an oncogene like c-erbB2 and drug sensitivity remains paramount.
The erbB2 gene encodes for HER-2/neu, a member of the erbB receptor tyrosine kinase family. This is a family of 4 transmembrane glycoproteins (erbB1, erbB2, erbB3, and erbB4) that are expressed on epithelial, mesenchymal, and neuronal cells18. ErbB receptors are activated in response to binding with multiple ligands produced in an autocrine fashion in the individual cells, or a paracrine fashion in the surrounding tissue. Ligand binding results in dimerization of the receptor, either with a twin receptor (homodimerization), or with one of its siblings (heterodimerization)18. This leads to phosphorylation of the intracellular tyrosine kinase residues, which serve as docking sites for various effectors and transcription factors that ultimately modulate various biological responses such as proliferation, survival, migration and differentiation18.
In a recent exploratory immunohistochemical analysis of HER-2/neu expression in advanced endometrial carcinoma performed by the GOG, HER-2/neu overexpression (either 2+ (moderate) or 3+ (strong)) was detected in 23 of 38 (61%) of the USPC tested11. These results are consistent with the HER-2/neu positivity previously reported by our own research group who found moderate-to-strong expression of HER-2/neu protein in 16 (62%) of 26 USPC samples evaluated7. High HER-2/neu expression levels in USPC have also been reported by Diane-Montez et al10, with 12 of the 25 (48%) USPC cases in the John Hopkins’ series demonstrating HER-2/neu overexpression. Of interest, in this study, a significant association of HER-2/neu overexpression with surgical staging was detected, with 81.8% advanced stage disease vs. 28.6% early stage disease showing HER-2/neu overexpression10. Importantly, while other studies have reported lower percentages of HER-2/neu overexpression in USPC patients19–20, in the majority, overexpression of the HER-2/neu oncogene has been associated with a shorter patient survival when compared to patients harboring tumors showing a normal amount of gene product7–10,19,20. While these reports suggest that overexpression of the HER-2/neu oncogene in USPC patients may be a marker for intrinsic multidrug resistance, as in other human cancers12–14, to our knowledge, no studies have yet analyzed the in vitro sensitivity/resistance of primary USPC cell lines to multiple cytotoxic drugs and whether overexpression of the HER-2/neu gene in these tumors may directly confer higher chemotherapy resistance.
We found USPC cell lines overexpressing HER-2/neu to have higher proliferation when compared to low HER-2/neu-expressing cell lines, and lower half maximum inhibitory concentration (IC50) when exposed to the majority of single-agent chemotherapies. Importantly, high HER-2/neu expressors were found to be significantly more sensitive than low HER-2/neu expressors to platinum compounds in vitro. With no exception, USPC cell lines showing low HER-2/neu expression, regardless of their fast or slow growth rate, were found to be significantly more resistant to platinum compounds when compared to high HER2/neu-expressor cell lines. Taken together, these results exclude the possibility that the different sensitivity to platinum compounds is secondary to a different growth rate in the two groups of USPC cell line studied, and also suggest that HER-2/neu overexpression results in increase in cell proliferation in vitro, potentially leading to a more rapid recovery from the cytotoxic effects of chemotherapeutic drugs in vivo.
These results were somewhat unexpected considering the abundant in vivo data showing worse prognosis in USPC patients harboring tumors overexpressing the HER-2/neu oncogene8–10 and the experimental evidence indicating a correlation between HER-2 overexpression and increased resistance to chemotherapy in other human tumors 21,22. On the other hand, similarly to our experimental results, other studies have previously shown increasing sensitivity to chemotherapy with increasing HER-2 expression levels23–25. While it is difficult to compare our in vitro experimental results in USPC with those obtained with human tumors from different organs and genetic backgrounds21,22, it is likely, as previously suggested25, that alterations in chemosensitivity in tumors are dependent on the combined effects of c-erbB2 overexpression and the intrinsic genetic alterations associated with a particular cell line studied. Consistent with this hypothesis, USPC-ARK-1, a primary cell line overexpressing HER-2/neu and sensitive to platinum compounds, was found in our assays to be highly resistant to paclitaxel, docetaxel and other microtubule inhibitors.
While the reason for the differences in resistance/sensitivity to chemotherapy between the high and low HER-2/neu expressors remains poorly understood, we hypothesized that USPC with low/negligible HER-2/neu expression may differ from their HER-2/neu-overexpressor counterparts not only in cell-cycle patterns but also in the activation of multiple molecular pathways and oncogene(s). To validate/exclude this hypothesis, gene expression profiling experiments to evaluate genomic differences between the two groups of primary USPC cell lines are underway in our laboratory.
Finally, when all USPC cell lines were considered as a group, all chemotherapy agents tested demonstrated lower IC50s when used in combination than as individual agents. These data confirm that chemotherapy combinations may better modulate drug responsiveness in vitro and likely in vivo. These findings may be particularly important for the treatment of USPC because even tumors found unresponsive to a large class of cytotoxic drugs (i.e., microtubule inhibitors) when used as single agents, (i.e., USPC-ARK- 1 in our experiments), may dramatically increase their response to therapy when exposed to drug combinations.
In summary, USPC cell lines overexpressing HER-2/neu showed higher proliferation when compared to low HER-2/neu expressing cell lines and lower half maximum inhibitory concentration (IC50) when exposed to the majority of single-agent chemotherapies. While a greater understanding of the molecular/genetic differences between USPC with high and low HER-2/neu expression will be necessary before tailoring of chemotherapy for improved clinical outcomes, these data suggest that the apparent lack of response to chemotherapy among patients with HER-2/neu positive tumors seen in clinical trials may be due to rapid tumor regrowth of surviving tumor cells following initial response to chemotherapy rather than intrinsic chemotherapeutic drug resistance at the time of chemotherapy treatment.
Acknowledgments
We wish to thank Sarah Suchy MS, for her technical support in the ChemoFx experiments.
Supported in part by grants from the Angelo Nocivelli, the Berlucchi and the Camillo Golgi Foundation, Brescia, Italy, NIH R01 CA122728-01A2 to AS, and grants 501/A3/3 and 0027557 from the Italian Institute of Health (ISS) to AS. This investigation was also supported by NIH Research Grant CA-16359 from the National Cancer Institute.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thun M, Xu J, Hao Y, Ward E, Siegel R, Jemal A. Cancer statistics, 2009. CA. 2009;59:225. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol.Oncol. 1983;15:10. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 3.Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6:93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Levenback C, Burke TW, Silva E, et al. Uterine papillary serous carcinoma (USPC) treated with cisplatin, doxorubicin, and cyclophosphamide (PAC) Gynecol. Oncol. 1992;46:317–321. doi: 10.1016/0090-8258(92)90224-7. [DOI] [PubMed] [Google Scholar]
- 5.Nicklin L, Copeland LJ. Endometrial papillary serous carcinoma: pattern of spread and treatment. Clin. Obstet. Gynecol. 1996;39:686–695. doi: 10.1097/00003081-199609000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz PE. The management of serous papillary uterine cancer. Schwartz PE. Current Opinion in Oncology. 2006;18(5):494–499. doi: 10.1097/01.cco.0000239890.36408.75. [DOI] [PubMed] [Google Scholar]
- 7.Santin AD, Bellone S, Van Stedum S, et al. Determination of HER-2/neu Status in Uterine Serous Papillary Carcinoma: Comparative Analysis of Immunohistochemistry and Fluorescence in situ Hybridization. Gynecol. Oncol. 2005;98:24–30. doi: 10.1016/j.ygyno.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 8.Santin AD, Bellone S, Van Stedum S, et al. Amplification of c-erbB2 Oncogene: a Major Prognostic Indicator in Uterine Serous Papillary Carcinoma. Cancer. 2005;104(7):1391–1397. doi: 10.1002/cncr.21308. [DOI] [PubMed] [Google Scholar]
- 9.Santin AD, Siegel ER, Bellone S, et al. Racial differences in overexpression of epidermal growth factor type II receptor (HER2/neu): a major prognostic indicator in Uterine Serous Papillary Cancer. Am. J. Obstet. Gynecol. 2005;192:813–818. doi: 10.1016/j.ajog.2004.10.605. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Montes TP, Ji H, Smith Sehdev AE, et al. Clinical significance of HER-2/neu overexpression in uterine serous carcinoma. Gynecol Oncol. 2006;100:139–144. doi: 10.1016/j.ygyno.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Grushko TA, Filiaci VL, Mundt AJ, Ridderstrale K, Olopade OI, Fleming GF Gynecologic Oncology Group. An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecologic Oncology. 2008;108(1):3–9. doi: 10.1016/j.ygyno.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright C, Angus B, Nicholson S, et al. Expression of c-erbB-2 oncoprotein: a prognostic indicator in human breast cancer. Cancer Res. 1989;49:2087–2090. [PubMed] [Google Scholar]
- 13.Berchuck A, Kamel A, Whitaker R, et al. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990;50:4087–4091. [PubMed] [Google Scholar]
- 14.Hetzel DJ, Wilson TO, Keeney GL, Roche PC, Cha SS, Podratz KC. HER-2/neu expression: a major prognostic factor in endometrial cancer. Gynecol Oncol. 1992;47:179–185. doi: 10.1016/0090-8258(92)90103-p. [DOI] [PubMed] [Google Scholar]
- 15.El-Sahwi K, Bellone S, Cocco E, Cargnel, et al. In vitro Activity of Pertuzumab in Combination with Trastuzumab in Uterine Serous Papillary Adenocarcinoma. Brit. J. Cancer. doi: 10.1038/sj.bjc.6605448. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brower SL, Fensterer JE, Bush JE. The ChemoFx® assay: an ex vivo chemosensitivity and resistance assay for predicting patient response to cancer chemotherapy. In: Mor G, Alvero AF, editors. Methods in Molecular Biology; Apoptosis and Cancer: Methods and Protocols. Totowa NJ: Humana Press; 2008. pp. 57–78. [DOI] [PubMed] [Google Scholar]
- 17.Gallion H, Christopherson WA, Coleman RL, et al. Progression-free interval in ovarian cancer and predictive value of an ex vivo chemoresponse assay. Int J Gynecol Cancer. 2006;16(1):194–201. doi: 10.1111/j.1525-1438.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- 18.Yarden Y, Sliwkowski MX. Untangling the erbB signaling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 19.Slomovitz BM, Broaddus RR, Burke TW. HER-2/neu overexpression and amplification in uterine papillary serous carcinoma. J Clin Oncol. 2004;22:2136. doi: 10.1200/JCO.2004.11.154. [DOI] [PubMed] [Google Scholar]
- 20.Morrison C, Zanagnolo V, Ramirez N, et al. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. J Clin Oncology. 2006;24(15):2376–2385. doi: 10.1200/JCO.2005.03.4827. [DOI] [PubMed] [Google Scholar]
- 21.Tsai CM, Chang KT, Perng RP, et al. Correlation of intrinsic chemoresistance of non-small-cell lung cancer cell lines with HER-2/neu gene expression but not with ras gene mutations. Journal of the National Cancer Institute. 1993;85(11):897–901. doi: 10.1093/jnci/85.11.897. [DOI] [PubMed] [Google Scholar]
- 22.Tsai CM, Yu D, Chang KT, et al. Enhanced chemoresistance by elevation of p185neu levels in HER-2/neu-transfected human lung cancer cells. Journal of the National Cancer Institute. 1995;87(9):682–684. doi: 10.1093/jnci/87.9.682. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa Y, Goto M, Hanai N, et al. Prediction of chemosensitivity using multigene analysis in head and neck squamous cell carcinoma. Oncology. 2007;73(1–2):104–111. doi: 10.1159/000120998. [DOI] [PubMed] [Google Scholar]
- 24.Orr MS, O’Connor PM, Kohn KW. Effects of c-erbB2 overexpression on the drug sensitivities of normal human mammary epithelial cells. J Natl Cancer Inst. 2000;92:987–994. doi: 10.1093/jnci/92.12.987. [DOI] [PubMed] [Google Scholar]
- 25.Pegram MD, Finn RS, Arzoo K, Beryt M, Pietras RJ, Slamon DJ. The effect of HER-2/neu overexpression on chemotherapeutic drug sensitivity in human breast and ovarian cancer cells. Oncogene. 1997;15:537–547. doi: 10.1038/sj.onc.1201222. [DOI] [PubMed] [Google Scholar]