Abstract
Mutacin 1140 is produced by Streptococcus mutans and belongs to the type A lantibiotic family. Experiments were done to optimize production of mutacin 1140 in minimal media enabling a more cost efficient downstream purification method. The development of a small volume fermentation method enabled a rapid screen of several variables in a standard shaking incubator. This method provided a fast approach for determining components that promote mutacin 1140 production in minimal media broth. Lactose was determined to be the optimal carbon source for mutacin 1140 production. High concentrations of CaCl2 (0.3% w/v) and MgSO4 (0.77% w/v) promoted an increase in mutacin 1140 production, while ZnCl2 and FeCl3 appeared to impair production. Optimization of mutacin 1140 production in minimal media resulted in more than a 100-fold increase in production compared to the base medium used to begin our optimizations. The yield has been estimated by RP-HPLC to be ~10 mg/L.
Keywords: Mutacin 1140, Lantibiotic, Streptococcus mutans, fermentation, minimal media
1. Introduction
Mutacin 1140 is a peptide belonging to a group of antibiotics called Type A lantibiotics, and is naturally produced by a strain of the common oral bacterium, Streptococcus mutans [1–3]. Lantibiotics are defined as lanthionine-containing antibacterial peptides with thioether bridges, called lanthionines, resulting from posttranslational modifications [4]. An increasing interest in lantibiotics has developed in recent years because of their antimicrobial mode of action towards a wide range of pathogenic Gram positive bacteria [3,5–9]. The mode of action of mutacin 1140 has been determined in previous studies. The essential cell wall molecule lipid II is sequestered into domains away from the sites that are required for cell wall synthesis [10,11].
Optimization of fermentation conditions for the production of the lantibiotic nisin has been well studied [12–15]. Moreover, fermentations have been optimized for the lantibiotics gallidermin and mutacin NY266 using complex media, such as yeast extract, high (5%) calcium chloride concentrations and using a large inoculum (10% v/v). These authors have reported production levels over 200 mg/L [16–20]. Using similar conditions and media composition our group has been able to achieve approximately 50 mg/L of mutacin 1140 in a 3L bioreactor controlling temperature, pH, and oxygen [21,22]. Extraction of mutacin 1140 from this complex medium by RP-HPLC methods is achievable, but not a commercially viable approach. In this paper we described a rapid approach for optimizing the production of the lantibiotic mutacin 1140 in minimal media using a standard shaking incubator.
2. Methods and materials
All media was purchased from Difco Laboratory (Detroit, MI) and chemicals were purchased from Fisher Scientific (Pittsburgh, PA) and were the highest grade, unless otherwise stated.
2.1 Bacterial Strains
We used two bacterial strains in this study: Streptococcus mutans JH1140 ATCC 55676 and Micrococcus luteus ATCC 272. S. mutans was used for the production of mutacin 1140. M. luteus was used as an indicator organism for detection of mutacin 1140 production [20,22].
S. mutans was first screened for colonies that would grow on minimal media and maintain antimicrobial activity. Colonies of S. mutans were picked from plates grown on Todd Hewitt-yeast extract (30 g Todd Hewitt broth/L, 3 g yeast extract/L) and streaked on modified M9 agar (M9 medium, supplemented with casamino acids (10 g/L), CaCl2 (5 g/L), glucose (40 g/L), NaHCO3 (1 g/L), and agar 15 g/L). It is important to note that supplements added to the modified M9 media that are normally present in the medium are listed as a final concentration and are not added in addition to the components normally found in the M9 medium. During our initial screen of medium components, casamino acids were determined to be essential for the antimicrobial activity of S. mutans. Individual colonies growing on modified M9 agar were lifted and screened for antimicrobial production using a standard inhibitory colony spot assay as outlined below. Colonies stabbed into a fresh modified M9 agar plate were overlaid with M. luteus indicator strain and each colony was also simultaneously streaked onto a fresh master plate. Ten colonies producing the largest clearing were used to repeat the assay. The final master plate of the colony that produced the largest zone was used for making glycerol stocks. Presumably, clearing is proportionate to mutacin 1140 production in the minimal media environment, thus this strain was used as the inoculum in the optimization of mutacin 1140 production in minimal media.
2.2 Rapid procedure for optimizing fermentation conditions
A stock solution of 100 μL aliquots of purified mutacin 1140 (provided by Oragenics Inc., Alachua FL) in 80% acetonitrile at a concentration of 10μg/mL was stored at 4°C and was use to compare mutacin 1140 production over different variables and across different samples. Small volume (20 mL) fermentations using a modified M9 minimal medium broth and a 10% inoculum of S. mutans was used as our base medium for optimizing each variable in a shaking incubator at 200 rpm at 37°C for 24 hours. As mentioned above, supplements added to the modified M9 media that are normally present in the medium are listed as a final concentration and are not added in addition to the components normally found in the M9 medium. The inoculum was started from a 400 μL glycerol stock (109 CFU (colony forming unit)/mL) grown in 40 mL of modified minimal media supplemented with 0.3% yeast extract (to help boost the inoculum growth rate) to an OD600 of 0.8 at 37°C.
The inoculum and fermentation media were analyzed for zinc by flame atomic absorption spectrophotometry with a Perkin-Elmer Analyst 800 instrument with a hollow cathode zinc lamp at a wavelength of 213.9 nm. Zinc atomic absorption standards were prepared from a stock solution (Aldrich). An equal volume of nitric acid was added to each sample (1 mL) and then transferred to a labeled Teflon tube. The samples were placed in an aluminum block heated to 90°C for 1 hour before analysis. Samples were compared to distilled water blank to determine background levels of possible contamination.
At completion of the fermentation, the samples were spun at 9000 g for 20 min and then the supernatants were collected. The collected supernatants were heated for 30 min at 65°C to kill any bacteria remaining. The resulting supernatant was assayed for mutacin 1140 production using the semiquantitative critical dilution method described below [23]. Five microliters of the resulting supernatants were stabbed in triplicate horizontally across a 100 mm plate and the mutacin 1140 stock solution was also stabbed in triplicate across each bioassay plate for comparison. Quantification of mutacin 1140 production was determined by the following formula: Mutacin production = diameter of culture liquor zone (mm)/diameter of mutacin 1140 stock solution zone (mm). The ratio of each zone was averaged and the standard deviation was calculated using the averaged ratios from each fermentation (n = 3). This approach enabled the comparison of each variable tested across numerous plates. The supernatant of the culture broth was also analyzed by RP-HPLC according to the method of Fiedler et al. (1987) with slight modifications [24], and is described in the Supporting Online Material.
2.3 Antimicrobial assay
A M. luteus semiquantitative critical dilution method is a qualitative assay for bactericidal activity. M. luteus is a mutacin sensitive strain with a nanomolar minimum inhibitory concentration (MIC). M. luteus was grown in Todd Hewitt-yeast extract (30 g Todd Hewitt broth/L, 3 g yeast extract/L) to an O.D.600nm of 0.2. Then, 400 μL of these cells were added to 10 mL of top agar (M9 media, casamino acids 10 g/L, and agar 7.5 g/L). 5 ml of melted top agar containing the standardized suspension were added to each Petri dish containing approximately 20 mL of modified M9 media agar. Before the plates were overlaid with the top agar containing the indicator strain, individual colonies of S. mutans were stabbed into the modified M9 medium agar and placed inverted into a candle jar for 48 hours. Following two days of incubation, the stabbed colonies were then overlaid with the top agar containing M. luteus. The plates were then allowed to dry before being inverted and placed in a candle jar overnight at 37°C. The following day the plates were checked to determine the relative size of the zone of inhibition created by the stabbed colonies. Zones of inhibitions were measured in units of millimeters. The semiquantitative critical dilution method was done as described above for the inhibitory colony spot assay, except that 5μL of the supernatant of the overnight fermentations was stabbed instead of a bacteria colony into Todd Hewitt-yeast extract agar plates (30 g Todd Hewitt Broth/L, 3 g yeast extract/L, and 15 g agar/L) and overlaid M. luteus in Todd Hewitt-yeast extract top agar (30 g Todd Hewitt Broth/L, 3 g yeast extract/L, and 7.5 g agar/L). Once the top agar on the Petri dish had solidified 5 μL of the cell free culture liquor was stabbed in triplicate on the plate. The plates were then allowed to dry before being inverted and placed in a candle jar overnight at 37°C. Zones of inhibitions were measured in units of millimeters. Supernatants from fermentations using our base medium was used as a positive control for mutacin 1140 production and each medium tested (minus the inoculum) was used as a negative control for antimicrobial activity. Colony forming units (CFU) were determined in duplicate by serial dilution and plating method. S. mutans grows in long chains, thus the CFU data is slightly variable. Therefore, CFU data showing a log difference in growth is only discussed in the results.
3. Results and Discussion
Fermentations at 37°C for a 24 hour time period using the modified M9 minimal media appeared to be the optimal temperature and time for determining the effects that supplemented components have on mutacin 1140 production (see Supporting Online Material).
3.1 Determination of the optimal concentration of calcium chloride for mutacin 1140 production
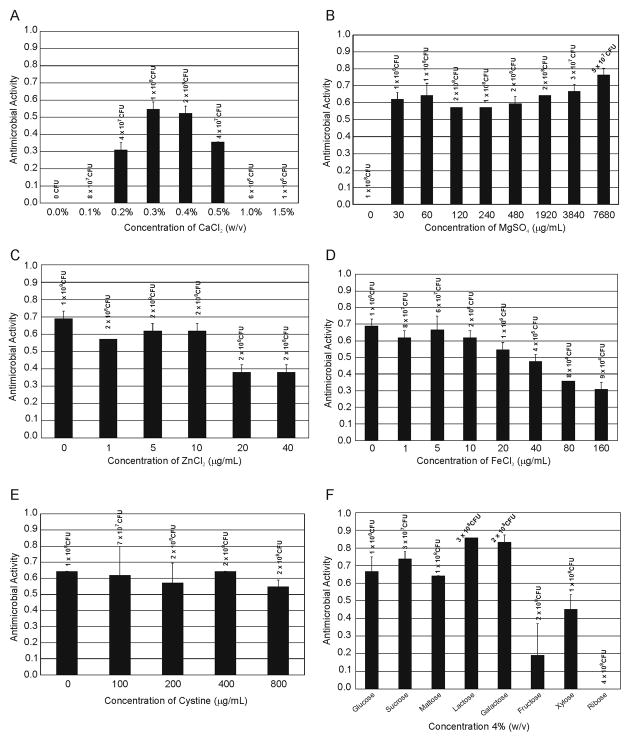
Earlier experiments in the complex medium yeast extract showed that a high concentration of CaCl2 (5% w/v) was optimal for mutacin 1140 production [21,22]. Due to the importance of calcium chloride for mutacin 1140 production in broth, CaCl2 was the first component optimized. To determine the optimal concentration for CaCl2, we first investigated the following percentages (w/v), 0, 0.1, 0.2, 0.3, 0.4, 0.5, 1.0, and 1.5%. The optimal concentration for mutacin 1140 production was 0.3% CaCl2 (Figure 1, panel A). There is approximately a 40% increase in zone diameter as compared to 0.2 and 0.5% CaCl2. Interestingly there appears to be a narrow window for promoting mutacin 1140 production. No activity was observed at 0.1% and 1% CaCl2 concentrations. CaCl2 also appears to be required for cell survival, since there were no CFUs when calcium was removed from the media. Furthermore, higher concentration of CaCl2 appeared to inhibit growth since there was an order of magnitude drop in cell density at 1.0 and 1.5% concentration compared to 0.5%. A 0.3% CaCl2 concentration was used as our base medium for the following optimization experiments.
Figure 1.
Optimization of mutacin 1140 production. A. CaCl2 concentrations ranging from 0.0 to 1.5 % were tested and the CFUs are listed above the concentrations. B. MgSO4 concentrations ranging from 0.0 to 7,680 μg/mL were tested and the CFUs are listed above the concentrations, C. ZnCl2 concentrations ranging from 0.0 to 40 μg/mL were tested and the CFU are listed above the concentrations, D. FeCl3 concentrations ranging from 0.0 to 160 μg/mL were tested and the CFUs are listed above the concentrations, E. Cystine concentrations ranging from 0.0 to 800 μg/mL were tested and the CFUs are listed above the concentrations, F. Monosaccharide and disaccharide sugars at 4% (w/v) concentrations were tested and the CFUs are listed above each sugar tested.
3.2 Determination of the optimal concentration of trace elements for mutacin 1140 production
Several other inorganic salts can be tested for their effect on mutacin 1140 production, but for the scope of this study the inorganic salts tested were MgSO4, ZnCl2, and FeCl3. Magnesium ions are required in a variety of enzymatic reactions, including DNA replication. Mutacin 1140 production was absent when MgSO4 was not added (Figure 1, panel B). Interestingly, there was cellular growth without the addition of MgSO4. Presumably, the inoculum provided a sufficient source of magnesium ions for growth. There is an interesting dip in mutacin 1140 production between 60 and 480 μg/mL of magnesium sulfate. These experiments were repeated showing the same phenomenon. Optimal production of mutacin 1140 occurred at 7,680 μg/mL (0.77% w/v) of MgSO4. However, the benefit of a high concentration of MgSO4 has never been mentioned in the production of other lantibiotics. The role of zinc ions had been shown to be important for the production of other lantibiotics [25–27]. Zinc is important for the activity of the post translational modification enzyme responsible for the formation of the lanthionine rings. Interestingly, mutacin 1140 production decreases when ZnCl2 is supplements at concentrations between 20 and 40 μg/mL (Figure 1, panel C). Given the CFUs at these concentrations, the cells appear to be healthy since they grew. Also of interest is that mutacin 1140 production was best when no zinc was supplemented in the media. If zinc is a requirement for the formation of the thioether linkages between the cysteine sulfhydryl groups and the didehydro amino acids, then presumably a small amount of zinc present in the inoculum is sufficient for enzymatic activity. Flame atomic absorption spectrophotometry revealed a low level of zinc present in the fermentation media (34+/−32 ng/mL), while the inoculum does contain a significant amount of zinc (351+/−2 ng/mL). Nonetheless, supplementing additional zinc should be avoided. Iron ions have an important role in the catalytic sites of several bacterial enzymes. Supplementing FeCl3 between 0 and 10 μg/mL had no effect on mutacin 1140 production, while supplementing iron in the media, particularly at concentrations above 10 μg/mL, had a pronounced effect on S. mutans viability and mutacin 1140 production (Figure 1, panel D). It is interesting to note that there was a significant amount of mutacin 1140 produced when the media was supplemented with 80 μg/mL given that the cell density was >1000 fold less than the media containing no supplemented iron. FeCl3 appears to inhibit the growth of S. mutans, but it may also inactivate other cellular activities that have a detrimental effect on the production of mutacin 1140. Therefore, future experiments will explore whether the addition of FeCl3 to the fermentation at a later time point with a higher cell density will promote a greater yield of mutacin 1140.
3.3 Cystine Supplementation
Currently, the concentration of casamino acids in the base media is 10 mg/mL. Two amino acids, cysteine and tryptophan, do not survive the hydrolysis procedure of casein that is used for making casamino acids. Given that four of the 22 amino acids found in mutacin 1140 are cysteines, an experiment was designed to determine whether the addition of cystine would have an effect on the production of mutacin 1140. Cysteine was shown to boost the production of the lantibiotic gallidermin when supplemented in the fermentation media [17]. The following concentrations of cystine were tested; 0 μg/mL, 100 μg/mL, 200 μg/mL, 400 μg/mL, and 800 μg/mL. The disulfide link between the two cysteines in cystine is readily reduced by the bacterium to give the corresponding thiol amino acid. The addition of cystine to our minimal M9 production medium had no significant effect on the production of mutacin 1140 (Figure 1, panel E). Possibly supplementing cystine may be important as the yield of mutacin 1140 is improved and will again be explored in future fermentations.
3.4 Determination of optimal carbon source
The base medium for the production of mutacin 1140 contains 4% glucose, which may not be the optimal carbon source for mutacin 1140 production. Several monosaccharides and disaccharides sugars were tested to determine optimal carbon source. The monosaccharides glucose, galactose, fructose, xylose, and ribose, as well as the disaccharides sucrose, maltose, and lactose were tested at a 4% concentration (w/v). Production of mutacin 1140 was enhanced with the addition of lactose and galactose (Figure 1, panel F). Carbon sources fructose, xylose, and ribose resulted in no or low mutacin 1140 production, while the CFUs suggest that these carbon sources did support growth. Only after we optimize mutacin 1140 production in a controlled bioreactor will we determine the optimal concentration of lactose and galactose in the fermentation media.
3.5 Optimized media
A fermentation at 37°C for 24 hrs using all the optimized variables in the modified M9 media, supplemented with 1% casamino acids, 0.1% NaHCO3, 0.3% CaCl2, 0.77% MgSO4, and 4% lactose, resulted in the supernatant having an antimicrobial activity greater than 1.0, demonstrating that production in a shaking incubator exceeds the concentration of the mutacin 1140 standard (10 mg/L). These results were confirmed by RP-HPLC (supplemental data). The procedure was scaled from a 20 mL fermentation volume to a 500 mL fermentation volume in a 1 L bottle, which resulted in the same level of production (Figure 2). Translation of the procedure to 500 mL is important for future optimization studies in a controlled bioreactor. A serial 5 fold dilution assay of the 500 mL fermentation media also resulted in the same level of activity as was observed in the mutacin 1140 standard. The antimicrobial activity units for each five fold dilution of the mutacin 1140 standard and for the 500 mL fermentation are shown in Figure 2. This shows that a 125 fold dilution results in an antimicrobial activity of 0.36, which is comparable to the activity we saw in our base modified M9 medium, containing 0.5% CaCl2 and 4% glucose. Therefore, the small volume fermentations in a shaking incubator method for optimizing mutacin 1140 provided a rapid means of increasing the production by more than 100 fold.
Figure 2.
The semiquantitative critical dilution method of the mutacin standard and the 500 mL fermentation. The samples were serially diluted five-fold and the units of antimicrobial activity were calculated by taking the ratio of the diameter of each zone to the diameter of the first zone of the mutacin 1140 standard.
4. Conclusions
Several lantibiotics have been known for decades but they have not been extensively tested for their potential usefulness for treating infections. The principal reason for this is due to the difficulty of obtaining these molecules in cost effective amounts and purity that would enable their testing for the treatment of infections. One major draw back is the use of complex medium generally used for their production, which obstructs the development of an effective approach to extract and purify them from the culture liquor. The small volume minimal media fermentation method described in this paper provides a useful method for optimizing the production of mutacin 1140 and may also be useful in the optimization of other antimicrobial substances.
Supplementary Material
Acknowledgments
This work was supported in part by grant R15 GM 085720 - 01 from NIH-NIAID. The authors thank Dr Lloyd Bennet at Mississippi State University’s College of Veterinary Medicine Pathobiology and Population Medicine Department for his help in collecting the flame atomic absorption spectrophotometry data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hillman JD, Johnson KP, Yaphe BI. Characterization of a Streptococcus mutans Bacteriocin with Novel Properties. J Dent Res. 1983;62:241–241. [Google Scholar]
- 2.Hillman JD, Johnson KP, Yaphe BI. Isolation of a Streptococcus mutans Strain Producing a Novel Bacteriocin. Infect Immun. 1984;44:141–144. doi: 10.1128/iai.44.1.141-144.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hillman JD, Novak J, Sagura E, Gutierrez JA, Brooks TA, Crowley PJ, Hess M, Azizi A, Leung KP, Cvitkovitch D. Genetic and biochemical analysis of mutacin 1140, a lantibiotic from Streptococcus mutans. Infect Immun. 1998;66:2743–2749. doi: 10.1128/iai.66.6.2743-2749.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee C, Paul M, Xie LL, van der Donk W. Biosynthesis and Mode of Action of Lantibiotics. Chem Rev. 2005;105:633–683. doi: 10.1021/cr030105v. [DOI] [PubMed] [Google Scholar]
- 5.Abee T, Krockel L, Hill C. Bacteriocins: Modes of action and potentials in food preservation and control of food poisoning. Int J Food Microbiol. 1995;28:169–185. doi: 10.1016/0168-1605(95)00055-0. [DOI] [PubMed] [Google Scholar]
- 6.Hansen JN. Nisin as a Model Food Preservative. Crit Rev Food Sci Nutr. 1994;34:69–93. doi: 10.1080/10408399409527650. [DOI] [PubMed] [Google Scholar]
- 7.Kramer NE, Smid EJ, Kok J, de Kruijff B, Kuipers OP, Breukink E. Resistance of Gram-positive bacteria to nisin is not determined by Lipid II levels. FEMS Microbiol Lett. 2004;239:157–161. doi: 10.1016/j.femsle.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Millette M, Le Tien C, Smoragiewicz W, Lacroix M. Inhibition of Staphylococcus aureus on beef by nisin-containing modified alginate films and beads. Food Control. 2007;18:878–884. [Google Scholar]
- 9.O’Mahony T, Rekhif N, Cavadini C, Fitzgerald GF. The application of a fermented food ingredient containing ‘variacin’, a novel antimicrobial produced by Kocuria varians, to control the growth of Bacillus cereus in chilled dairy products. J Appl Microbiol. 2001;90:106–114. doi: 10.1046/j.1365-2672.2001.01222.x. [DOI] [PubMed] [Google Scholar]
- 10.Hasper HE, Kramer NE, Smith JL, Hillman JD, Zachariah C, Kuipers OP, de Kruijff B, Breukink E. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science. 2006;313:1636–1637. doi: 10.1126/science.1129818. [DOI] [PubMed] [Google Scholar]
- 11.Smith L, Hasper H, Breukink E, Novak J, Cerkasov J, Hillman JD, Wilson-Stanford S, Orugunty RS. Elucidation of the Antimicrobial Mechanism of Mutacin 1140. Biochemistry. 2008;47:3308–3314. doi: 10.1021/bi701262z. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez DC, Perez VH, Justo OR, Alegre RM. Effect of the extremely low frequency magnetic field on nisin production by Lactococcus lactis subsp lactis using cheese whey permeate. Process Biochemistry. 2006;41:1967–1973. [Google Scholar]
- 13.Xia L, Chung YK, Yang ST, Yousef AE. Continuous nisin production in laboratory media and whey permeate by immobilized Lactococcus lactis. Process Biochemistry. 2005;40:13–24. [Google Scholar]
- 14.Guerra NP, Pastrana L. Modelling the influence of pH on the kinetics of both nisin and pediocin production and characterization of their functional properties. Process Biochemistry. 2002;37:1005–1015. [Google Scholar]
- 15.de Arauz LJ, Jozala AF, Mazzola PG, Penna TCV. Nisin biotechnological production and application: a review. Trends in Food Science & Technology. 2009;20:146–154. [Google Scholar]
- 16.Kempf M, Theobald U, Fiedler HP. Influence of dissolved O2 on the fermentative production of gallidermin by Staphylococcus gallinarum. Biotechnol Lett. 1997;19:1063–1065. [Google Scholar]
- 17.Kempf M, Theobald U, Fiedler HP. Correlation between the consumption of amino acids and the production of the antibiotic gallidermin by Staphylococcus gallinarum. Biotechnol Lett. 1999;21:959–963. [Google Scholar]
- 18.Kempf M, Theobald U, Fiedler HP. Economic improvement of the fermentative production of gallidermin by Staphylococcus gallinarum. Biotechnol Lett. 1999;21:663–667. [Google Scholar]
- 19.Kempf M, Theobald U, Fiedler HP. Production of the antibiotic gallidermin by Staphylococcus gallinarum - development of a scale-up procedure. Biotechnol Lett. 2000;22:123–128. [Google Scholar]
- 20.Nicolas G, Auger I, Beaudoin M, Halle F, Morency H, LaPointe G, Lavoie MC. Improved methods for mutacin detection and production. J Microbiol Meth. 2004;59:351–361. doi: 10.1016/j.mimet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Smith JL, Orugunty R, Pollock J, Hillman JD. Optimization of the Production and Purification of Mutacin 1140 for the Manufacture of Clinical Grade Antibiotic. American Society for Microbiology, South East Branch; St. Petersburg, Florida: 2005. [Google Scholar]
- 22.Chaney N, Wilson-Stanford S, Kastrantas J, Dahal N, Smith L. Rapid Method for Extracting the Antibiotic Mutacin 1140 from the Complex Fermentation Medium Yeast Extract. Can J Microbiol. 2009;55:1261–1266. doi: 10.1139/w09-091. [DOI] [PubMed] [Google Scholar]
- 23.Parrot M, Charest M, Lavoie MC. Production of mutacin like substances by Streptococcus mutans. Can J Microbiol. 1989;35:366–372. doi: 10.1139/m89-056. [DOI] [PubMed] [Google Scholar]
- 24.Fiedler H-P, Hörner T, Wörn A. Separation of polypeptide antibiotics by reversed-phase high-performance liquid chromatography. Chromatographia. 1987;24:433–438. [Google Scholar]
- 25.Cheng F, Takala TM, Saris PEJ. Nisin biosynthesis in vitro. J Mol Microbiol Biotechnol. 2007;13:248–254. doi: 10.1159/000104754. [DOI] [PubMed] [Google Scholar]
- 26.Li B, Yu JPJ, Brunzelle JS, Moll GN, van der Donk WA, Nair SK. Structure and mechanism of the lantibiotic cyclase involved in nisin biosynthesis. Science. 2006;311:1464–1467. doi: 10.1126/science.1121422. [DOI] [PubMed] [Google Scholar]
- 27.Okeley NM, Paul M, Stasser JP, Blackburn N, van der Donk WA. SpaC and NisC, the cyclases involved in subtilin and nisin biosynthesis, are zinc proteins. Biochemistry. 2003;42:13613–13624. doi: 10.1021/bi0354942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.