Table 3.
Survey of Oxidative Hydrolysis Conditions for Hydrosilane 37.
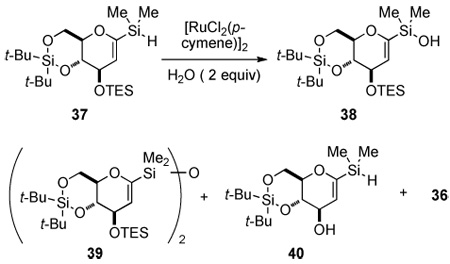 | ||||||||
|---|---|---|---|---|---|---|---|---|
| yield, %g |
||||||||
| entrya | catalyst, mol % |
solvent | time, h | 37 | 38 | 39 | 40 | 36 |
| 1b | 4 | CH3CN | 4 | - | 73 | - | - | - |
| 2c | 4 | CH3CN | 1 | - | 70 | - | - | - |
| 3d | 8 | CH3CN | 2 | - | 47 | - | - | - |
| 4e,f | 3 | CH3CN | 3 | 19 | 52 | 3 | 23 | - |
| 5 | 8 | n-BuCN | 2 | - | 87 | 2 | - | - |
| 6 | 3 | n-BuCN | 4 | - | 84 | 4 | - | - |
| 7 | 3 | THF | 4 | - | 54 | 5 | - | 27 |
| 8 | 4 | PhCN | 4 | - | 90 | 4 | - | - |
| C6H6/ | ||||||||
| 9 | 3 | CH3CN | 1 | - | 86 | 7 | - | - |
| (1:1) | ||||||||
| C6H6/ | ||||||||
| 10h | 3 | CH3CN | 1 | - | 84 | 8 | - | - |
| (1:1) | ||||||||
All the reactions were carried out on 100 mg scale, unless otherwise stated.
110 mg scale
564 mg scale.
950 mg scale.
[Ir(cod)Cl]2, H2O (2.0 equiv) were used.
TES ether cleavage was observed.
Yield of isolated product.
The reaction was run on 1.6 mmol scale and the yield of 38 is of analytically pure material.
