Abstract
The release of fecal pollution into surface waters may create environmental reservoirs of feces-derived microorganisms, including pathogens. Clostridium perfringens is a commonly used fecal indicator that represents a human pathogen. The pathogenicity of this bacterium is associated with its expression of multiple toxins; however, the prevalence of C. perfringens with various toxin genes in aquatic environments is not well characterized. In this study, C. perfringens spores were used to measure the distribution of fecal pollution associated with suspended sediments in the nearshore waters of Lake Michigan. Particle-associated C. perfringens levels were greatest adjacent to the Milwaukee harbor and diminished in the nearshore waters. Species-specific PCR and toxin gene profiles identified 174 isolates collected from the suspended sediments, surface water, and sewage influent as C. perfringens type A. Regardless of the isolation source, the beta2 and enterotoxin genes were common among isolates. The suspended sediments yielded the highest frequency of cpe-carrying C. perfringens (61%) compared to sewage (38%). Gene arrangement of enterotoxin was investigated using PCR to target known insertion sequences associated with this gene. Amplification products were detected in only 9 of 90 strains, which suggests there is greater variability in cpe gene arrangement than previously described. This work presents evidence that freshwater suspended sediments and sewage influent are reservoirs for potentially pathogenic cpe-carrying C. perfringens spores.
Commonly used fecal indicator bacteria (FIB), such as fecal coliforms, Escherichia coli, and enterococci, are considered to be short-lived in the aquatic environment (8, 51), and this complicates the determination of the long-term fate of pollution in complex freshwater and marine systems. Clostridium perfringens has spore-forming capabilities, which allow for survival in harsh environments after deposition and facilitate prolonged detection in aquatic environments (15, 27). C. perfringens has an extensive history as a FIB, based on its association with the gastrointestinal tract of humans and other animals and the presence of its spores in sewage (14, 15, 39, 40, 44). Due to its survival, size, and association with particles, this organism has been considered a good surrogate for tracking Cryptosporidium oocysts in aquatic systems (42).
Aquatic sediments are considered a repository of fecal pollution. Thus, the accumulation of C. perfringens spores in sediments may relate to the affinity of this bacterium for particles in surface waters (5, 11, 12, 33). In sediments of marine and brackish waters, C. perfringens persists, while the levels of other indicators can decrease by orders of magnitude over the short time frames of days or weeks (3, 15). Levels recovered from the sediments of extreme environments range from 5,000 to 9,000 CFU g−1 and demonstrate that this bacterium can be recovered from long-term (e.g., for years) fecal pollution reservoirs under diverse environmental conditions (17, 27, 39). The occurrence of particle-associated FIB is considered to be an important factor in the overall transport of fecal pollution into surface waters. The association of FIB with particles is thought to prolong environmental survival by protecting cells against cold water temperatures, UV radiation, grazing, and low nutrient concentrations (7, 12, 15, 51). While sediment reservoirs have been documented, little is known about suspended sediment deposition and fluxes in freshwater environments and how these impact the fate and diversity of C. perfringens in the aquatic environment.
C. perfringens is associated with 14 toxins, which accounts for the diverse range of toxin profiles that have been described for this organism (29, 30, 49, 50). Four of these genes, which code for the alpha, beta, epsilon, and iota toxins, are used to characterize C. perfringens into five different toxin types (A through E) (48, 49). The presence of alpha toxin (phospholipase C) defines C. perfringens and corresponds to type A. Other toxin genes of interest, such as enterotoxin, beta2, and netB, are not associated with a specific toxin type, but they can relate to overall virulence and differences among C. perfringens strains (29, 30, 48). Enterotoxin is important due its connection with disease in both humans and animals (41). In humans, type A isolates that produce enterotoxin encoded by the cpe gene cause food-borne illnesses, sporadic diarrhea, and antimicrobial drug-associated diarrhea (22, 53). Consequently, the role of enterotoxin in human disease has led to comprehensive analyses of C. perfringens from multiple hosts and potential environmental reservoirs to characterize the source of this toxin. Overall, it is estimated that only 2 to 5% of C. perfringens isolates worldwide carry the cpe gene (6, 18, 43, 56). These results have been collected from meat products (6, 18, 43, 56), animals (9, 18, 21, 23, 29, 37, 43, 56), and soils (36). There has been some indication that humans are a primary source of enterotoxin-positive C. perfringens, based upon the prevalence of individuals in which the cpe gene is detected in fecal samples (∼18%) (24).
In this study, the dispersion pattern of particle-associated C. perfringens spores was characterized from suspended sediments that were transported from the Milwaukee River basin into the nearshore waters of Lake Michigan. A collection of isolates was obtained from sewage samples and the aquatic environment to assess the prevalence of six toxin genes. The main objectives were to illustrate the distribution, survival, and toxin diversity of C. perfringens associated with fecal pollution. This work demonstrates the potential for fecal pollution inputs to create transient toxin reservoirs in freshwater aquatic systems.
MATERIALS AND METHODS
Study area.
The Milwaukee River basin drains 2,300 km2 of mixed land usage (e.g., urban, suburban, and agricultural lands) and is home to over 1 million people. The Milwaukee, Menomonee, and Kinnickinnic Rivers are the primary tributaries of the basin and converge just prior to discharge into Lake Michigan (46). The Milwaukee harbor is a break wall-enclosed freshwater estuary that has three openings (i.e., Main Gap, North Gap, and South Gap) to Lake Michigan. The older infrastructure of Milwaukee made this system prone to combined sewage overflows (CSO) that occur during periods of heavy rainfall. Reductions in CSO frequency from 50 to ∼2 per year are the result of infrastructure improvements over the last 20 years. Approximate discharge from the Milwaukee River basin was calculated from the combined flow data for the individual rivers as obtained from the USGS (USGS site codes 04087000, 04087142, and 0407159).
Water sampling.
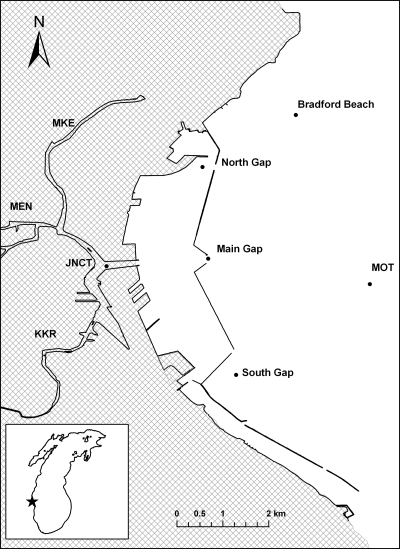
Surface water samples were collected at the convergence of the three rivers (junction) and each of the three harbor openings following storm events and under base flow conditions (Fig. 1). Samples were collected between June and October 2007. These samples were characterized for Escherichia coli, fecal coliforms, and C. perfringens by using standard methods (2, 3, 54). Turbidity was measured using a portable 2020e turbidity meter (La Motte, Chestertown, MD). Additional water parameters were characterized with a YSI 660 sonde device (YSI Incorporated, Yellow Springs, OH).
FIG. 1.
Location of sediment traps and surface water collection sites in the Milwaukee harbor and nearshore waters of Lake Michigan. Each river is listed: Milwaukee River (MKE), Menomenee River (Men), and Kinnickinnic River (KKR), in addition to the convergence of the three rivers (i.e., junction [JNCT]). The star indicates the location of the Milwaukee harbor on the west coast of Lake Michigan. The coast is shown in gray. (Based on data from the NOAA National Geophysical Data Center and The Great Lakes Information Network.)
Sediment trap deployment and sample collection.
Sediment traps were deployed in the nearshore waters of Lake Michigan at various distances from the Milwaukee harbor (Fig. 1). Four paired sediment traps were deployed at three locations. One trap from each pair was used for microbiological analyses, and the other was used for radiochemical analyses. Suspended sediments settling from the water column were captured in cylindrical sediment traps (15-cm diameter). These traps were suspended on a steel cable that was held vertical by a bottom weight (135 to 180 kg) and a subsurface buoy. Sediment traps deployed at the South Gap (lat 43.00.212, long 87′52.695; depth, 8 m) and Bradford Beach (lat 43.03.252, long 87.51.902; depth, 9 m) sites. The sediment traps were placed 4 m below the surface of the water. Two traps were deployed at the Milwaukee outer trap (MOT) location (lat 43.00.232, long 87.52.697; depth, 15 m) and placed at 4 and 13 m below the surface of the water. Sediment traps were deployed at the end of April 2007 and serviced approximately every 3 weeks until the end of October. The suspended sediment samples were collected on 10 May, 30 May, 20 June, 11 July, 1 August, 21 and 22 August, 24 September, and 26 and 29 October, and the sediment traps were redeployed on the same day. Only the traps at South Gap and Bradford Beach were serviced on 21 August, with MOT serviced on 22 August due to inclement weather. Similarly, the MOT site was serviced on 26 October and South Gap and Bradford Beach on 29 October due again to inclement weather. The sample from the Bradford Beach site for 21 August to 24 September was lost because of equipment failure. During the season, only one CSO occurred, on 20 August 2007, after 9.04 cm of rain.
Continuous water parameters were recorded at the MOT location by using an YSI 6600 sonde device (Yellow Springs, OH) deployed at 13 m and equipped with sensors for temperature, turbidity, chlorophyll fluorescence, pH, conductivity, and dissolved oxygen. Water parameter data for the other two locations was supplemented by a series of instrumented buoys associated with Great Lakes Urban Coastal Observing System (GLUCOS) deployed in the Milwaukee Bight (13). Each buoy consisted of a temperature sensor string and one or two multiparameter YSI 6600 sondes (Yellow Springs, OH) equipped as previously described.
Sample processing for fecal indicators and C. perfringens.
Sediment samples from the traps were processed for three fecal indicators: fecal coliforms, E. coli, and C. perfringens. Sediment samples were diluted (1:1 or 1:10) with sterile water, sonicated (60 s), and vortex mixed (30 s). Each sediment sample (0.1 to 1 ml) was filtered through a 0.45-μm-pore-size 47-mm mixed esters filter (Millipore, Billerica, MA) and placed on m-FC-agar (Difco, Sparks, MD) for fecal coliform enumeration or modified m-TEC agar (Difco, Sparks, MD) for E. coli enumeration (1, 54). An aliquot (1 ml) of each suspended sediment sample used for microbiological analysis was dried at 60°C for 48 h to calculate counts per gram of sediment.
A separate dilution was made for the enumeration of C. perfringens spores. Sediment samples were diluted (1:1 or 1:10) with sterile water, sonicated (60 s), and vortex mixed (30 s). Samples were heat shocked for 20 min at 60°C and then placed on ice for 20 min. Samples (0.1 to 1 ml) were filtered through a 0.45-μm-pore-size 47-mm mixed esters filter (Millipore, Billerica, MA) and then placed on mCP medium following the method of Armon and Payment (2). The mCP plates were incubated at 44.5°C ± 1°C for 24 h in a GasPak anaerobic jar (BD, Franklin Lakes, NJ). After incubation, the filters were exposed to the vapors of ammonium hydroxide for 20 to 30 s. Levels of vegetative C. perfringens were not estimated with this method. Only straw-colored colonies that turned pink to magenta were counted as C. perfringens. An aliquot (1 ml) of each suspended sediment sample used for microbiological analyses was dried at 60°C for 48 h to calculate counts per gram of sediment. Between 5 and 15 colonies presumptively characterized as C. perfringens were chosen for isolation. These colonies were transferred to mCP medium to confirm characteristic biochemical reactions (e.g., sucrose utilization and acid phosphatase activity) and then isolated for purity on brain heart infusion agar (Difco, Sparks, MD). A subset of isolates (n = 19) was further characterized using API 20A (bioMerieux, l'Etoile, France) to confirm identity as C. perfringens (46). A total of 109 suspended sediment and 7 surface water bacterial isolates were collected. Influent samples from the two Milwaukee area wastewater treatment plants (WWTPs; Jones Island [JI] and South Shore [SS]) were used to obtain sewage-associated C. perfringens as detailed above, resulting in collection of 58 isolates (29 from each treatment plant).
Molecular characterization of C. perfringens isolates.
The identities of the isolates recovered on mCP from suspended sediments, surface water, and sewage were determined using a species-specific PCR. DNA was extracted from the isolates by a boiling method (18). Type strains ATCC 3626 and ATCC 12916 were used as positive controls. PCR was carried out using primers targeting the 16S rRNA gene of C. perfringens (CLPER-F, 5′-AGATGGCATCACATTCAAC-3′; CLPER-R, 5′-GCAAGGGATGTCAAGTGT-3′) (31). PCR mixtures consisted of a 25-μl total volume with 2× Master Mix (Qiagen, Valencia, CA), a 40 pmol final concentration of primers, and 2 μl of template DNA from each isolate. PCR conditions were as previously published (46). The ∼800-bp PCR products were analyzed on a 1.5% agarose gel.
After molecular confirmation as C. perfringens, the toxin profile was determined for each isolate. Six toxin genes were targeted, cpa, cpb, cpb2, etx, ia, and cpe, using the primer sequences reported by Garmory et al. (21) and Heikinheimo and Korkeala (23), which yielded PCR products of 400, 196, 655, 446, 233, and 567 bp, respectively. When assessing the multiplex PCR method for toxin typing, interference between the enterotoxin and alpha toxin primer sets was observed. Consequently, the six primers sets were used separately to eliminate primer interference and to correctly identify the toxin genotype of each isolate. PCRs were carried out in a 25-μl total volume using 2× Master Mix (Qiagen, Valencia, CA), a 40 pmol final concentration of forward and reverse primers, and 2 μl of cell lysates. PCR conditions were as follows: 95°C for 2 min, 35 cycles of 94°C for 60 s, 55°C for 60 s, and 72°C for 60 s, and an elongation step at 72°C for 10 min. PCR products were analyzed on a 2% agarose gel.
Restriction enzyme digests were performed on the beta and enterotoxin PCR products to verify the presence of the gene and correctly identify the toxin profile. MseI (New England Biolabs, Ipswich, MA) was used to digest the cpb gene PCR products, and the cpb amplification product from C. perfringens ATCC 3626 was utilized as the control. BanI (New England Biolabs, Ipswich, MA) was used to digest cpe gene PCR products, with the amplification product from C. perfringens ATCC 12916 as the control. Restriction digests were carried out following the manufacturer's instructions. Restriction digests were examined on 4% agarose gels and compared against a 1-kb ladder (Invitrogen, Carlsbad, CA) to determine the fragment size.
Characterization of cpe location using IS element-anchored PCR.
The cpe gene-positive C. perfringens isolates were analyzed with a previously described assay to characterize association with different insertion sequence (IS) elements (45). Confirmation of presence of the enterotoxin gene was performed in a separate PCR using the primer pair 3F (5′-GATAAAGGAGATGGTTGGATATTAGG-3′) and 4R (5′-GAGTCCAAGGGTATGAGTTAGAAG-3′), which amplifies a larger fragment than the initial primer pair used in this study. The IS-anchored PCR utilizes IS elements that are downstream from the enterotoxin locus on the chromosome (IS1470) or associated with plasmids (IS1151 or IS1470-like). The primers IS1470R1.3 (5′-CTTCTTGATTACAAGACTCCAGAAGAG-3′) and cpe4F (5′-TTAGAACAGTCCTTAGGTGATGGAG-3′) were used to determine if the locus was of chromosomal origin. Assessment of the enterotoxin locus of plasmid origin was performed with two separate primer pairs targeting either the IS1470-like sequence (IS1470-likeR1.6, 5′-CTTTGTGTACACAGCTTCGCCAATGTC-3′) and cpe4F or the IS1151 sequence (IS1151R0.8, 5′-ATCAAAATATGTTCTTAAAGTACGTTC-3′) and cpe4F primers. PCRs were carried out in 50-μl volumes using 2× Master Mix (Qiagen, Valencia, CA), a 60 pmol final primer concentration, and 5 μl of template DNA from each isolate. The PCR conditions used were as follows: 94°C for 2 min, 40 cycles of 94°C for 30 s, 61°C for 30 s, and 68°C for 90 s, and extension for 8 min at 68°C. Products were analyzed on a 1.5% agarose gel and compared against a 1-kb ladder (Invitrogen, Carlsbad, CA) to estimate product size.
Statistical analyses.
When necessary, the bacterial counts were log10 transformed. Data comparisons using analysis of variance, linear regression, t tests, and a chi-square test were performed using MiniTab, release 15 (MiniTab, Inc.).
RESULTS
River discharge and water quality of the Milwaukee harbor after storm events.
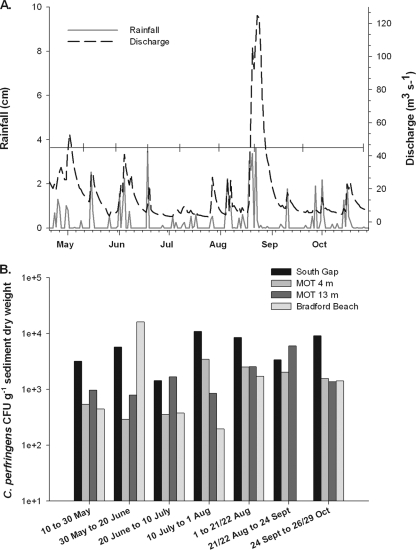
For 2007, the storm events that occurred from May until late August are shown in Fig. 2 A. The impact from each event on the hydrograph lasted approximately 2 to 15 days, depending on the intensity of the rainfall. Levels of E. coli, C. perfringens spores, and fecal coliforms varied greatly between storm events and base flow conditions in the Milwaukee harbor (Table 1). All of the collection sites had 5- to 10-fold-higher levels of FIB following rain events than under base flow conditions. The junction site, which is the confluence of the three rivers leading to Lake Michigan, typically had higher levels of FIB than the three gap sites of the harbor whether sampled during rain events or base flow conditions. C. perfringens spore levels in all surface water samples were much lower than E. coli levels. Overall, these findings demonstrate that the Milwaukee River basin consistently contributes fecal pollution to the Milwaukee harbor and nearshore waters of Lake Michigan.
FIG. 2.
(A) Total rainfall data were obtained from the NOAA National Weather Service. Discharge into Lake Michigan was calculated from USGS stations along the three Milwaukee area tributaries. Brackets indicate sediment trap deployment periods. (B) Counts of culturable C. perfringens expressed as log CFU/g−1 (dry weight) of suspended sediment.
TABLE 1.
Average FIB loads in surface waters of Milwaukee harbor in 2007
| Site | Event type (n) | Avg CFU/100 ml (SD) |
||
|---|---|---|---|---|
| Fecal coliforms | E. coli | C. perfringens | ||
| Junction | Base flow (5) | 2,017 (1,715) | 235 (291) | 19 (13) |
| Rain (8) | 10,180 (6,752) | 1756 (1,640) | 61 (41) | |
| Main Gap | Base flow (3) | 195 (333) | 15 (23) | <5 |
| Rain (7) | 5,864 (7,526) | 741 (963) | 37 (27) | |
| South Gap | Base flow (3) | 212 (345) | 29 (37) | NDa |
| Rain (8) | 1,934 (3,504) | 180 (203) | 21 (26) | |
| North Gap | Base flow (4) | 84 (77) | 14 (17) | <5 |
| Rain (7) | 2,243 (5,155) | 101 (153) | 15 (19) | |
ND, not detected.
Prevalence of C. perfringens associated with freshwater suspended sediments.
Suspended sediments were captured at sites with various proximities (0 to 3 km) to the Milwaukee harbor, and this allowed for characterization of particle-associated fecal indicators in nearshore waters. C. perfringens spores were consistently recovered from the sediment traps at high levels (Fig. 2B), while the levels of the other fecal indicators fluctuated over time. E. coli ranged from 30 to 1.14 × 104 CFU g−1, and fecal coliforms ranged from 59 to 8.2 × 103 CFU g−1, with mean levels of both indicators approximately 2-fold lower than that of C. perfringens spores. Recovery of particle-associated C. perfringens spores was related to the proximity to the source. This trend was most evident at the South Gap location, with C. perfringens counts ranging from 3,000 to 11,000 CFU g−1 (dry weight) of sediment (Fig. 2B). Overall, the levels at the South Gap location were significantly higher (P < 0.05) than at the other three locations, excluding one sample captured at Bradford Beach (i.e., 10 to 30 May), which had very high C. perfringens levels. The sediment trap deployed near the beach environment (Bradford Beach) generally had levels of C. perfringens lower than what was detected near the Milwaukee harbor.
Rainfall and C. perfringens spore levels from the sediment samples were not significantly correlated, suggesting that there were constant inputs from the watershed in the absence of storm events. However, the residual impact from the 20 August CSO corresponded to sustained elevated counts recovered in the MOT 4-m sample for the collection period following the CSO, indicating a time lag between large-scale pollution events and transport of particle-attached FIB into nearshore waters. Nevertheless, there were no clear correlations between total rainfall, total stream flow, sediment accumulation, or site and counts, indicating that multiple factors influence the distribution of particle-associated C. perfringens spores.
Toxin gene profiles of C. perfringens isolates.
The toxin gene profiles of the C. perfringens isolates were examined to assess if these three environments serve as possible reservoirs of potentially pathogenic strains. C. perfringens isolates obtained from sewage influent were used to represent human fecal pollution released into surface waters. A total of 116 sediments and surface water isolates and 58 sewage isolates were analyzed for the presence of cpa, cpb, cpb2, etx, ia, and cpe. All strains were classified as C. perfringens based on amplification of the 16S rRNA gene with species-specific primers and detection of the cpa gene. The cpb gene was not detected in any of the isolates; however, primers targeting this toxin gene did produce an ∼200-bp fragment in 54% of the isolates. A restriction digest with MseI demonstrated that the amplification product was not from the cpb gene. The sequence of the nonspecific amplification product had 90 to 91% identity to an unknown gene annotated as the phosphate ABC transporter from the C. perfringens ATCC 13124 complete genome (accession number CP000246). All strains were classified as type A, since none of the isolates possessed cpb, etx, or ia toxin genes.
The cpe and cpb2 toxin genes were detected at high frequencies in the different environments (Table 2). The cpb2 gene was most frequently observed in sewage isolates originating from the South Shore WWTP and was evenly distributed across the sewage and suspended sediments (Table 2). Isolates positive for the cpe gene were obtained from all environments, with a significantly higher percentage from suspended sediment samples than from sewage (chi-square, P < 0.05). Forty-two isolates were positive for both toxin genes.
TABLE 2.
Toxin gene profiles of Clostridium perfringens isolates from environmental samples and sewage
| C. perfringens isolate source | No. screened | No. (%) positive with toxin gene profilea |
|||
|---|---|---|---|---|---|
| A | A-e | A-β2 | A-e-β2 | ||
| South Shore sewage | 29 | 5 (17) | 2 (7) | 13 (45) | 9 (31) |
| Jones Island sewage | 29 | 12 (41) | 8 (28) | 6 (21) | 3 (10) |
| South Gap SSb | 43 | 4 (9) | 19 (44) | 6 (14) | 14 (33) |
| MOT SS, 4 m | 28 | 6 (21) | 14 (50) | 2 (7) | 6 (21) |
| MOT SS, 13 m | 25 | 9 (36) | 3 (12) | 5 (20) | 8 (32) |
| Bradford Beach SS | 13 | 6 (46) | 1 (8) | 4 (31) | 2 (15) |
| Milwaukee estuary | 7 | 3 (43) | 1 (14) | 3 (43) | NDc |
| Total | 174 | 45 (24.7) | 48 (27.6) | 39 (22.4) | 42 (24.1) |
The letter A refers to the alpha toxin gene e refers to the presence of the enterotoxin gene, and β2 refers to the presence of the gene encoding the β2 toxin.
SS, suspended sediment samples.
ND, not detected.
IS element-anchored PCR for enterotoxin location.
The large number of strains positive for the cpe gene warranted an investigation of the location of this locus among the isolates. All of the positive isolates were analyzed with a second set of primers targeting an internal region of the gene to confirm its presence. Twelve of the 90 isolates did not yield a cpe gene product with these primers, which may have been associated with a change in the sequence at the position of the reverse primer. Regardless, all 90 cpe gene-positive strains were further examined with the IS-anchored PCR. Six of the sediment isolates appeared to posses the IS1470-like plasmid and one isolate was positive for the IS1151 plasmid (Table 3). Only two sewage isolates were found to have the cpe gene on the chromosome adjacent to IS1470. The location of the cpe gene could not be determined for the majority of isolates, suggesting that the arrangement of this toxin gene in isolates recovered from the environment is more diverse than previously characterized.
TABLE 3.
IS element associations for cpe gene-positive isolates
| Source | No. of samples | No. of cpe-positive isolates associated with IS element or unclassified |
|||
|---|---|---|---|---|---|
| IS1151 | IS1470-like | IS1470 | Unclassified | ||
| Sewage | 22 | NDa | ND | 2 | 20 |
| MOT, 4 m | 20 | ND | 2 | ND | 18 |
| MOT, 13 m | 11 | 1 | 2 | ND | 8 |
| South Gap | 33 | ND | 2 | ND | 31 |
| Bradford Beach | 3 | ND | ND | ND | 3 |
| Estuary | 1 | ND | ND | ND | 1 |
ND, not detected with IS element-anchored PCR.
DISCUSSION
Understanding the fate of fecal pollution in the environment is important for protecting human health. C. perfringens is commonly detected in surface waters contaminated with fecal pollution (3, 5, 38, 39). A recent epidemiological study of freshwater beaches demonstrated adverse health effects when average C. perfringens levels were above 10 CFU/100 ml, illustrating that low levels of this indicator can be associated with a human health risk (57). The link between C. perfringens levels and negative human health outcomes supports the use of this organism as a surrogate for human pathogens, specifically, protozoan and enteric human viruses (47).
Pollution plume dynamics involve numerous factors as the contamination moves from a riverine system into a coastal zone. Previous research has shown that dispersion of material from episodic rain events is dependent upon wind-driven processes, current formation, dilution, and storm intensity in the nearshore environment (16). In our study system, as the pollution plume from the Milwaukee River basin was released into the open waters of Lake Michigan, dilution made it difficult to detect culturable FIB (51). Subsequently, fecal pollution impacts on recreational waters were not easily determined (52). The absence of FIB in overlying water samples indicates the importance of collecting suspended sediments to assess the fate of fecal pollution when the signal becomes diluted. The proximity to the source of fecal pollution has been shown to impact detection of C. perfringens spores (17). The highest loading of particle-associated C. perfringens was captured at the South Gap location, which receives constant fecal pollution from storm water, small rain events, and disturbance of harbor sediments. The MOT 4- and 13-m traps were found to have higher levels from midsummer to early fall compared with spring; this may be an effect of seasonal processes, such as differences in water temperatures, prevailing winds, and resuspension events.
Attachment of FIB to particles was considered an important factor in the overall transport of fecal pollution in aquatic systems (11, 12). Previous studies have illustrated the dominance of C. perfringens spores as a particle-attached FIB quantified in surface waters (5, 11, 12). Particles in the nearshore of Lake Michigan have been shown to have residence times in the water column ranging from 0.2 to 4.8 days (32). Overall lake circulation patterns for the southern basin of Lake Michigan prevent long-term benthic sediment accumulation in the nearshore (16). These two factors allow us to consider particles captured in the sediment traps as recently deposited material (55). In general, the particle-associated C. perfringens spores captured in sediment traps represent the previous 3 weeks in the nearshore suspended sediment record.
The residence time and long survival of particle-attached C. perfringens spores were evident, based on ease of recovery and high levels from suspended sediment samples captured over ∼20- to 30-day deployments. Others have repeatedly indicated the ability to recover C. perfringens spores from sediments long after initial fecal pollution inputs (17, 27, 39). The accumulation of particle-associated C. perfringens indicates the possibility of creating reservoirs of this potential pathogen in aquatic environments.
C. perfringens can be classified into five different types (A to E) based upon the combination of the four (alpha, beta, epsilon, and iota) toxins they possess (47). In this study, a collection of 174 C. perfringens isolates from environmental sources and sewage were identified as type A. These results agree with the majority of previous research studies demonstrating a dominance of C. perfringens type A isolates from numerous hosts and food sources (4, 18, 29). Our results match closely with samples recovered from soil environments (∼99% type A) (36). The absence of the other four C. perfringens types is most likely due to little or no input from fecal pollution sources containing these types. Even though much of the upstream areas of the Milwaukee River basin are dedicated to agricultural activities, indicating the study area does receive a complex mixture of fecal pollution, this was not reflected in the toxin types of C. perfringens isolates. A larger survey of isolates from suspended sediments, surface waters, sewage influent, and agricultural wastes may reveal the presence of other toxin types at low abundances in these different environments. The presence of the different toxin types can be reflective of the specific environment and sources of fecal pollution (9). The limited molecular characterization of C. perfringens from different aquatic environments and sewage makes it difficult to determine the impact of environmental stressors on survival and on genetic diversity.
Approximately half of the environmental and sewage isolates were positive for the mobile toxin genes cpe and cpb2. Type A strains isolated from various animals and food products have a high prevalence of the cpb2 gene (6, 21, 43, 56). The presence of the cpb2 gene was significantly higher (P < 0.001) in the isolates from the SS WWTP, which receives only sanitary sewage, than in isolates collected from the JI WWTP, which receives both sanitary and storm water (e.g., nonpoint and nonhuman sources of fecal pollution) from the combined sewer system. These findings suggest a link between the presence of the cpb2 gene and human-associated C. perfringens, and this was supported by detection of this gene in humans with sporadic diarrhea (29). Greater than half of the isolates carry the cpb2 gene and lack the cpe gene, even though previous research demonstrated both genes can be located on the same plasmid (20) and with multiple toxin types (21, 29).
A majority of human illnesses caused by C. perfringens have been correlated with the presence of enterotoxin (35, 48). Yet, the environmental or animal source of enterotoxin-positive strains has not been determined. In this study, ∼52% of all C. perfringens isolates from the freshwater environment and sewage were cpe positive, and this was 10-fold higher than generally reported (6, 19, 41, 47). These cpe gene-carrying isolates may reside in healthy individuals at low levels (24, 48) and could contribute to the detection in wastewater treatment plants and feces-polluted aquatic systems. In wastewater influent, feces-derived microorganisms have already been subjected to considerable selective pressures, which could account for the increased frequency of cpe-positive isolates from sewage compared with past reports of what can be found in humans. C. pefringens leaves the human host predominantly in the spore form (10), which may have some relationship to the preservation of mobile toxin genes in strains isolated from sewage and sediments.
Possessing the cpe gene appears to be common among C. perfringens isolates from the freshwater environment, and sequential inputs of fecal pollution create secondary reservoirs of this potentially pathogenic bacterium. Clostridium botulinum has been detected in aquatic environments (28) with a differential occurrence of botulinum neurotoxins compared to clinical isolates, suggesting differential survival of specific toxin-producing strains in aquatic environments (19, 25, 26). The impacts of variable environmental conditions experienced in freshwater and marine systems on toxin gene and plasmid carriage are unknown and need to be examined further.
Our previous work on the diversity of overlapping polymorphisms in the 16S rRNA gene of C. perfringens isolates from sewage influent, polluted water, and suspended sediments indicated a direct link with sewage pollution (46). The attempts to characterize the diversity in the enterotoxin locus for all positive strains provided limited information. Only 10% of the isolates corresponded with the previously identified IS elements. Prior research has demonstrated that the IS element-anchored PCR method leaves numerous cpe-positive isolates uncharacterized (24, 34, 36, 43). The presence of the unclassified enterotoxin-carrying isolates from diverse hosts and environments suggests there are multiple arrangements of the enterotoxin locus that still need to be characterized.
Overall, our results demonstrate that there is a high prevalence of isolates positive for cpe and cpb2 toxin genes. These findings may relate to the enrichment of these isolates in the aquatic environment and sewage influenced by intrinsic properties related to these genotypes. These results could also be influenced by the method and type of medium used for isolation. In this study, the mCP method was used to enumerate and isolate C. perfringens from suspended sediments, sewage, and surface water samples. This method has been shown to be highly selective for isolating a diverse collection of C. perfringens isolates (46).
In the aquatic environment, the presence of C. perfringens spores was reflective of long-term pollution inputs from a watershed with multiple land uses. This bacterium was easily recovered from sewage and freshwater environments with a high prevalence of isolates positive for the cpe gene. This work signifies that freshwater sediments and sewage inputs are reservoirs of enterotoxin-carrying C. perfringens spores.
Acknowledgments
We gratefully acknowledge the crew of the R/V Neeskay for field support. We thank Kim Weckerly, Don Szmania, and Robert Paddock for assistance in sampling suspended sediments. The maps of the sampling sites were provided by Kim Weckerly.
This work was funded by the NOAA Oceans and Human Health Initiative (extramural grant number NA05NOS4781243).
Footnotes
Published ahead of print on 25 June 2010.
REFERENCES
- 1.American Public Health Association. 1998. Standard methods for the examination of water and wastewater. American Public Heath Association, American Water Works Association, and the Water Environment Federation. APHA, Washington, DC.
- 2.Armon, R., and P. Payment. 1988. A modified m-Cp medium for enumerating Clostridium perfringens from water samples. Can. J. Microbiol. 34:78-79. [DOI] [PubMed] [Google Scholar]
- 3.Ashbolt, N. J., G. S. Grohmann, and C. S. W. Kueh. 1993. Significance of specific bacterial pathogens in the assessment of polluted receiving waters of Sydney, Australia. Water Sci. Technol. 27:449-452. [Google Scholar]
- 4.Baums, C. G., U. Schotte, G. Amtsberg, and R. Goethe. 2004. Diagnostic multiplex PCR for toxin genotyping of Clostridium perfringens isolates. Vet. Microbiol. 100:11-16. [DOI] [PubMed] [Google Scholar]
- 5.Brookes, J. D., M. R. Hipsey, M. D. Burch, R. H. Regel, L. G. Linden, C. M. Ferguson, and J. P. Antenucci. 2005. Relative value of surrogate indicators for detecting pathogens in lakes and reservoirs. Environ. Sci. Technol. 39:8614-8621. [DOI] [PubMed] [Google Scholar]
- 6.Bueschel, D. M., B. H. Jost, S. J. Billington, H. T. Trinh, and J. G. Songer. 2003. Prevalence of cpb2, encoding beta2 toxin, in Clostridium perfringens field isolates: correlation of genotype with phenotype. Vet. Microbiol. 94:121-129. [DOI] [PubMed] [Google Scholar]
- 7.Burkhardt, W., K. R. Calci, W. D. Watkins, S. R. Rippey, and S. J. Chirtel. 2000. Inactivation of indicator microorganisms in estuarine waters. Water Res. 34:2207-2214. [Google Scholar]
- 8.Byamukama, D., R. L. Mach, F. Kansiime, M. Manafi, and A. H. Farnleitner. 2005. Discrimination efficacy of fecal pollution detection in different aquatic habitats of a high-altitude tropical country, using presumptive coliforms, Escherichia coli, and Clostridium perfringens spores. Appl. Environ. Microbiol. 71:65-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai, Y., J. Gao, X. Wang, T. Chai, X. Zhang, H. Duan, S. Jiang, B. A. Zucker, and G. Schlenker. 2008. Clostridium perfringens toxin types from freshwater fishes in one water reservoir of Shandong Province of China, determined by PCR. Dtsch. Tierarztl. Wochenschr. 115:292-294, 296-297. [PubMed] [Google Scholar]
- 10.Carman, R. J., S. Sayeed, J. H. Li, C. W. Genheimer, M. F. Hiltonsmith, T. D. Wilkins, and B. A. McClane. 2008. Clostridium perfringens toxin genotypes in the feces of healthy North Americans. Anaerobe 14:102-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Characklis, G. W., M. J. Dilts, O. D. Simmons, C. A. Likirdopulos, L. A. H. Krometis, and M. D. Sobsey. 2005. Microbial partitioning to settleable particles in stormwater. Water Res. 39:1773-1782. [DOI] [PubMed] [Google Scholar]
- 12.Cizek, A. R., G. W. Characklis, L. A. Krometis, J. A. Hayes, O. D. Simmons, S. Di Lonardo, K. A. Alderisio, and M. D. Sobsey. 2008. Comparing the partitioning behavior of Giardia and Cryptosporidium with that of indicator organisms in stormwater runoff. Water Res. 42:4421-4438. [DOI] [PubMed] [Google Scholar]
- 13.Consi, T. R., T. F. Hansen, and J. V. Klump. 2007. GLUCOS: The Great Lakes Urban Coastal Observing System. A radio-linked buoy network for real-time monitoring of water quality in an urban freshwater coastal zone. Sea Technol. 48:39-43. [Google Scholar]
- 14.Cox, P., M. Griffith, M. Angles, D. Deere, and C. Ferguson. 2005. Concentrations of pathogens and indicators in animal feces in the Sydney watershed. Appl. Environ. Microbiol. 71:5929-5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies, C. M., J. A. H. Long, M. Donald, and N. J. Ashbolt. 1995. Survival of fecal microorganisms in marine and fresh water sediments. Appl. Environ. Microbiol. 61:1888-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eadie, B. J., J. A. Robbins, J. V. Klurnp, D. J. Schwab, and D. N. Edgington. 2008. Winter spring storms and their influence on sediment resuspension, transport, and accumulation patterns in southern Lake Michigan. Oceanography 21:118-135. [Google Scholar]
- 17.Edwards, D. D., G. A. McFeters, and M. I. Venkatesan. 1998. Distribution of Clostridium perfringens and fecal sterols in a benthic coastal marine environment influenced by the sewage outfall from McMurdo Station, Antarctica. Appl. Environ. Microbiol. 64:2596-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erol, I., M. Goncuoglu, N. D. Ayaz, F. S. B. Ormanci, and G. Hildebrandt. 2008. Molecular typing of Clostridium perfringens isolated from turkey meat by multiplex PCR. Lett. Appl. Microbiol. 47:31-34. [DOI] [PubMed] [Google Scholar]
- 19.Fach, P., S. Perelle, F. Dilasser, J. Grout, C. Dargaignaratz, L. Botella, J. M. Gourreau, F. Carlin, M. R. Popoff, and V. Broussolle. 2002. Detection by PCR-enzyme-linked immunosorbent assay of Clostridium botulinum in fish and environmental samples from a coastal area in northern France. Appl. Environ. Microbiol. 68:5870-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher, D. J., K. Miyamoto, B. Harrison, S. Akimoto, M. R. Sarker, and B. A. McClane. 2005. Association of beta2 toxin production with Clostridium perfringens type A human gastrointestinal disease isolates carrying a plasmid enterotoxin gene. Mol. Microbiol. 56:747-762. [DOI] [PubMed] [Google Scholar]
- 21.Garmory, H. S., N. Chanter, N. P. French, D. Bueschel, J. G. Songer, and R. W. Titball. 2000. Occurrence of Clostridium perfringens beta 2-toxin amongst animals, determined using genotyping and subtyping PCR assays. Epidemiol. Infect. 124:61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison, B., D. Raju, H. S. Garmory, M. M. Brett, R. W. Titball, and M. R. Sarker. 2005. Molecular characterization of Clostridium perfringens isolates from humans with sporadic diarrhea: evidence for transcriptional regulation of the beta2-toxin-encoding gene. Appl. Environ. Microbiol. 71:8362-8370. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Heikinheimo, A., and H. Korkeala. 2005. Multiplex PCR assay for toxinotyping Clostridium perfringens isolates obtained from Finnish broiler chickens. Lett. Appl. Microbiol. 40:407-411. [DOI] [PubMed] [Google Scholar]
- 24.Heikinheimo, A., M. Lindstrom, P. E. Granum, and H. Korkeala. 2006. Humans as reservoir for enterotoxin gene-carrying Clostridium perfringens type A. Emerg. Infect. Dis. 12:1724-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hielm, S., J. Bjorkroth, E. Hyytia, and H. Korkeala. 1998. Prevalence of Clostridium botulinum in Finnish trout farms: pulsed-field gel electrophoresis typing reveals extensive genetic diversity among type E isolates. Appl. Environ. Microbiol. 64:4161-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hielm, S., E. Hyytia, A. B. Andersin, and H. Korkeala. 1998. A high prevalence of Clostridium botulinum type E in Finnish freshwater and Baltic Sea sediment samples. J. Appl. Microbiol. 84:133-137. [DOI] [PubMed] [Google Scholar]
- 27.Hill, R. T., I. T. Knight, M. S. Anikis, and R. R. Colwell. 1993. Benthic distribution of sewage sudge indicated by Clostridium perfringens at a deep ocean dump site. Appl. Environ. Microbiol. 59:47-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huss, H. H. 1980. Distribution of Clostridium botulinum. Appl. Environ. Microbiol. 39:764-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jost, B. H., S. J. Billington, H. T. Trinh, D. M. Bueschel, and J. G. Songer. 2005. Atypical cpb2 genes, encoding beta2-toxin in Clostridium perfringens isolates of nonporcine origin. Infect. Immun. 73:652-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keyburn, A. L., J. D. Boyce, P. Vaz, T. L. Bannam, M. E. Ford, D. Parker, A. Di Rubbo, J. I. Rood, and R. J. Moore. 2008. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 4:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kikuchi, E., Y. Miyamoto, S. Narushima, and K. Itoh. 2002. Design of species-specific primers to identify 13 species of Clostridium harbored in human intestinal tracts. Microbiol. Immun. 46:353-358. [DOI] [PubMed] [Google Scholar]
- 32.Klump, J. V., D. N. Edgington, D. C. Szmania, B. E. Brown, and K. A. Orlandini. 2003. Sampling methods and approaches using radionuclide tracers in the study of sediment resuspension and cross margin transport in the nearshore of the Laurentia Great Lakes. Int. J. Sediment Res. 18:266-277. [Google Scholar]
- 33.Krometis, L. A. H., G. W. Characklis, O. D. Simmons, M. J. Dilts, C. A. Likirdopulos, and M. D. Sobsey. 2007. Intra-storm variability in microbial partitioning and microbial loading rates. Water Res. 41:506-516. [DOI] [PubMed] [Google Scholar]
- 34.Lahti, P., A. Heikinheimo, T. Johansson, and H. Korkeala. 2008. Clostrdium perfringens type A strains carrying a plasmid-borne enterotoxin gene (genotype IS1151-cpe or IS1470-like-cpe) as a common cause of food poisoning. J. Clin. Microbiol. 46:371-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, J. H., and B. A. McClane. 2006. Further comparison of temperature effects on growth and survival of Clostridium perfringens type A isolates carrying a chromosomal or plasmid-borne enterotoxin gene. Appl. Environ. Microbiol. 72:4561-4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, J. H., S. Sayeed, and B. A. McClane. 2007. Prevalence of enterotoxigenic Clostridium perfringens isolates in Pittsburgh (Pennsylvania) area soils and home kitchens. Appl. Environ. Microbiol. 73:7218-7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin, Y. T., and R. Labbe. 2003. Enterotoxigenicity and genetic relatedness of Clostridium perfringens isolates from retail foods in the United States. Appl. Environ. Microbiol. 69:1642-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipp, E. K., J. L. Jarrell, D. W. Griffin, J. Lukasik, J. Jacukiewicz, and J. B. Rose. 2002. Preliminary evidence for human fecal contamination in corals of the Florida Keys, U.S.A. Mar. Pollut. Bull. 44:666-670. [DOI] [PubMed] [Google Scholar]
- 39.Lisle, J. T., J. J. Smith, D. D. Edwards, and G. A. McFeters. 2004. Occurrence of microbial indicators and Clostridium perfringens in wastewater, water column samples, sediments, drinking water, and Weddell seal feces collected at McMurdo Station, Antarctica. Appl. Environ. Microbiol. 70:7269-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matches, J. R., J. Liston, and D. Curran. 1974. Clostridium perfringens in the environment. Appl. Microbiol. 28:655-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McClane, B. A. 1996. An overview of Clostridium perfringens enterotoxin. Toxicon 34:1335-1343. [DOI] [PubMed] [Google Scholar]
- 42.Medema, G. J., M. Bahar, and F. M. Schets. 1997. Survival of Cryptosporidium parvum, Escherichia coli, faecal enterococci and Clostridium perfringens in river water: influence of temperature and autochthonous microorganisms. Water Sci. Technol. 35:249-252. [Google Scholar]
- 43.Miki, Y., K. Miyamoto, I. Kaneko-Hirano, K. Fujiuchi, and S. Akimoto. 2008. Prevalence and characterization of enterotoxin gene-carrying Clostridium perfringens isolates from retail meat products in Japan. Appl. Environ. Microbiol. 74:5366-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miwa, N., T. Nishina, S. Kubo, and K. Fujikura. 1996. Nested polymerase chain reaction for detection of low levels of enterotoxigenic Clostridium perfringens in animal feces and meat. J. Vet. Med. Sci. 58:197-203. [DOI] [PubMed] [Google Scholar]
- 45.Miyamoto, K., Q. Y. Wen, and B. A. McClane. 2004. Multiplex PCR genotyping assay that distinguishes between isolates of Clostridium perfringens type A carrying a chromosomal enterotoxin gene (cpe) locus, a plasmid cpe locus with an IS1470-like sequence, or a plasmid cpe locus with an IS1151 sequence. J. Clin. Microbiol. 42:1552-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mueller-Spitz, S. R., L. B. Stewart, and S. L. McLellan. 2010. Reliability of mCP method for identification of Clostridium perfringens from faecal polluted aquatic environments. J. Appl. Microbiol. 108:1994-2002. [DOI] [PubMed] [Google Scholar]
- 47.Payment, P., and E. Franco. 1993. Clostridium perfringens and somatic coliphages as indicators of the efficiency of drinking water treatment for viruses and protozoan cysts. Appl. Environ. Microbiol. 59:2418-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petit, L., M. Gibert, and M. R. Popoff. 1999. Clostridium perfringens: toxinotype and genotype. Trends Microbiol. 7:104-110. [DOI] [PubMed] [Google Scholar]
- 49.Rood, J. I. 1998. Virulence genes of Clostridium perfringens. Annu. Rev. Microbiol. 52:333-360. [DOI] [PubMed] [Google Scholar]
- 50.Rood, J. I., and S. T. Cole. 1991. Molecular genetics and pathogenesis of Clostridium perfringens. Microbiol. Rev. 55:621-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schultz-Fademrecht, C., M. Wichern, and H. Horn. 2008. The impact of sunlight on inactivation of indicator microorganisms both in river water and benthic biofilms. Water Res. 42:4771-4779. [DOI] [PubMed] [Google Scholar]
- 52.Scopel, C. O., J. Harris, and S. L. McLellan. 2006. Influence of nearshore water dynamics and pollution sources on beach monitoring outcomes at two adjacent Lake Michigan beaches. J. Great Lakes Res. 32:543-552. [Google Scholar]
- 53.Sparks, S. G., R. J. Carman, M. R. Sarker, and B. A. McClane. 2001. Genotyping of enterotoxigenic Clostridium perfringens fecal isolates associated with antibiotic-associated diarrhea and food poisoning in North America. J. Clin. Microbiol. 39:883-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.U.S. Environmental Protection Agency. 2000. Improved enumeration methods for recreational water quality indicators: enterococci and Escherichia coli. EPA/821/R-97/004. U.S. Environmental Protection Agency, Washington, DC.
- 55.Waples, J. T., K. A. Orlandini, D. N. Edgington, and J. V. Klump. 2004. Seasonal and spatial dynamics of Th-234/U-238 disequilibria in southern Lake Michigan. J. Geophys. Res. Oceans doi: 10.1029/2003JC00204. [DOI]
- 56.Wen, Q. Y., and B. A. McClane. 2004. Detection of enterotoxigenic Clostridium perfringens type A isolates in American retail foods. Appl. Environ. Microbiol. 70:2685-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiedenmann, A., P. Kruger, K. Dietz, J. M. Lopez-Pila, R. Szewzyk, and K. Botzenhart. 2006. A randomized controlled trial assessing infectious disease risks from bathing in fresh recreational waters in relation to the concentration of Escherichia coli, intestinal enterococci, Clostridium perfringens, and somatic coliphages. Environ. Health Perspect. 114:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]