Abstract
Magnetotactic bacteria synthesize intracellular magnetosomes comprising membrane-enveloped magnetite crystals within the cell which can be manipulated by a magnetic field. Here, we report the first example of tellurium uptake and crystallization within a magnetotactic bacterial strain, Magnetospirillum magneticum AMB-1. These bacteria independently crystallize tellurium and magnetite within the cell. This is also highly significant as tellurite (TeO32−), an oxyanion of tellurium, is harmful to both prokaryotes and eukaryotes. Additionally, due to its increasing use in high-technology products, tellurium is very precious and commercially desirable. The use of microorganisms to recover such molecules from polluted water has been considered as a promising bioremediation technique. However, cell recovery is a bottleneck in the development of this approach. Recently, using the magnetic property of magnetotactic bacteria and a cell surface modification technology, the magnetic recovery of Cd2+ adsorbed onto the cell surface was reported. Crystallization within the cell enables approximately 70 times more bioaccumulation of the pollutant per cell than cell surface adsorption, while utilizing successful recovery with a magnetic field. This fascinating dual crystallization of magnetite and tellurium by magnetotactic bacteria presents an ideal system for both bioremediation and magnetic recovery of tellurite.
Magnetotactic bacteria are known to synthesize magnetosomes that comprise membrane-enveloped magnetic crystals (e.g., Fe3O4 and Fe3S4) (4, 6, 23). These crystals allow them to be directly manipulated with magnetic force (35, 40). This biomineralization process is highly regulated by the cell, rendering the crystals highly chemically pure. However, there have been reports of magnetite and iron sulfide magnetosome crystals containing manganese and copper, respectively, found in environmental magnetotactic bacteria, but the nonferrous metal quantities within the crystals were not consistent or controlled (5, 15, 30). Recently, the controlled cobalt doping of magnetite magnetosome crystals was also reported in magnetotactic bacteria (37). Until now, however, there has been no report of other toxic metal or metalloid (e.g., tellurium) being taken up by magnetotactic bacteria. Additionally, all reports so far show metal incorporated into the magnetosome. There has been no report of separate crystallization independent from the magnetite within magnetotactic bacteria. In this study, we investigate the tellurium uptake and crystallization in a magnetotactic bacterial strain, Magnetospirillum magneticum AMB-1.
The rare metalloid tellurium is used as an alloy component in a wide range of high-technology products, including optical discs, solar cell materials, and thermoelectric elements (27, 42). Although it is naturally rare, due to its use in the technologies described, it is becoming more and more in demand and is therefore highly commercially desirable. This chalcogen is rarely found in the nontoxic elemental state Te(0), but is more commonly present in the environment in its highly toxic soluble oxyanion form, tellurite(IV) (9). The increasing use of tellurium has indirectly led to the increased contamination of water with tellurite, which raises serious concerns for both ecological systems and mammalian health (12, 17). There is thus a growing need to recover tellurium from the environment to both (i) remove a toxic chemical and (ii) recycle a valuable element.
The recovery of metals and metalloids using microorganisms has emerged as a potentially attractive (33, 34, 47) and environment-friendly alternative to conventional techniques such as reclamation treatments (46). The crystallization of target molecules within the microorganisms has the potential to provide some advantages over other bioremediation technology, such as increased accumulation efficiency over cell surface adsorption or nonprecipitous uptake within the cell, decreasing the toxicity of the pollutant by reductive (or oxidative) precipitation/crystallization, and additional potential to utilize the pollutant product for further material applications. The uptake and crystallization of Pb(II), Ag(I), Au(III), U(VI), Se(IV), and other metals by many species has been well documented previously (11, 14, 16, 19, 24). Some bacteria in particular have been the subject of increased study due to the reduction and crystallization of tellurite within or onto the cell (2, 16, 25, 45). However, although these bacteria have been adapted and utilized for the accumulation of various metals and metalloids, cell recovery remains a bottleneck in this approach. Therefore, modification of the system is needed to improve this step.
Recently, the potential of combining a cell surface modification technology with magnetic recovery of heavy metals using magnetotactic bacteria was proposed (40). Here, we present the serendipitous discovery of the simultaneous biomineralization of tellurium and magnetite as discrete nanoparticles within magnetotactic bacteria during cell growth. This has great potential for development of a bioremediation system that incorporates tellurite removal from polluted water with magnetic recovery.
MATERIALS AND METHODS
Determination of the MIC of tellurite for M. magneticum AMB-1 growth.
M. magneticum AMB-1 was microaerobically cultured in magnetic spirillum growth medium (MSGM) at 28°C as previously described (39, 41). Microaerobic conditions were established by purging the cultures (250 ml) with argon gas for 5 min. The MIC of tellurite for M. magneticum AMB-1 in MSGM was determined by growing the cells in various concentrations of tellurite: 0 (control), 10, 20, 40, 60, 80, and 100 μg/ml. The cells were directly counted with a hemocytometer and an optical microscope (Olympus BH-2) for 6 days.
Tellurite removal using magnetotactic bacteria.
For tellurite removal, M. magneticum AMB-1 was inoculated into the medium containing tellurite and grown until the stationary phase. The medium was filtered (pore size, 0.45 μm), dried, and dissolved with a nitric acid solution (0.1 N) at 180°C. These samples were analyzed with an atomic absorption spectrophotometer (AA-6700; Shimadzu, Japan) (214.3 nm). All assays were performed in triplicate.
TEM and EDX analyses of M. magneticum AMB-1 grown in different tellurite concentrations.
M. magneticum AMB-1 cells grown in the presence of tellurite were observed by transmission electron microscopy (TEM) (H7100; Hitachi, Japan). Cells harvested from MSGM containing tellurite (0 and 40 μM) were washed with phosphate-buffered saline (PBS) buffer (pH 7.4) and spotted onto 150-mesh copper grids (Nisshin EM). The samples were analyzed by TEM operated at an accelerating voltage of 100 kV. To measure the number of magnetite and tellurium crystals, over 30 cells were manually counted.
To verify the biomineralization of tellurium within the cell, ultrathin-section TEM analysis was also conducted. Magnetotactic bacteria in the stationary phase cultured in the presence of 20 μM tellurite were washed and fixed overnight with 2% glutaraldehyde in a 0.05 M sodium cacodylate buffer. After being washed overnight with 0.05 M sodium cacodylate buffer, the material was postfixed with 2% osmium tetroxide for 2 h at 4°C, washed with Milli-Q at 4°C, dehydrated with ethanol, and embedded in Epon 812. Ultrathin sections were obtained from several blocks, stained with lead citrate and uranyl acetate, and observed in a JEOL JEM1200EX electron microscope operated at 80 kV. Scanning TEM (STEM)-energy-dispersive X-ray spectrometry (EDX) analysis was performed with a JEOL JEM-2500SE electron microscope operating at 200 kV and was performed with a beam current of 10 nA for 20 s (live time). Selected-area electron diffraction SAED analysis was conducted with TEM (H-9000NAR; Hitachi, Japan) at 200 kV.
Magnetic recovery assay of tellurium crystal-containing M. magneticum AMB-1.
A magnetic recovery assay was conducted to verify the ability to recover M. magneticum AMB-1. Cells cultured in the presence of tellurite were harvested and adjusted to 1.0 × 108 cells/ml MSGM. Six milliliters of each sample was transferred to separate glass test tubes (10 mm in diameter and 7.6 cm in height), and each test tube was sealed with a rubber bung. A cylindrical neodymium-boron magnet (3,710 G) was used to recover the cells by attracting them to one side of the tube. At the designated times (1, 2, 3, 4, 5, 7, 8, 13, 15, and 20 h), culture medium from each test tube was collected by inserting a syringe through the rubber bung and by extracting the culture medium (20 μl) near the surface. A cell count was performed against the extracted culture medium samples. In addition, the removed cells and tellurium concentration were also measured at the endpoint for further verification.
RESULTS
Tellurite tolerance and uptake in M. magneticum AMB-1.
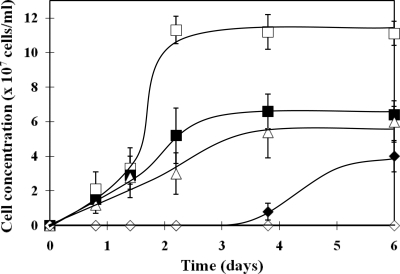
The toxic effect of tellurite on the growth of M. magneticum AMB-1 was investigated at various concentrations of tellurite (Fig. 1). Cells cultured in MSGM containing 10 and 20 μM tellurite showed similar growth rates, with stationary-phase cell densities of approximately 6.0 × 107 cells/ml, which was approximately half the number of cells grown in the absence of tellurite. However, when the cells were cultured in higher tellurite concentrations, a slow growth rate (40 μM) or complete growth inhibition (60 μM) was observed (data for 80 and 100 μM not shown). Based on these observations, it was concluded that the MIC of tellurite for M. magneticum AMB-1 was 60 μM.
FIG. 1.
Tolerance of M. magneticum AMB-1 to tellurite. Cells grown in different concentrations of tellurite were directly counted (□, 0 μM; ▪, 10 μM; ▵, 20 μM; ⧫, 40 μM; and ⋄, 60 μM). The average values from three independent experiments were obtained. Error bars show standard deviations.
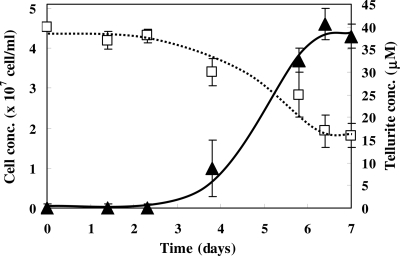
The time course of tellurite concentrations in the cell and in the medium was measured (Fig. 2). For cells grown in 40 μM tellurite, the cell growth and tellurite uptake were saturated at 7 days. The final cell density gradually decreased with increasing concentrations of tellurite in the medium (Table 1). The most effective condition for tellurite removal with respect to proportion of initial concentration was 20 μM tellurite, where 58.8% of the initial tellurite and 1.8 × 108 tellurite molecules were accumulated by the cells. On the other hand, in the medium containing 40 μM tellurite, 38.6% of the initial tellurite was accumulated by the cells, which accounts for 2.7 × 108 tellurite molecules per cell, which is the maximum tellurite concentration accumulated.
FIG. 2.
Tellurite removal during magnetotactic bacterial cell growth. Tellurite removal using magnetotactic bacteria (□, dashed line) and cell growth (▴, black line) was evaluated in the presence of 40 μM tellurite for 7 days. The average values from three independent experiments were obtained. Error bars show standard deviations.
TABLE 1.
Tellurium recovery using magnetotactic bacteria
| Initial tellurite concn in MSGM (μM) | Final cell density (×107 cells/ml) | Recovery of tellurium from MSGM (%) | No. of recovered tellurium molecules/cell | Avg no. of particles/cella |
|
|---|---|---|---|---|---|
| Magnetite | Tellurium | ||||
| 0 | 12.4 | 23.6 ± 4.1 | |||
| 10 | 6.5 | 45.2 | 4.2 × 107 | 15.1 ± 3.3 | 8.2 ± 4.8 |
| 20 | 4.0 | 58.8 | 1.8 × 108 | 11.7 ± 3.5 | 14.8 ± 7.6 |
| 40 | 3.5 | 38.6 | 2.7 × 108 | 9.4 ± 4.2 | 32.2 ± 9.6 |
The data are shown as means ± standard deviations.
In order to compare this method with the cell surface adsorption of tellurium, tellurium adsorption onto a magnetotactic bacteria cell membrane modified with hexahistidine-tagged outer membrane protein (MspI) in 40 μM tellurite was also evaluated based on the previously described EDTA wash method (40). Approximately 3.6 × 106 tellurium atoms per cell were adsorbed. This value is similar to that of Cd2+ adsorption onto the modified cell surface (3.8 × 106) (40), which is 2 orders of magnitude less than the accumulation achieved by crystallization within the cell presented in this study.
Analysis of simultaneous biomineralization of discrete tellurium and magnetite nanoparticles within M. magneticum AMB-1.
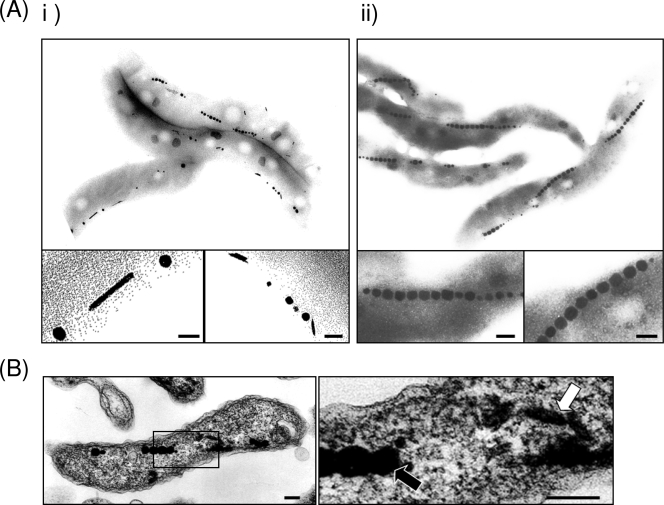
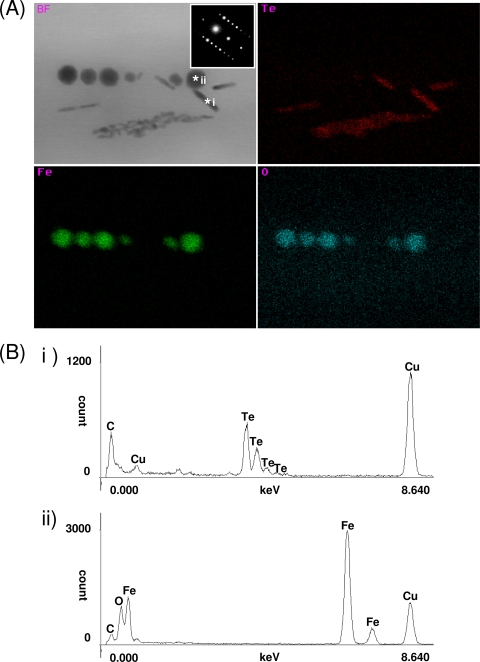
To verify the accumulation of tellurium within M. magneticum AMB-1, TEM analysis was conducted on unstained samples. Rod-shaped nanostructures (∼15 nm in diameter by ∼200 nm in length) in addition to the magnetite magnetosome crystals were observed (Fig. 3 A and B). The number and the size of these crystals within the cell increased with increasing initial concentration of tellurite in the medium (Table 1). Additionally, the morphological change of magnetite crystals within the magnetotactic bacteria grown in the presence of tellurite was not observed. Elemental analysis using STEM-EDX on these samples reveals that the magnetosomes are composed of magnetite (black arrow) and the nanorods contain tellurium (white arrow), and both are present within the same cell. It should also be noted that tellurium nanorods were not observed in either the periplasmic space or on the cell surface (Fig. 3B). Results from representative M. magneticum strain AMB-1 with biominerals containing tellurium are presented in Fig. 4A. Te, Fe, and O element mapping of magnetotactic bacteria clearly shows crystals containing either Te or Fe and O. The spot scan shows that C, O, Fe, and Te were the only elements present (the Cu and C were from the copper TEM grid and carbon coating, respectively) (Fig. 4B). Tellurium in the rod-shaped crystals (*i) and Fe and O in magnetite (*ii) were concentrated within the magnetotactic bacteria. In addition, the linear electron diffraction pattern of spots, which is identical to the tellurium single crystalline form (the Joint Committee on Powder Diffraction Standards no. 36-1452), was observed from the rod-shaped crystal by SAED analysis. Therefore, these analyses show that there is no tellurium doping of the magnetite and suggest the rod-shaped crystals are composed of pure elemental crystalline tellurium(0), which seems to be reduced from tellurite(IV) in the medium.
FIG. 3.
Transmission electron micrographs of magnetotactic bacteria grown in the presence of tellurite. (A) Magnetotactic bacteria grown (i) in the presence of tellurite (40 μM) and (ii) in its absence. The scale bar indicates 100 nm. (B) Ultrathin-sectioned micrograph of magnetotactic bacteria grown in the presence of tellurite. The image on the right is a magnification of the square area from the left image. Black and white arrows indicate magnetite and tellurium, respectively. The scale bar indicates 100 nm.
FIG. 4.
STEM-EDX and SAED analyses for magnetite and tellurium within magnetotactic bacteria. (A) Bright-field (BF) STEM image with SAED patterns of a rod-shaped crystal and STEM-EDX maps of Te, Fe, and O taken using a probe size of approximately 2 nm; (B) spot scans of *i and *ii as representations of tellurium and magnetite, respectively.
Magnetic recovery of magnetotactic bacteria cultured in the presence of tellurite.
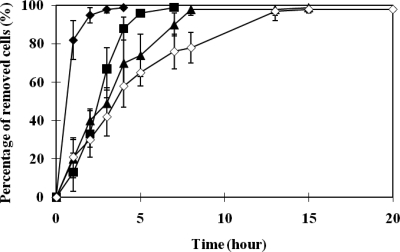
The fact that magnetotactic bacteria can be recovered using a magnetic field significantly increases the potential to utilize them for bioremediation. The magnetic recovery assay shows that approximately 100% of the bacteria grown in 10 μM tellurite were successfully recovered within 7 h (Fig. 5). However, the time for magnetic cell recovery gradually increased with increasing concentrations of tellurite. For cells grown in 40 μM tellurite, total cell recovery required 15 h. The amount of tellurium depleted from the medium containing 40 μM tellurite was also determined, and a total of approximately 33.6% of the tellurium was accumulated in the cells. Thus, in addition to the finding that magnetotactic bacteria can be utilized for bioremediation, in the presence of tellurite, we have demonstrated that the cells can be efficiently recovered.
FIG. 5.
Magnetic recovery assay of tellurium crystal-containing M. magneticum AMB-1. The percentage of recovered cells is calculated from the initial cell numbers (1.0 × 108/ml) by counting the number of dispersed cells left within the culture medium. In addition, the number of cells recovered by magnetic force was also verified by counting the cells recovered at the endpoints. M. magneticum AMB-1 (0 μM (⧫), 10 μM (▪), 20 μM (▴), and 40 μM (⋄)) was cultured and assayed with the respective concentrations of tellurite. The average values from three independent experiments were obtained. Error bars show standard deviations.
DISCUSSION
Tellurite is known to be toxic to most microorganisms at concentrations as low as 10 μM (38, 45). The model Gram-negative bacterium Escherichia coli O157 (wild type) has a MIC of 4.5 μM for tellurite. However, a specific resistant isolate has shown a MIC of 4.6 mM (44). The MIC of tellurite for M. magneticum AMB-1 was determined to be 60 μM, which shows M. magneticum AMB-1 to be 13 times more resistant to tellurite than E coli (wild type). Although M. magneticum AMB-1 does not display resistance as high as that of some tellurite-resistant organism, the cells are capable of tellurite reduction and crystallization mechanisms within the cell (Fig. 4).
A number of genetic tellurite resistance determinants that can be found in the bacterial chromosome have been identified in different bacteria (9). Although these determinants mediate tellurite resistance by an as-yet-unknown mechanism, several genes such as those coding for nitrate reductase (narGHIJ; E. coli) (1) and dihydrolipoamide dehydrogenase (lpdA; Aeromonas hydrophila) (8), the tellurite resistance gene trgAB (Rhodobactor sphaeroides) (28), and the toxic anion resistance gene telA (R. sphaeroides) (28) were suggested to be linked to the reduction of tellurite. Within the whole-genomic information on M. magneticum AMB-1 (22), lpdA (Amb3963, 4e−88; Amb2321, 1e−63; and Amb1666, 1e−41), trgB (Amb1307, 5e−26), narH (Amb3289, 3e−21; Amb3542, 2e−18; Amb1649, 4e−17; and Amb3377, 4e−13) were found and presumed to be the respective homologous gene. Although further study is necessary, these genes may play a role in the reduction of tellurite within the magnetotactic bacteria. Recently, tellurite was suggested to serve as an electron acceptor for anaerobic growth of some prokaryotes during production of the tellurium(0) crystal, while no dissimilatory electron transport to tellurium compounds is known to date (10). To understand the tellurium precipitation mechanism within the magnetotactic bacteria, further biochemical and genetic studies are required.
Tellurium is an interesting element. A metalloid, it has properties likening it to metals such as iron, as well as its fellow chalcogens such as oxygen and sulfur, having various oxidation states and a similar ionic radius. A gradual decrease of magnetite crystal formation was seen for cells grown in higher tellurite concentrations (Table 1). This was also verified by the magnetic recovery assay, as cells recovered from a higher tellurite concentration contained more tellurium crystals and fewer magnetite crystals, rendering them less magnetic, thus demanding a longer recovery time (Fig. 5). These observations suggest tellurium inhibits magnetosome formation or that the tellurium crystallization system competes to some extent with the magnetosome synthesis system.
Interestingly, many (not all) rod-shaped tellurium nanocrystals were observed along the magnetite crystal chain within magnetotactic bacteria (Fig. 3). Within other microorganisms, internal tellurium crystals are formed close to the cell's periphery, conform to the contour of the cell envelope, and randomly form in the cytoplasmic space (7, 29, 32, 44). This leads to questions of how these nanorods interact with magnetosome components such as the actin-like protein MamK (18, 43) and thus questions about the possibility of whether or not these nanorods are forming within magnetosome-like vesicles. However, tellurium crystals surrounded by membranous structure were not observed by TEM analysis of thin-sectioned samples in this study.
In addition to tellurium affecting magnetosome synthesis and tellurium crystal localization, the fact that tellurium crystals form independently of magnetite crystals is also very curious. There have been reports of nonferrous metal incorporation (e.g., manganese and cobalt) into magnetite crystals in magnetosomes (15, 37). In this research, tellurite has been reduced and crystallized independently of magnetite crystals, contrary to the partial oxidation and incorporation into magnetosomes that occur for ferrous ions. Perhaps then tellurium should be considered to be behaving like the other chalcogens. Thiosulfate (S2O32−) is frequently taken up by bacteria and reduced to elemental sulfur (S0) to form sulfur globules (20). Indeed there have been reports of sulfur globule formation occurring in several magnetotactic bacteria, although the mechanism is still unknown (3, 36). There are literature reports of tellurium substitution of sulfur in amino acids such as cysteine in tellurium-resistant fungi (26, 31). Therefore, as tellurium can be considered to be an analogue to sulfur, we tentatively suggest that the tellurium nanorods described in this report are analogues to sulfur globules.
Recent research in material sciences has focused on preparing nanorods of either TeO2 or Te using expensive and hazardous organic coordinating solvents, as well as some complexing agents (13, 21). Crystallization within magnetotactic bacteria may also yield diverse tellurium nanomaterials that can be generated under mild conditions using tellurite from the environment and without requiring the use of either harsh chemical or physical methods.
Conclusion.
We have reported the first account of tellurite uptake by magnetic bacteria and demonstrated successful biomineralization of discrete crystals of tellurium and magnetite within the same cell, and thus efficient magnetic recovery was verified. This discovery could lead to development of a novel tellurium recovery system. Therefore, we believed that magnetotactic bacteria provide new advantages for the development of various biorecovery technologies, the bioremediation of polluted water, and also new methods of tellurium nanomaterial synthesis.
Acknowledgments
This work was partially supported in part by Grant-in-Aid for Scientific Research (A) no. 18206084.
We thank T. Takai, Tokyo University of Agriculture and Technology, for much experimental support and K. Akiyama, Hanaichi Co., Ltd., Japan, for technical support with TEM and STEM-EDX.
Footnotes
Published ahead of print on 25 June 2010.
REFERENCES
- 1.Avazéri, C., R. Turner, J. Pommier, J. Weiner, G. Giordano, and A. Verméglio. 1997. Tellurite reductase activity of nitrate reductase is responsible for the basal resistance of Escherichia coli to tellurite. Microbiology 143:1181-1189. [DOI] [PubMed] [Google Scholar]
- 2.Baesman, S. M., T. D. Bullen, J. Dewald, D. Zhang, S. Curran, F. S. Islam, T. J. Beveridge, and R. S. Oremland. 2007. Formation of tellurium nanocrystals during anaerobic growth of bacteria that use Te oxyanions as respiratory electron acceptors. Appl. Environ. Microbiol. 73:2135-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazylinski, D., A. Dean, T. Williams, L. Long, S. Middleton, and B. Dubbels. 2004. Chemolithoautotrophy in the marine, magnetotactic bacterial strains MV-1 and MV-2. Arch. Microbiol. 182:373-387. [DOI] [PubMed] [Google Scholar]
- 4.Bazylinski, D. A., R. B. Frankel, B. R. Heywood, S. Mann, J. W. King, P. L. Donaghay, and A. K. Hanson. 1995. Controlled biomineralization of magnetite Fe3O4 and Greigite Fe3S4 in a magnetotactic bacterium. Appl. Environ. Microbiol. 61:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazylinski, D. A., A. J. Garratt-Reed, A. Abedi, and R. B. Frankel. 1993. Copper association with iron sulfide magnetosomes in a magnetotactic bacterium. Arch. Microbiol. 160:35-42. [Google Scholar]
- 6.Blakemore, R. P., D. Maratea, and R. S. Wolfe. 1979. Isolation and pure culture of a freshwater magnetic spirillum in chemically defined medium. J. Bacteriol. 140:720-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghese, R., F. Borsetti, P. Foladori, G. Ziglio, and D. Zannoni. 2004. Effects of the metalloid oxyanion tellurite (TeO32−) on growth characteristics of the phototrophic bacterium Rhodobacter capsulatus. Appl. Environ. Microbiol. 70:6595-6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro, M., R. Molina, W. Díaz, S. Pichuantes, and C. Vásquez. 2008. The dihydrolipoamide dehydrogenase of Aeromonas caviae ST exhibits NADH-dependent tellurite reductase activity. Biochem. Biophys. Res. Commun. 375:91-94. [DOI] [PubMed] [Google Scholar]
- 9.Chasteen, T., D. Fuentes, J. Tantaleán, and C. Vásquez. 2009. Tellurite: history, oxidative stress, and molecular mechanisms of resistance. FEMS Microbiol. Rev. 33:820-832. [DOI] [PubMed] [Google Scholar]
- 10.Csotonyi, J., E. Stackebrandt, and V. Yurkov. 2006. Anaerobic respiration on tellurate and other metalloids in bacteria from hydrothermal vent fields in the eastern Pacific Ocean. Appl. Environ. Microbiol. 72:4950-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deplanche, K., and L. E. Macaskie. 2008. Biorecovery of gold by Escherichia coli and Desulfovibrio desulfuricans. Biotechnol. Bioeng. 99:1055-1064. [DOI] [PubMed] [Google Scholar]
- 12.Dopp, E., L. M. Hartmann, A. M. Florea, A. W. Rettenmeier, and A. V. Hirner. 2004. Environmental distribution, analysis, and toxicity of organometal(loid) compounds. Crit. Rev. Toxicol. 34:301-333. [DOI] [PubMed] [Google Scholar]
- 13.Gautam, K. U., and C. N. R. Rao. 2004. Controlled synthesis of crystalline tellurium nanorods, nanowires, nanobelts and related structures by a self-seeding solution process. J. Mater. Chem. 14:2530-2535. [Google Scholar]
- 14.Junier, P., M. Frutschi, N. Wigginton, E. Schofield, J. Bargar, and R. Bernier-Latmani. 2009. Metal reduction by spores of Desulfotomaculum reducens. Environ. Microbiol. 11:3007-3017. [DOI] [PubMed] [Google Scholar]
- 15.Keim, C., U. Lins, and M. Farina. 2009. Manganese in biogenic magnetite crystals from magnetotactic bacteria. FEMS Microbiol. Lett. 292:250-253. [DOI] [PubMed] [Google Scholar]
- 16.Klonowska, A., T. Heulin, and A. Vermeglio. 2005. Selenite and tellurite reduction by Shewanella oneidensis. Appl. Environ. Microbiol. 71:5607-5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi, A., and Y. Ogra. 2009. Metabolism of tellurium, antimony and germanium simultaneously administered to rats. J. Toxicol. Sci. 34:295-303. [DOI] [PubMed] [Google Scholar]
- 18.Komeili, A., Z. Li, D. K. Newman, and G. J. Jensen. 2006. Magnetosomes are cell membrane invaginations organized by the actin-like protein MamK. Science 311:242-245. [DOI] [PubMed] [Google Scholar]
- 19.Law, N., S. Ansari, F. Livens, J. Renshaw, and J. Lloyd. 2008. Formation of nanoscale elemental silver particles via enzymatic reduction by Geobacter sulfurreducens. Appl. Environ. Microbiol. 74:7090-7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, Y., M. Dashti, A. Prange, F. Rainey, M. Rohde, W. Whitman, and J. Wiegel. 2007. Thermoanaerobacter sulfurigignens sp. nov., an anaerobic thermophilic bacterium that reduces 1 M thiosulfate to elemental sulfur and tolerates 90 mM sulfite. Int. J. Syst. Evol. Microbiol. 57:1429-1434. [DOI] [PubMed] [Google Scholar]
- 21.Liu, Z., Z. Hu, J. Liang, S. Li, Y. Yang, S. Peng, and Y. Qian. 2004. Size-controlled synthesis and growth mechanism of monodisperse tellurium nanorods by a surfactant-assisted method. Langmuir 20:214-218. [DOI] [PubMed] [Google Scholar]
- 22.Matsunaga, T., Y. Okamura, Y. Fukuda, A. T. Wahyudi, Y. Murase, and H. Takeyama. 2005. Complete genome sequence of the facultative anaerobic magnetotactic bacterium Magnetospirillum sp. strain AMB-1. DNA Res. 12:157-166. [DOI] [PubMed] [Google Scholar]
- 23.Matsunaga, T., T. Suzuki, M. Tanaka, and A. Arakaki. 2007. Molecular analysis of magnetotactic bacteria and development of functional bacterial magnetic particles for nano-biotechnology. Trends Biotechnol. 25:182-188. [DOI] [PubMed] [Google Scholar]
- 24.Mikheenko, I., M. Rousset, S. Dementin, and L. Macaskie. 2008. Bioaccumulation of palladium by Desulfovibrio fructosivorans wild-type and hydrogenase-deficient strains. Appl. Environ. Microbiol. 74:6144-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore, M., and S. Kaplan. 1992. Identification of intrinsic high-level resistance to rare-earth oxides and oxyanions in members of the class Proteobacteria: characterization of tellurite, selenite, and rhodium sesquioxide reduction in Rhodobacter sphaeroides. J. Bacteriol. 174:1505-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moroder, L. 2005. Isosteric replacement of sulfur with other chalcogens in peptides and proteins. J. Pept. Sci. 11:187-214. [DOI] [PubMed] [Google Scholar]
- 27.Nilges, T., S. Lange, M. Bawohl, J. M. Deckwart, M. Janssen, H. D. Wiemhofer, R. Decourt, B. Chevalier, J. Vannahme, H. Eckert, and R. Weihrich. 2009. Reversible switching between p- and n-type conduction in the semiconductor Ag10Te4Br3. Nat. Mater. 8:101-108. [DOI] [PubMed] [Google Scholar]
- 28.O'Gara, J., M. Gomelsky, and S. Kaplan. 1997. Identification and molecular genetic analysis of multiple loci contributing to high-level tellurite resistance in Rhodobacter sphaeroides 2.4.1. Appl. Environ. Microbiol. 63:4713-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ollivier, P., A. Bahrou, S. Marcus, T. Cox, T. Church, and T. Hanson. 2008. Volatilization and precipitation of tellurium by aerobic, tellurite-resistant marine microbes. Appl. Environ. Microbiol. 74:7163-7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pósfai, M., P. Buseck, D. Bazylinski, and R. Frankel. 1998. Iron sulfides from magnetotactic bacteria: structure, composition, and phase transitions. Am. Mineral. 83:1469-1481. [Google Scholar]
- 31.Ramadan, S., A. Razak, A. Ragab, and M. Elemeleigy. 1989. Incorporation of tellurium into amino-acids and proteins in a tellurium-tolerant fungi. Biol. Trace Elem. Res. 20:225-232. [DOI] [PubMed] [Google Scholar]
- 32.Rathgeber, C., N. Yurkova, E. Stackebrandt, J. T. Beatty, and V. Yurkov. 2002. Isolation of tellurite- and selenite-resistant bacteria from hydrothermal vents of the Juan de Fuca Ridge in the Pacific Ocean. Appl. Environ. Microbiol. 68:4613-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruta, L., C. Paraschivescu, M. Matache, S. Avramescu, and I. Farcasanu. 2010. Removing heavy metals from synthetic effluents using “kamikaze” Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 85:763-771. [DOI] [PubMed] [Google Scholar]
- 34.Shibasaki, S., H. Maeda, and M. Ueda. 2009. Molecular display technology using yeast—arming technology. Anal. Sci. 25:41-49. [DOI] [PubMed] [Google Scholar]
- 35.Simmons, S. L., D. A. Bazylinski, and K. J. Edwards. 2006. South-seeking magnetotactic bacteria in the Northern Hemisphere. Science 311:371-374. [DOI] [PubMed] [Google Scholar]
- 36.Spring, S., R. Amann, W. Ludwig, K. Schleifer, H. van Gemerden, and N. Petersen. 1993. Dominating role of an unusual magnetotactic bacterium in the microaerobic zone of a freshwater sediment. Appl. Environ. Microbiol. 59:2397-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staniland, S., W. Williams, N. Telling, G. Van Der Laan, A. Harrison, and B. Ward. 2008. Controlled cobalt doping of magnetosomes in vivo. Nat. Nanotechnol. 3:158-162. [DOI] [PubMed] [Google Scholar]
- 38.Summers, A. O., and G. A. Jacoby. 1977. Plasmid-determined resistance to tellurium compounds. J. Bacteriol. 129:276-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka, M., A. Arakaki, and T. Matsunaga. 2010. Identification and functional characterization of liposome tubulation protein from magnetotactic bacteria. Mol. Microbiol. 76:480-488. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka, M., Y. Nakata, T. Mori, Y. Okamura, H. Miyasaka, H. Takeyama, and T. Matsunaga. 2008. Development of a cell surface display system in a magnetotactic bacterium, Magnetospirillum magneticum AMB-1. Appl. Environ. Microbiol. 74:3342-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka, M., Y. Okamura, A. Arakaki, T. Tanaka, H. Takeyama, and T. Matsunaga. 2006. Origin of magnetosome membrane: proteomic analysis of magnetosome membrane and comparison with cytoplasmic membrane. Proteomics 6:5234-5247. [DOI] [PubMed] [Google Scholar]
- 42.Tang, Z., Z. Zhang, Y. Wang, S. C. Glotzer, and N. A. Kotov. 2006. Self-assembly of CdTe nanocrystals into free-floating sheets. Science 314:274-278. [DOI] [PubMed] [Google Scholar]
- 43.Taoka, A., R. Asada, L. F. Wu, and Y. Fukumori. 2007. Polymerization of the actin-like protein MamK, which is associated with magnetosomes. J. Bacteriol. 189:8737-8740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor, D. 1999. Bacterial tellurite resistance. Trends Microbiol. 7:111-115. [DOI] [PubMed] [Google Scholar]
- 45.Taylor, D. E., M. Rooker, M. Keelan, L. K. Ng, I. Martin, N. T. Perna, N. T. Burland, and F. R. Blattner. 2002. Genomic variability of O islands encoding tellurite resistance in enterohemorrhagic Escherichia coli O157:H7 isolates. J. Bacteriol. 184:4690-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tordoff, G. M., A. J. Baker, and A. J. Willis. 2000. Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 41:219-228. [DOI] [PubMed] [Google Scholar]
- 47.Yamada, A., N. Miyagishima, and T. Matsunaga. 1997. Tellurite removal by marine photosynthetic bacteria. J. Mar. Biotechnol. 1:46-49. [Google Scholar]