Abstract
The applicability of 454 pyrosequencing to characterize bacterial biofilm communities from two water meters of a drinking water distribution system was assessed. Differences in bacterial diversity and composition were observed. A better understanding of the bacterial ecology of drinking water biofilms will allow for effective management of water quality in distribution systems.
Drinking water distribution systems (DWDS) are interesting model systems for studies of microbial diversity and ecosystem functions in engineered environments. DWDS biofilms in particular have received much focus due to the importance of potable water delivery to end-point consumers (8). Both cultivation-based and molecular approaches have been used to reveal bacterial communities from different locations or pipe materials within a DWDS and from DWDS that differ in source water and nutrients. Studies of bacterial communities throughout DWDS have indicated that populations can differ drastically from source water to tap water (7). Activities of microbial growth and the presence of potential opportunistic pathogens have been detected in tap water, faucets, and showerheads, etc., all end points of the DWDS (8, 14). Many studies attribute the survival of opportunistic pathogens in DWDS to resistance mechanisms such as cell wall permeability and biofilm formation (9, 10). The type of bacterial communities present in a DWDS and the disinfection regime applied may also be factors influencing pathogen retention in the DWDS. Nitrifying microorganisms, for example, can contribute to the depletion of monochloramine and subsequently lead to increased overall microbial growth (7).
Compared to the analysis of microbial communities in source water and end-point drinking water, investigations related to the delivery process are difficult due to limited access and the high cost involved in sampling within the DWDS. To overcome these challenges, several studies have used model DWDS to mimic full-scale DWDS so as to address treatment effects on water quality. The researchers in these studies reported that episodic chlorination may accelerate the development of microbial communities with increased resistance to disinfectants (4) and that bacterial diversity can also affect disinfection efficacy and pathogen survival (1). In addition, the occurrence of bacterial community succession was observed in a model DWDS (17), which indicated the importance of long-term monitoring of bacterial biofilm development in the DWDS. These findings also demonstrated the need for the direct study of microbial communities within the DWDS, which would complement studies of the source water and end points, give a more complete view of the microbial ecology of the DWDS, and lend insights for subsequent monitoring of the DWDS.
Biofilms obtained from water meters can be a good alternative to those obtained from distribution water pipes to study biofilms in DWDS because the water meters can be obtained relatively easily from private households. Moreover, the biofilms in the water meters not only represent the microbial communities at the point during which potable water is being delivered to consumers, but also serve as a means to study biofilm development in the DWDS during the water delivery process. As a preliminary study, we explored the possibility of analyzing biofilms from water meters by using pyrosequencing technology. We obtained biofilms from two water meters in a water distribution network to evaluate whether the biofilms in the water meters represented the microbial ecology of the DWDS. Furthermore, we aimed to assess the potential use of 454 pyrosequencing as an effective approach for the detection of microorganisms in the DWDS for subsequent large-scale monitoring of microbial community changes in the DWDS.
Water meters WDMS_A and WDMS_B were collected from two private households in Urbana-Champaign, IL, in October and December 2008, respectively, and disassembled to collect samples for bacterial analysis. To confirm bacterial growth in the DWDS, a plastic strainer that had a brown biofilm-like coating was obtained from a separate water meter for scanning electron microscopy (SEM) analysis. The plastic strainer was fixed as described previously (3) and dried with a CO2 critical point dryer (Tousimis, MD). The plastic strainer was sputter coated with gold-palladium and viewed with a Philips XL30 field emission environmental scanning electron microscope (FEI, OR). SEM analysis of the plastic strainer revealed different features of biofilm morphology in different fields of the surface. In one field (Fig. 1 A), extracellular matrix material was present on the plastic strainer, and this material was also observed previously in showerheads (8). In another field (Fig. 1B), sparse populations of mainly rod-shaped bacteria with a polar flagellum were observed alongside the deposits on the surface of the plastic strainer. This observation was different from that in a DWDS pipe (16), where bacteria of different morphologies (e.g., rod-shaped and coccoid) and higher cell density were observed. These observed differences could be attributed to the reduced surface area and different substratum material for the plastic strainer compared to the DWDS pipe.
FIG. 1.
SEM images of extracellular matrix material (A) and rod-shaped bacteria (B) on the surface of the plastic strainer obtained from a water meter.
Based on the SEM results, we further addressed whether it was possible to sample the water meter biofilms and study their bacterial diversity. Biofilms from the inner surfaces and components of the water meters were obtained using sterile cotton swabs. The swabs were suspended in 1× phosphate-buffered saline (PBS), and the biofilms were dislodged via vortexing. The suspensions were then centrifuged to collect the pellets. DNA was extracted from the pellets using a chemical and enzymatic DNA extraction protocol as described previously (22) and was amplified with bacterium-specific forward primer 27F (5′-Fusion A-Barcode-CA linker-AGAGTTTGATCCTGGCTCAG-3′) and reverse primer 534R (5′-Fusion B-TC linker-ATTACCGCGGCTGCTGGC-3′) (10a). 454 pyrosequencing was carried out with a 454 Life Sciences GS FLX series genome sequencer (Roche, Switzerland).
The sequences were trimmed (resulting in an average sequence length of 230 bp), and merged alignments of the sequences aligned via the Infernal aligner from the Ribosomal Database Project (RDP) pyrosequencing pipeline (http://pyro.cme.msu.edu/) and the NAST alignment tool from Greengenes (6) were obtained via software developed by the biotechnology center at the University of Illinois (http://acai.igb.uiuc.edu/bio/merge-nast-infernal.html). RDP Classifier was used for taxonomical assignments of the aligned 454 pyrosequences at the 95% confidence level.
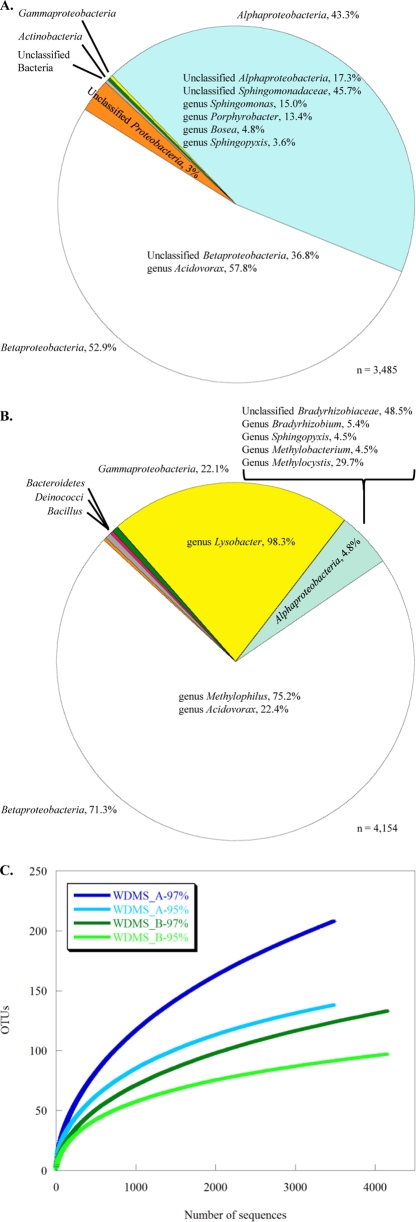
The total bacterial communities of the two water meters were analyzed for the number of operational taxonomic units (OTUs), rarefaction, and species richness by using the DOTUR program (21). Since the current length restriction for sequences obtained from pyrosequencing is a challenge to the estimation of microbial richness, the numbers of OTUs were estimated at two different cutoffs, 95 and 97% 16S rRNA gene sequence similarity. Approximately 3,485 and 4,154 16S rRNA pyrotags were obtained for WDMS_A and WDMS_B, respectively. Based on this number of pyrotags, 208 and 133 OTUs (defined at 97% gene similarity) were found in WDMS_A and WDMS_B, respectively. The more conservative approach (defined at 95% gene similarity) yielded 110 and 89 OTUs. The rarefaction curve approximated toward a plateau after ∼3,500 pyrotags were sequenced, therefore indicating that enough sample coverage was obtained in this study (Fig. 2 C). The species richness estimators (Chao1 and the abundance-based coverage estimator [ACE]) showed that WDMS_B had a lower level of diversity than WDMS_A (Table 1). We classified the bacterial compositions of the water meter biofilms into Firmicutes, Deinococcus-Thermus, Bacteroidetes, Actinobacteria, and Proteobacteria, with the majority of the sequences from the Proteobacteria classified to the genus level. The taxonomic distribution of our data is in agreement with other findings for bacterial populations in the DWDS environment (7, 18).
FIG. 2.
(A and B) Phylogenetic compositions represented by bacterial sequences from water meters WDMS_A (A) and WDMS_B (B). The percentage of bacteria from each division was calculated, and results of ≥3% are shown. The composition and percentage of each bacterial genus are shown in the sector of the respective class of Proteobacteria. (C) Rarefaction analysis of the two water meters at levels of 95 and 97% 16S rRNA gene similarity.
TABLE 1.
Bacterial diversity in the two water meters
| Water meter | Total no. of OTUs at: |
Chao1 estimate of richness (95% CI)a at: |
ACE estimate of richness (95% CI) at: |
|||
|---|---|---|---|---|---|---|
| 97% sequence similarity | 95% sequence similarity | 97% sequence similarity | 95% sequence similarity | 97% sequence similarity | 95% sequence similarity | |
| WDMS_A | 208 | 110 | 341 (284-441) | 136 (120-173) | 339 (292-412) | 138 (124-168) |
| WDMS_B | 133 | 89 | 202 (167-273) | 116 (99-158) | 206 (174-262) | 116 (102-148) |
95% CI, 95% confidence interval.
It was further observed that the two water meters had different bacterial community compositions. The bacterial composition in WDMS_A consisted of two major bacterial populations from the Betaproteobacteria (53%) and Alphaproteobacteria (43%), together with minor populations from the Gammaproteobacteria (Fig. 2A). In WDMS_A, the main population of Betaproteobacteria belonged to the genus Acidovorax (58%), and mainly Sphingomonas-like sequences represented the Alphaproteobacteria (Fig. 2A). In contrast, the Betaproteobacteria population predominated (at >70%) in the WDMS_B microflora, with Gammaproteobacteria (at >20%) being the other main population (Fig. 2B). Alphaproteobacteria were present at only approximately 5% of the WDMS_B microflora, and other bacterial groups were present at much lower proportions (Fig. 2B). In WDMS_B, members of the genus Methylophilus constituted 75% of the Betaproteobacteria, and this genus was not detected in WDMS_A (Fig. 2A and B). Bacteria of the genus Lysobacter constituted the main population (98%) of Gammaproteobacteria (Fig. 2B).
Evaluation at the genus level showed that members of the genera Sphingomonas and Acidovorax were the two predominant bacterial groups in water meter WDMS_A. These two genera comprise metabolically diverse species capable of using a wide range of naturally occurring compounds. With respect to the DWDS, members within these two genera have been implicated in copper pipe corrosion (5) and demonstrated to enhance biofilm formation by other bacterial groups (23) and could be potential opportunistic pathogens (15, 24). However, due to the short read lengths of the sequences obtained, the precise species identities of the members cannot be distinguished, and these sequences could represent ubiquitous members in the environment that may not pose risks to human health.
In both water meters, a few methanotrophs belonging to the family Methylococcaceae of the Gammaproteobacteria were detected (data not shown). Methane was detected in the groundwater that serves as the source water for Urbana-Champaign and could have possibly favored the occurrence of methanotrophs in the DWDS (13). Methanotrophs can play a role in the nitrogen cycle through mechanisms such as their coexistence with heterotrophic nitrifiers via cross-feeding of metabolites (19). In the presence of environmental gradients of oxygen and methane, methanotrophs can also support denitrification by both creating anoxic environments conducive for denitrification during methane oxidation and releasing organic substances that can be used by coexisting heterotrophic denitrifiers (19). The association between the two groups of microorganisms has led to the suggestion of using methane as an external carbon source for biological denitrification of nitrate-contaminated waters (19).
Unlike WDMS_A, WDMS_B had a relatively high abundance of members of the genus Methylophilus. The genus Methylophilus consists of a group of bacteria that oxidize methyl compounds but not methane. An abundance of Methylophilus bacteria in cave water that receives a high flux of methane was observed previously (2). It was suggested that the methylotrophs possibly fed on methanol produced by the methanotrophs during methane oxidation (2, 20) or that the methylotroph-like sequences were in fact from as yet uncultured methanotrophs in the Betaproteobacteria (2). Since members of the genus Methylophilus can use nitrate as a nitrogen source (11), it is also possible that the Methylophilales detected in water meter WDMS_B can carry out denitrification using the methanol supplied by the methanotrophs. Methanol-dependent denitrification by members of the family Methylophilaceae was reported recently, which suggested the potential role of this group of bacteria in both nitrogen and carbon cycling (12). However, as this study examined only 16S rRNA gene amplicons retrieved from genomic DNA, it remains unknown whether the methanotrophs and methylotrophs present in the water meters were functionally active or whether there are such biogeochemical associations among them.
A temporal analysis of water quality around the sampling area indicated that turbidity, pH, and chlorine levels were relatively stable throughout October and December (see Fig. S1 in the supplemental material). Other parameters like ammonia and nitrite concentrations, however, fluctuated from 1 to 0.1 mg/liter during the sampling period (see Fig. S1 in the supplemental material). Although water quality data were available during this period, we were unable to correlate water quality data with the differences in the bacterial communities because of the small number of water meters analyzed. Future studies would aim to address this possible correlation by analyzing a larger number of water meters along with the water quality data. This additional information would allow us to corroborate whether the bacterial populations in the water meters are reflective of the water geochemistry in the area. Such analysis would then lend insight into when the DWDS may become compromised and allow for improvement in subsequent disinfection strategies or the removal of certain substrates that bacteria can use for growth.
A comprehensive analysis of the composition of the bacterial community in the DWDS in relation to the disinfection strategy applied is essential for improving the management of the DWDS. In most DWDS studies based on small-subunit rRNA clone libraries, the limited number of clones sequenced could not represent the complete picture of the diversity and complex interactions of the bacterial populations in the DWDS. The 454 pyrosequencing approach, on the other hand, allows for high-throughput sequencing in a single sequencing run. Our preliminary study has demonstrated the feasibility of sampling water meters as a means to examine DWDS biofilms and the applicability of 454 pyrosequencing, although the DWDS bacterial populations were assigned only to the genus level of the taxonomic rank. With increasing improvements in the read lengths provided by 454 pyrosequencing, we may be able to obtain better taxonomic identification of the bacterial populations in the DWDS in the near future. Thus, the 454 pyrosequencing approach can be further used to analyze DWDS bacterial communities obtained at different times (e.g., before and after a chlorination event or at time points reflecting seasonal variation) and from different locations. Such studies will help better characterize the microbial ecology in the DWDS and provide insights into treatment efficacy for drinking waters.
Supplementary Material
Acknowledgments
We thank the crew at Illinois American Waters for obtaining the two water meters, Scott J. Robinson from the ITG group of Beckman Institute for his assistance with the environmental SEM, and the staff from the Biotechnology Center at UIUC for their assistance with 454 pyrosequencing.
This work (project no. 4116) was supported by the Water Research Foundation.
Footnotes
Published ahead of print on 25 June 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Batte, M., B. Koudjonou, P. Laurent, L. Mathieu, J. Coallier, and M. Prevost. 2003. Biofilm responses to ageing and to a high phosphate load in a bench-scale drinking water system. Water Res. 37:1351-1361. [DOI] [PubMed] [Google Scholar]
- 2.Chen, Y., L. Q. Wu, R. Boden, A. Hillebrand, D. Kumaresan, H. Moussard, M. Baciu, Y. H. Lu, and J. C. Murrell. 2009. Life without light: microbial diversity and evidence of sulfur- and ammonium-based chemolithotrophy in Movile Cave. ISME J. 3:1093-1104. [DOI] [PubMed] [Google Scholar]
- 3.Clark, M. E., R. E. Edelmann, M. L. Duley, J. D. Wall, and M. W. Fields. 2007. Biofilm formation in Desulfovibrio vulgaris Hildenborough is dependent upon protein filaments. Environ. Microbiol. 9:2844-2854. [DOI] [PubMed] [Google Scholar]
- 4.Codony, F., J. Morato, and J. Mas. 2005. Role of discontinuous chlorination on microbial production by drinking water biofilms. Water Res. 39:1896-1906. [DOI] [PubMed] [Google Scholar]
- 5.Critchley, M. M., R. Pasetto, and R. J. O'Halloran. 2004. Microbiological influences in ‘blue water’ copper corrosion. J. Appl. Microbiol. 97:590-597. [DOI] [PubMed] [Google Scholar]
- 6.DeSantis, T. Z., Jr., P. Hugenholtz, K. Keller, E. L. Brodie, N. Larsen, Y. M. Piceno, R. Phan, and G. L. Andersen. 2006. NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res. 34:W394-W399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichler, S., R. Christen, C. Holtje, P. Westphal, J. Botel, I. Brettar, A. Mehling, and M. G. Hofle. 2006. Composition and dynamics of bacterial communities of a drinking water supply system as assessed by RNA- and DNA-based 16S rRNA gene fingerprinting. Appl. Environ. Microbiol. 72:1858-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feazel, L. M., L. K. Baumgartner, K. L. Peterson, D. N. Frank, J. K. Harris, and N. R. Pace. 2009. Opportunistic pathogens enriched in showerhead biofilms. Proc. Natl. Acad. Sci. U. S. A. 106:16393-16399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman, R., H. Geier, K. M. Weigel, J. Do, T. E. Ford, and G. A. Cangelosi. 2006. Roles for cell wall glycopeptidolipid in surface adherence and planktonic dispersal of Mycobacterium avium. Appl. Environ. Microbiol. 72:7554-7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grobe, S., J. Wingender, and H. C. Flemming. 2001. Capability of mucoid Pseudomonas aeruginosa to survive in chlorinated water. Int. J. Hyg. Environ. Health 204:139-142. [DOI] [PubMed] [Google Scholar]
- 10a.Hamady, M., J. J. Walker, J. K. Harris, N. J. Gold, and R. Knight. 2008. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat. Methods 5:235-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins, O., D. Byrom, and D. Jones. 1987. Methylophilus—a new genus of methanol-utilizing bacteria. Int. J. Syst. Bacteriol. 37:446-448. [Google Scholar]
- 12.Kalyuhznaya, M. G., W. Martens-Habbena, T. Wang, M. Hackett, S. M. Stolyar, D. A. Stahl, M. E. Lidstrom, and L. Chistoserdova. 2009. Methylophilaceae link methanol oxidation to denitrification in freshwater lake sediment as suggested by stable isotope probing and pure culture analysis. Environ. Microbiol. Rep. 1:385-392. [DOI] [PubMed] [Google Scholar]
- 13.Kirk, M. F., T. R. Holm, J. Park, Q. Jin, R. A. Sanford, B. W. Fouke, and C. M. Bethke. 2004. Bacterial sulfate reduction limits natural arsenic contamination in groundwater. Geology 32:953-956. [Google Scholar]
- 14.Kormas, K. A., C. Neofitou, M. Pachiadaki, and E. Koufostathi. 2010. Changes of the bacterial assemblages throughout an urban drinking water distribution system. Environ. Monit. Assess. 165:27-38. [DOI] [PubMed] [Google Scholar]
- 15.Koskinen, R., T. Ali-Vehmas, P. Kampfer, M. Laurikkala, I. Tsitko, E. Kostyal, F. Atroshi, and M. Salkinoja-Salonen. 2000. Characterization of Sphingomonas isolates from Finnish and Swedish drinking water distribution systems. J. Appl. Microbiol. 89:687-696. [DOI] [PubMed] [Google Scholar]
- 16.Liu, W., H. Wu, Z. Wang, S. L. Ong, J. Y. Hu, and W. J. Ng. 2002. Investigation of assimilable organic carbon (AOC) and bacterial regrowth in drinking water distribution system. Water Res. 36:891-898. [DOI] [PubMed] [Google Scholar]
- 17.Martiny, A. C., T. M. Jorgensen, H. J. Albrechtsen, E. Arvin, and S. Molin. 2003. Long-term succession of structure and diversity of a biofilm formed in a model drinking water distribution system. Appl. Environ. Microbiol. 69:6899-6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathieu, L., C. Bouteleux, S. Fass, E. Angel, and J. C. Block. 2009. Reversible shift in the alpha-, beta- and gamma-proteobacteria populations of drinking water biofilms during discontinuous chlorination. Water Res. 43:3375-3386. [DOI] [PubMed] [Google Scholar]
- 19.Modin, O., K. Fukushi, and K. Yamamoto. 2007. Denitrification with methane as external carbon source. Water Res. 41:2726-2738. [DOI] [PubMed] [Google Scholar]
- 20.Qiu, Q., R. Conrad, and Y. Lu. 2009. Cross-feeding of methane carbon among bacteria on rice roots revealed by DNA-stable isotope probing. Environ. Microbiol. Rep. 1:355-361. [DOI] [PubMed] [Google Scholar]
- 21.Schloss, P. D., and J. Handelsman. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt, T. M., E. F. DeLong, and N. R. Pace. 1991. Analysis of a marine picoplankton community by 16S rRNA gene cloning and sequencing. J. Bacteriol. 173:4371-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simoes, L. C., M. Simoes, and M. J. Vieira. 2007. Biofilm interactions between distinct bacterial genera isolated from drinking water. Appl. Environ. Microbiol. 73:6192-6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanetti, F., G. De Luca, and S. Stampi. 2000. Recovery of Burkholderia pseudomallei and B. cepacia from drinking water. Int. J. Food Microbiol. 59:67-72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.