Abstract
In this study, a methylotrophic bacterium, Methylobacterium rhodesianum MB 126, was used for the production of the chiral compound (R)-3-hydroxybutyrate (R-3HB) from methanol. R-3HB is formed during intracellular degradation of the storage polymer (R)-3-polyhydroxybutyrate (PHB). Since the monomer R-3HB does not accumulate under natural conditions, M. rhodesianum was genetically modified. The gene (hbd) encoding the R-3HB-degrading enzyme, R-3HB dehydrogenase, was inactivated in M. rhodesianum. The resulting hbd mutant still exhibited low growth rates on R-3HB as the sole source of carbon and energy, indicating the presence of alternative pathways for R-3HB utilization. Therefore, transposon mutagenesis was carried out with the hbd mutant, and a double mutant unable to grow on R-3HB was obtained. This mutant was shown to be defective in lipoic acid synthase (LipA), resulting in an incomplete citric acid cycle. Using the hbd lipA mutant, we produced 3.2 to 3.5 mM R-3HB in batch and 27 mM (2,800 mg liter−1) in fed-batch cultures. This was achieved by sequences of cultivation conditions initially favoring growth, then PHB accumulation, and finally PHB degradation.
Enantiomeric purity of a product or building block is often a prerequisite for its application in the health care field. Due to their chirality and the presence of two functional groups (i.e., hydroxyl and carbonic acid), (R)-3-hydroxyalkanoates are valuable building blocks for the synthesis of pharmaceutical products, such as carbapenem or macrolide antibiotics (36; for a review, see reference 9). (R)-3-hydroxyalkanoates can be obtained by hydrolysis of poly-(R)-3-hydroxyalkanoates (PHAs), which are synthesized as carbon storage polymers under conditions of nutrient limitation by many bacterial species (3). Poly-(R)-3-hydroxybutyrate (PHB), a homopolymer of (R)-3-hydroxybutyrate (R-3HB), is the most common naturally occurring PHA. For the recovery of the chiral monomers, chemical hydrolysis and a variety of biotechnological processes have been tested. The biotechnological processes include the in vitro or in vivo depolymerization of PHA using wild-type or genetically engineered microorganisms (23, 24, 30, 34, 37). Also, direct pathways for (R)-3-hydroxyalkanoate synthesis in non-PHA-producing strains have been established (15, 25). For cultivation of the (R)-3-hydroxyalkanoate-producing bacteria, sugars and alkanoates have typically been used as carbon sources.
In this study, production of R-3HB from methanol using a facultative methylotrophic bacterium, Methylobacterium rhodesianum MB 126, was assessed. Methanol, a cheap bulk chemical, is usually synthesized from natural gas or coal via syngas. In the near future, it will be possible to synthesize methanol in huge amounts directly from methane, a main component of biogas and natural gas, or from the greenhouse gas carbon dioxide (31). Thus, methanol is a promising substrate for new biotechnological processes. Members of the genus Methylobacterium are able to use reduced one-carbon compounds, such as methanol, as sole sources of carbon and energy. M. rhodesianum MB 126 accumulates PHB under conditions of excess methanol and limiting concentrations of nitrogen, phosphorus, or oxygen (1, 7). Upon provision of sufficient nutrients and in the absence of a carbon source, PHB is remobilized and used as a source of carbon and energy. In Methylobacterium, R-3HB is formed during intracellular degradation of PHB (Fig. 1). Under natural conditions, R-3HB does not accumulate, as it is rapidly channeled into the central metabolic pathways via the enzyme R-3HB dehydrogenase (21) and possibly other yet-unknown enzymes. Therefore, to achieve accumulation of the monomer, the key idea of this study was to enhance the metabolic fluxes toward R-3HB and to block R-3HB consumption, using genetic-engineering techniques and optimizing cultivation conditions.
FIG. 1.
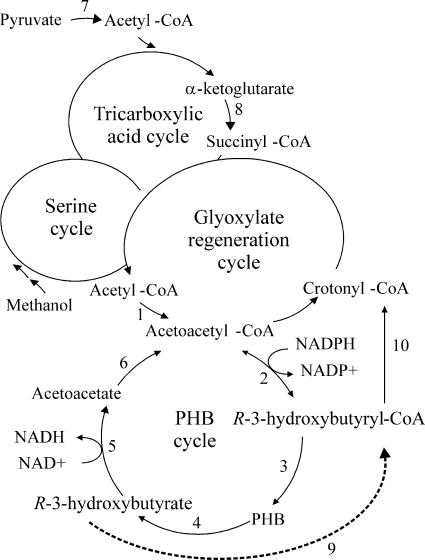
Simplified scheme of carbon assimilation cycles in Methylobacterium (modified from reference 21). Reactions of the PHB cycle are given in detail. PHB cycle enzymes are as follows: 1, β-ketothiolase; 2, NADPH-linked acetoacetyl-CoA reductase; 3, PHB synthase; 4, PHB depolymerase; 5, R-3HB dehydrogenase; 6, acetoacetate-succinyl-CoA transferase. Lipoic acid-dependent enzymes are as follows: 7, pyruvate dehydrogenase; 8, α-ketoglutarate dehydrogenase. Enzymes of the proposed alternative R-3HB utilization pathway are as follows: 9, acyl-CoA synthetase (putative enzyme [dotted line]); 10, (R)-specific enoyl-CoA hydratase.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The relevant characteristics and references for the strains and plasmids used in this work are listed in Table 1. E. coli strains were grown in Luria-Bertani (LB) medium. M. rhodesianum MB 126 and Methylobacterium extorquens AM1 were cultivated routinely in mineral medium (18) containing 125 mM methanol (standard medium). Batch cultures of Methylobacterium were cultivated aerobically in shaking flasks at 200 rpm and 30°C. Growth was recorded by optical density measurements at 600 nm (OD600). These measurements were verified by gravimetrical determinations. Fed-batch cultivations of M. rhodesianum were performed in a Labfors bioreactor (Infors, Bottmingen, Switzerland; 2-liter working volume) at 30°C and a constant pH of 7.0, maintained by the automatic addition of either 1 M NaOH or 17.5% aqueous ammonia. Aqueous ammonia was applied during growth phases, whereas NaOH was used during the other phases of the experiment. The stirrer speed was 300 rpm, and the aeration rate was 3.0 liters min−1. Methanol as a carbon source was added periodically after depletion. To induce PHB accumulation or PHB degradation, the addition of the source of nitrogen or carbon, respectively, was stopped (17).
TABLE 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Description or sequence (5′-3′)a | Reference or source |
|---|---|---|
| Strains | ||
| M. rhodesianum MB 126 | Wild-type strain | UFZb |
| M. extorquens AM1 | Wild-type strain | DSM 1338 |
| M. rhodesianum hbd mutant | MB 126 derivative; hbd::Kmr | This study |
| M. rhodesianum hbd lipA mutant | MB 126 derivative; Δhbd; transposon mutant (lipA::miniTn5-gfp) | This study |
| E. coli DH5α | λ− φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | 16 |
| E. coli HB101 | supE44 hsdS20(rB− mB−) recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 | 5 |
| E. coli S17-1 λ pir | λ pir lysogen of S17-1 (Tpr Smrthi pro hsdR hsdM+recA RP4::2-Tc::Mu-Km::Tn7) | 38 |
| Plasmids | ||
| pUC19 | E. coli cloning vector; Apr | 45 |
| pCM80 | M. extorquens expression vector; Tcr | 27 |
| pCM157 | cre expression plasmid for removal of loxP-flanked regions; Tcr | 26 |
| pCM184 | Allelic exchange vector with loxP sites flanking Kmr gene; Tcr Kmr | 26 |
| pRK2013 | Helper plasmid for triparental mating; Kmr | 14 |
| pAG408 | Suicide delivery vector pAG408 carrying a miniTn5 derivative with a promoterless gfp gene; Kmr Gmr | 39 |
| Primers | ||
| hF-Bgl | TCA GAT CTG CAT CGA ACT CGC CAT CG | This study |
| hR-Not | CTG CGG CCG CGA TGA TCT GGT CCC ACT TCT C | This study |
| hF-Apa | AGG GCC CAC ATG AAG GCG AAT GGC TG | This study |
| hR-Sac | AGA GCT CAT GTT GGC ACC GGT GAT CTG | This study |
| lipF2 | AGT CTA GAC AAG GCC TTA AGT CAG GGA TG | This study |
| lipR | TGA ATT CTG ATG CGG AAG GAT GGC ATT C | This study |
| Tn5 | GGC CAG ATC TGA TCA AGA GA | 22 |
| TnoutR | CCG CAC TTG TGT ATA AGA GTC | 35 |
| hF-hi1 | ACT AAG CTT AAG TCG AGA TAG GAG CGC ATC | This study |
| hR-xb1 | AGT CTA GAG ACG ATG ATC GAG GCA TTC ACT | This study |
Restriction sites used for cloning are in boldface.
UFZ, Helmholtz Centre for Environmental Research-UFZ, Leipzig, Germany.
For screening of potential M. rhodesianum mutants defective in R-3HB utilization, colonies obtained on standard medium agar were transferred two or three times on mineral medium agar containing 8 mM R-3HB (selective medium), as well as on standard medium agar (controls). Antibiotics were used at the following concentrations: ampicillin, 100 μg ml−1; cefoxitin, 50 μg ml−1; rifamycin, 50 μg ml−1; gentamicin, 10 μg ml−1 (Escherichia coli) or 50 μg ml−1 (Methylobacterium); kanamycin, 50 μg ml−1; and tetracycline, 20 μg ml−1.
DNA amplification.
For PCRs, genomic DNA isolated from M. rhodesianum MB 126 or M. extorquens AM1 was used as a template. Pfu DNA polymerase (Promega GmbH, Mannheim, Germany) was used for PCR amplification of inserts for cloning, and Taq DNA polymerase (Promega) was used for PCR amplification in test reactions (e.g., colony PCR). The primers used in this study are listed in Table 1. For primer design, the gapped genome sequence of M. extorquens AM1 and the sequences of published PHB cycle genes (21) were used. To locate the minitransposon insertion site in the hbd mutant, the semirandom, two-step PCR protocol (ST-PCR) strategy (13) was used as described elsewhere (35). ST-PCR was performed with primer Tn5 (22) or Tn5outR (35), targeting the minitransposon, and a random primer (35).
Vector construction.
To generate a gene replacement vector for inactivation of the hbd gene, the 0.6-kb 5′ region and the 0.38-kb 3′ region of the M. rhodesianum MB 126 hbd gene were amplified with primers hF-Bgl/hR-Not and hF-Apa/hR-Sac, respectively, and cloned into plasmid pCM184 (26), so that the hbd regions flanked the kanamycin resistance gene. In complementation experiments, genes from M. extorquens AM1 were used in place of those from M. rhodesianum MB 126 because of the earlier availability of sequence information. For complementation of the hbd lipA mutant with lipA, a 1.11-kb fragment containing the lipA gene of M. extorquens AM1, including its putative promoter region, was amplified with primers lipF2 and lipR and cloned between the XbaI-EcoRI sites of pCM80, behind the mxaF promoter (27). When present on a plasmid, the mxaF promoter acts essentially constitutively (12). For complementation of the hbd lipA mutant with hbd, a 1.14-kb fragment containing the hbd gene of M. extorquens AM1, including its putative promoter region, was amplified with primers hF-hi1 and hR-xb1 and cloned between the HindIII-XbaI sites of pCM80.
Conjugational plasmid transfer into Methylobacterium.
Plasmids were transferred into Methylobacterium by triparental mating, using E. coli DH5α bearing the respective vector as the donor and E. coli HB101 bearing plasmid pRK2013 as the helper strain. The three strains were grown to late exponential phase, pelleted, resuspended in standard medium, and mixed at a 1:1:4 ratio of donor, helper, and recipient strain. The mixture was plated on LB medium agar containing 125 mM methanol and incubated overnight at 30°C. The grown cell patches were scraped from the plates and streaked on selective standard medium agar containing cefoxitin (or rifamycin for M. extorquens AM1) and the appropriate selective antibiotic (kanamycin, gentamicin, or tetracycline). The plates were incubated for 2 to 4 days until resistant colonies appeared.
Tn5 mutagenesis.
Transposon mutagenesis of the hbd mutant of M. rhodesianum was carried out by conjugational transfer of vector pAG408 bearing the minitransposon miniTn5-gfp (39) into the hbd mutant. The E. coli S17-1 λ pir donor strain containing pAG408 and the recipient hbd mutant strain were grown to exponential phase, pelleted, and resuspended in standard medium. A 1:2 mixture of donor and recipient was plated on standard medium agar and incubated overnight at 30°C. The grown cell patches were scraped from the plates, resuspended in standard medium to a cell number of 108 to 109 ml−1, and plated on selective standard medium agar containing cefoxitin, kanamycin, and gentamicin. The plates were incubated for 2 to 4 days until kanamycin- and gentamicin-resistant colonies appeared.
Preparation of crude extracts.
M. rhodesianum wild-type or mutant strains were grown in 100 ml standard medium containing the appropriate antibiotics in 500-ml flasks to an OD600 of 1.8 to 2.0. Cells were harvested by centrifugation at 8,000 × g for 10 min; washed once with 50 ml 20 mM Tris-HCl, pH 7.8; and resuspended in the same buffer. The cells were broken by three cycles of ultrasonication using a Branson sonifier 250 (intensity, 4; 20% duty cycle; 5 min). The supernatant obtained after centrifugation at 10,000 × g was used as a crude extract.
Enzyme assays.
R-3HB dehydrogenase activity was determined photometrically by monitoring the reduction of NAD+ at 340 nm and 37°C (4). The reaction mixture contained 50 mM Tris-HCl, pH 7.8, 2 mM NAD+, 20 mM R-3HB, and crude extract. One unit of dehydrogenase activity was defined as the reduction of 1 μmol NAD+ per min, corresponding to the oxidation of 1 μmol R-3HB per min, using an extinction coefficient of NADH of 6.22 mM−1 cm−1 at 340 nm. Hydroxypyruvate dehydrogenase activity was assayed as described by Chistoserdova and Lidstrom (11).
Sequence analysis.
The gapped genome of M. extorquens AM1 was obtained from the Integrated Genomics public website (http://www.integratedgenomics.com/genomereleases.html#list6). Genome sequences from other Methylobacterium species were obtained from GenBank (http://www.ncbi.nlm.nih.gov). Sequences were compared to other published sequences by using the National Center for Biotechnology Information BLASTP search tool (http://www.ncbi.nlm.nih.gov/BLAST/). Amino acid sequences were aligned with the ClustalW program located at the European Bioinformatics Institute website (http://www.ebi.ac.uk/clustalw/). Analysis of putative promoter regions was carried out with the Neural Network Promoter Prediction tool (http://www.fruitfly.org/seq_tools/promoter.html) or Promscan (http://molbiol-tools.ca/promscan/).
Analytical methods.
R-3HB and methanol were analyzed by high-performance liquid chromatography (HPLC) (Shimadzu) using a Rezex ROA-Organic Acid H+ column (300 by 3 mm; Phenomenex, Aschaffenburg, Germany) at 20°C, with both refractive index and photo array detection. The mobile phase was 2.5 mM H2SO4 pumped at a flow rate of 0.6 ml min−1. Under these conditions, R-3HB was eluted after 14.3 min and had a detection limit of about 0.02 mM. For measurement of intracellular R-3HB, samples of 30 to 50 ml culture broth were centrifuged, and cells were washed and resuspended in 2 ml of distilled water. After incubation at 95°C for 5 min, 200 μl of 25 mM H2SO4 was added. After a final centrifugation step, R-3HB was determined in the supernatant (see above). PHB was determined after acid propanolysis of lyophilized cells as described by Riis and Mai (33). The products of propanolysis (R-3HB propyl esters) were analyzed by gas chromatography using an HP 6890 GC system from Agilent Technologies (Waldbronn, Germany) with an Optima FFAP column (Macherey & Nagel, Düren, Germany) and a flame ionization detector. Bacterial dry weight was determined gravimetrically from samples of 1 to 5 ml of culture broth after overnight oven drying at 105°C. Nitrogen in the culture supernatant was measured according to the Kjeldahl method. Protein concentrations were determined as described by Bradford (6), using bovine serum albumin as the standard.
RESULTS
Properties of the mutant defective in R-3HB dehydrogenase.
To generate an M. rhodesianum strain capable of producing R-3HB, we first blocked the known downstream enzyme, the R-3HB dehydrogenase, by site-directed inactivation of the hbd gene using the allelic exchange vector pCM184 (26). The resulting hbd mutant carried a loxP-flanked kanamycin cassette provided by pCM184, which replaced a central 48-bp fragment of the hbd gene. The disruption of hbd by the kanamycin cassette and the absence of the vector within the genome of the mutant were confirmed by diagnostic PCR. To generate an unmarked derivative of the hbd mutant, the loxP-flanked kanamycin cassette was removed with the cre expression plasmid pCM157 (26). The kanamycin-sensitive hbd mutant was subsequently cured from pCM157 by cultivation at 37°C in medium lacking tetracycline.
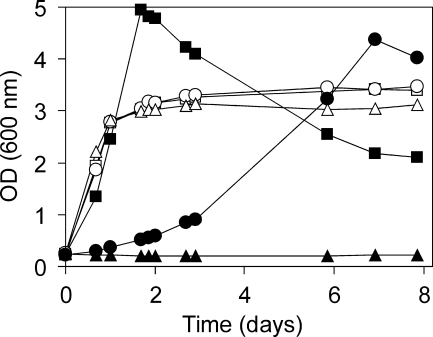
Unexpectedly, the hbd mutant was still able to grow in minimal medium on R-3HB as the sole source of carbon and energy. However, growth of the hbd mutant was significantly slower than that of the wild-type strain, while with methanol, no difference in growth rate was observed (Fig. 2).
FIG. 2.
Growth of M. rhodesianum wild-type and mutant strains in batch cultures with methanol (open symbols) or R-3HB (closed symbols). Squares, wild type; circles, hbd mutant; triangles, hbd lipA mutant.
R-3HB dehydrogenase activity was assayed in crude extracts of methanol-grown cells. The reduction of R-3HB dehydrogenase activity from 0.25 U mg−1 of protein in the wild type to below the detection limit of the assay (0.01 U mg−1 of protein) in the hbd mutant demonstrated the successful inactivation of this downstream enzyme. In a control experiment, the activity of hydroxypyruvate dehydrogenase, a serine cycle enzyme (11), was measured and amounted to 4 to 5.5 U mg−1 in crude extracts of both strains. R-3HB excretion by the wild-type strain and the hbd mutant was assessed. Surprisingly, in the culture supernatants of both strains, R-3HB either was below the detection limit or was detected only in very small amounts (0.02 to 0.3 mM). To exclude the possibility that R-3HB accumulated intracellularly, broken cells were also assayed for R-3HB; however, R-3HB concentrations did not exceed those of the corresponding supernatants. For comparison, we blocked the hbd gene in a second strain, M. extorquens AM1. It was found that this hbd mutant exhibited the same properties as our hbd mutant of strain M. rhodesianum MB 126, i.e., slow growth with R-3HB and little or no excretion of R-3HB during growth on methanol. All of these results indicated the presence of further downstream enzymes or alternative pathways for R-3HB utilization.
Generation of a transposon mutant unable to grow with R-3HB.
To detect further genes involved in R-3HB utilization, the M. rhodesianum hbd mutant was subjected to transposon mutagenesis using the minitransposon miniTn5-gfp, which contains the promoterless green fluorescent protein (GFP) gene and confers kanamycin and gentamicin resistance (39). Kanamycin- and gentamicin-resistant clones obtained on standard medium agar containing methanol were mainly nonfluorescent. Fluorescence is only found with mutants in which the minitransposon has inserted into an actively transcribed gene (39). Therefore, both fluorescent and nonfluorescent clones were investigated further. Clones were screened for the inability to grow with R-3HB while retaining the ability to grow with methanol. Of about 1,000 screened clones, one nonfluorescent mutant exhibiting these growth features was obtained and chosen for further experiments.
Analysis of the minitransposon insertion site.
The minitransposon insertion site in the M. rhodesianum transposon mutant was located by ST-PCR between positions 597 and 598 of an open reading frame containing 1,005 nucleotides. The amino acid sequence derived from this open reading frame shares 97 to 98.5% identity with the putative lipoic acid synthase (LipA) proteins of other Methylobacterium species (Methylobacterium chloromethanicum CM4 [accession number B7KRC9], M. extorquens PA1 [accession number ABY31190], M. extorquens AM1 [accession number YP_002964025], and Methylobacterium populi BJ001 [accession number YP_001925613]). The identity of the M. rhodesianum LipA sequence with biochemically characterized lipoic acid synthases is 76% with LipA from Rhizobium etli (40), 45% with LipA from E. coli (32), and 43% with LipA from the archaeon Sulfolobus solfataricus (accession number AAK43259). The 134-bp intergenic region upstream of the lipA gene in M. rhodesianum has high probability for a sigma 70-type promoter (Neural Network Promoter Prediction score, 0.86) but might also contain a sigma 54-type promoter (Promscan score, 66).
Partial sequencing of the adjacent genes showed that in M. rhodesianum, the lipA gene is located downstream of a cell wall hydrolase gene and upstream of a gene annotated as a cyclase/dehydrase, as in other Methylobacterium species. In M. rhodesianum, the stop codon of lipA is separated by only 6 bp from the start codon of the cyclase/dehydrase gene, suggesting cotranscription of the two genes.
Properties of the hbd lipA mutant.
The transposon mutant (hbd lipA mutant) of M. rhodesianum did not grow on R-3HB (Fig. 2) or other multicarbon compounds, i.e., ethanol, pyruvate, acetoacetate, racemic (RS)-3HB, succinate, or fructose, when either compound was supplied as the sole source of carbon and energy. In contrast, the wild-type strain could grow on each of these substrates. However, the hbd lipA mutant still grew on the one-carbon compound methanol (Fig. 2) and was therefore used for R-3HB production (see below). In batch cultures of the hbd lipA mutant containing both R-3HB and methanol, the R-3HB concentration slowly decreased, indicating that the hbd lipA mutant was still able to metabolize R-3HB to some degree.
In the hbd lipA mutant, the promoterless gfp gene of the minitransposon was found to be oriented in the same direction as the lipA gene, thus potentially allowing transcription of gfp from the lipA promoter (39). Indeed, colonies of the hbd lipA mutant showed weak GFP-mediated fluorescence on standard medium agar containing both R-3HB and methanol, but not on agar containing only methanol, indicating higher transcription rates of the lipA gene in the presence of a multicarbon compound.
The hbd lipA mutant could be complemented by plasmid pCM80 carrying the lipA gene from M. extorquens AM1. In lipoic acid-free mineral medium with R-3HB as the sole source of carbon and energy, growth of the hbd lipA mutant carrying pCM80-lipA was similar to that of the hbd mutant with or without the empty vector pCM80 (data not shown). No growth of the hbd lipA mutant with R-3HB was observed in mineral medium supplied with 5 ng ml−1 or 2 μg ml−1 α-lipoic acid.
hbd Complementation of the hbd lipA mutant.
To investigate the effect of an inactivated lipA gene alone, a mutant defective in lipoic acid synthase was generated by complementation of the hbd lipA mutant with the hbd gene from M. extorquens AM1. R-3HB dehydrogenase activity was restored in this lipA mutant, demonstrating successful hbd complementation. The lipA mutant showed growth characteristics similar to those of the hbd lipA mutant, i.e., the strain grew on methanol, but not on multicarbon compounds. In batch cultures, R-3HB excretion by the lipA mutant amounted to about 40% of that obtained with the hbd lipA mutant.
R-3HB excretion by the hbd lipA mutant.
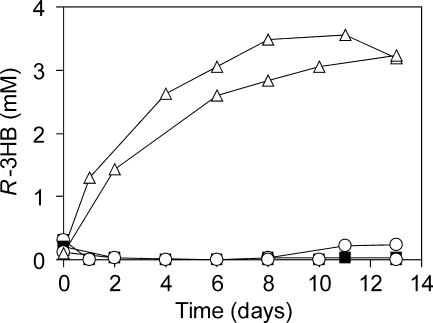
For R-3HB production in M. rhodesianum, cultivation was carried out as a sequence of conditions favoring (i) growth, (ii) PHB accumulation, and (iii) PHB degradation. Using batch cultivation, cells were grown in 200 ml standard medium to an OD600 of 3 to 3.5, harvested, and transferred to standard medium lacking a nitrogen source to induce PHB accumulation. During this step, strong aggregation of cells occurred. After 24 h, cell aggregates were harvested and transferred to 50 ml mineral medium lacking methanol to induce PHB degradation and R-3HB excretion. In the culture supernatants of the wild-type strain and the hbd mutant, the R-3HB concentrations ranged from the detection limit to very small amounts (up to 0.3 mM) (Fig. 3). In contrast, cells of the hbd lipA mutant excreted 1.3 mM R-3HB within 24 h of incubation. After 8 days, 2.8 to 3.5 mM R-3HB was obtained in the supernatant, corresponding to about 100 mg R-3HB g−1 cell dry weight. No further increase in the R-3HB concentration was observed.
FIG. 3.
R-3HB excretion by batch cultures of M. rhodesianum wild-type and mutant strains. The graph shows the PHB degradation phase of cells (3.3 g/liter [dry weight]) kept in mineral medium without methanol (see the text for details). For each strain, data for two independent experiments are depicted. Closed squares, wild type; open circles, hbd mutant; open triangles, hbd lipA mutant.
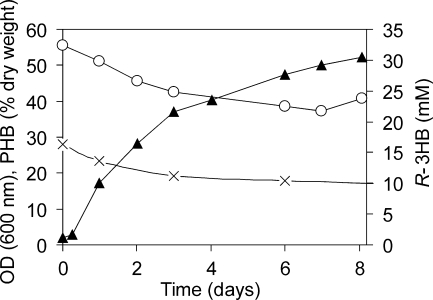
To obtain higher R-3HB yields, the hbd lipA mutant was cultivated in a bioreactor under more controlled conditions, i.e., constant pH and a high aeration rate, using the fed-batch mode. Here, transition between the different cultivation phases was achieved by omitting the further supply of methanol (shift to phase 2) or ammonium chloride (shift to phase 3) after depletion of these factors. The PHB accumulation phase was initiated after the culture had reached an OD600 of about 30 to 40, corresponding to about 10 to 15 g liter−1 cell dry weight. A slight further increase in optical density was observed during this phase. After reaching a constant OD600, the culture was shifted to the PHB degradation phase, during which R-3HB was excreted into the culture supernatant (Fig. 4). A maximum of 30.5 mM (3.2 g liter−1) R-3HB was obtained within 8 days of PHB degradation. This corresponds to 320 mg R-3HB per g cell dry weight.
FIG. 4.
R-3HB excretion by a bioreactor culture of the hbd lipA mutant. The graph shows the PHB degradation phase of cells (about 10 g/liter [dry weight]) kept in mineral medium without methanol (see the text for details). Open circles, optical density at 600 nm; crosses, PHB, expressed as percent of total cell dry weight; closed triangles, R-3HB.
For the hbd lipA mutant that accumulated PHB up to 30% of cell dry weight, a theoretical overall yield of 0.14 g R-3HB per g methanol was estimated, based on the theoretical PHB yield for methylotrophic bacteria reported by Yamane (44). Our experimental yields amounted to about 0.009 and 0.036 g R-3HB per g methanol in bioreactor and batch cultures, respectively.
DISCUSSION
In this study, a double mutant of M. rhodesianum MB 126 was generated that excreted R-3HB in substantially larger amounts than the wild type. In this mutant, the hbd gene and the lipA gene were inactivated. Inactivation of the R-3HB dehydrogenase alone, the only R-3HB-consuming enzyme known so far, was not sufficient to abolish growth on R-3HB as the sole source of carbon and energy. In contrast to our results, Korotkova and Lidstrom (21) described an hbd mutant of M. extorquens AM1, which they reported to be unable to grow with R-3HB. An explanation for our deviating observation, besides somewhat different culture conditions, could be that we possibly followed the extremely slow growth of our hbd mutant on R-3HB for a longer time.
For other bacterial species, different characteristics with respect to R-3HB metabolism have been described. An hbd mutant of Ensifer meliloti (formerly Rhizobium meliloti) was unable to grow with R-3HB (2, 8). In an hbd mutant of Cupriavidus necator, however, rapid remetabolization of R-3HB after its transient accumulation was found, suggesting the presence of R-3HB-metabolizing enzymes other than R-3HB dehydrogenase (37). In an E. coli strain, the ability to grow with R-3HB as the sole carbon source could be induced by introduction of so-far-uncharacterized genes from microbial communities (43). Those genes were assumed to be different from hbd, since they did not confer R-3HB dehydrogenase activity on E. coli.
In our study, the M. rhodesianum hbd mutant lost its ability to grow on R-3HB or other multicarbon compounds as the sole carbon source after additional transposon-mediated inactivation of the lipA gene. The M. rhodesianum mutant defective in the lipA gene alone showed the same auxotrophy as the hbd lipA mutant. This auxotrophy could be complemented by expression of the lipA gene in trans, demonstrating that it was exclusively due to the lack of the lipA gene. The close linkage between lipA and a cyclase/dehydrase-encoding gene located downstream in the M. rhodesianum genome suggests cotranscription of the two genes. Therefore, expression of the cyclase/dehydrase might also be inhibited by the minitransposon insertion. On the other hand, the cyclase/dehydrase could be transcribed from the promoter of the gentamicin resistance gene present at the 3′ end of the minitransposon. In any case, the exact function of the cyclase/dehydrase is not known, but a possible downstream effect of the transposon insertion is obviously not linked to the observed auxotrophy.
Bacterial lipA-null mutants are unable to synthesize α-lipoic acid, the cofactor of pyruvate dehydrogenase and α-ketoglutarate dehydrogenase (Fig. 1) (41), the latter of which is part of the tricarboxylic acid cycle. Thus, inactivation of lipA results in an incomplete tricarboxylic acid cycle. As shown by Van Dien et al. (42), M. extorquens AM1 mutants with a defective pyruvate dehydrogenase or α-ketoglutarate dehydrogenase were able to grow on one-carbon compounds (methanol) but not on multicarbon compounds (succinate and pyruvate), which is consistent with our results.
Accumulation of R-3HB by the M. rhodesianum hbd lipA mutant and the lipA mutant suggests that α-lipoic acid is involved in an alternative R-3HB metabolization route, most probably as the cofactor of α-ketoglutarate dehydrogenase. The highest R-3HB concentrations were achieved when both the hbd- and the lipA-dependent pathways were blocked. The putative lipA-dependent (hbd-independent) pathway for R-3HB utilization might proceed via (R)-3-hydroxybutyryl-coenzyme A (CoA), which can be converted to acetoacetyl-CoA or crotonyl-CoA and thus enter the glyoxylate regeneration cycle and the tricarboxylic acid cycle (Fig. 1) (10, 20). An enzyme converting R-3HB to (R)-3-hydroxybutyryl-CoA in Methylobacterium has not yet been described; however, the genomes of Methylobacterium strains contain genes for several enzymes with similarity to short-chain acyl-CoA synthetases that might catalyze this reaction.
E. coli lipA-null mutants are unable to grow in glucose minimal medium, and growth can be restored either by introduction of the plasmid-borne lipA gene or by supplementation with α-lipoic acid (32). In contrast, the growth defect induced by the lipA mutation in M. rhodesianum could not be complemented with exogenous α-lipoic acid. However, complementation by plasmid-borne lipA clearly showed that the growth defect in M. rhodesianum was exclusively due to inactivation of lipA. In E. coli, two different lipoyl-protein ligases, encoded by lplA and lipB, are used to attach lipoic acid to the lipoic acid-dependent enzymes. The lplA gene is necessary for the attachment of exogenously added lipoic acid, whereas the lipB gene product utilizes lipoyl groups generated via endogenous (lipA-mediated) biosynthesis (19, 28). The available Methylobacterium genome sequences contain genes with similarity to lipB, but no lplA-like genes. Therefore, unlike E. coli, Methylobacterium strains might be unable to use external α-lipoic acid.
For bacterial production of (R)-3-hydroxyalkanoates, mainly multicarbon compounds, such as sugars and alkanoates, have been used as growth substrates (15, 23, 34, 37). Among the microorganisms used was also a Methylobacterium strain, Methylobacterium sp. strain ZP24, which was grown on lactose (30). So far, the highest concentrations of R-3HB were reported with genetically engineered strains of E. coli, which were cultivated in LB medium containing glucose. In this way, Shiraki et al. (37) and Gao et al. (15) obtained R-3HB concentrations of 70 mM (7.3 g liter−1) and 115 mM (12 g liter−1) in the culture supernatant, respectively, which are higher than our maximum concentration. However, our study extends the range of substrates by qualifying the one-carbon compound methanol for (R)-3-hydroxyalkanoate production. Methanol is a cheap substrate that can be produced from virtually any kind of biomass, e.g., agricultural by-products or other cellulosic and lignocellulosic waste materials (29), as well as from carbon dioxide or methane as the main components of biogas or natural gas (31).
Despite its low price, efforts need to be made to increase the R-3HB yields from methanol. Our experimental R-3HB yields in bioreactor cultures of the hbd lipA mutant were significantly lower than the theoretical overall yield. This difference can be mainly attributed to the high aeration rate in the bioreactor, which caused a strong stripping of methanol from the culture liquid. However, technical measures, such as (i) the condensation of exhaust methanol gas and refeeding or (ii) the reduction of the aeration rate by using pure oxygen or elevated pressure, are available to solve the stripping problem in subsequent studies. Therefore, the experimental R-3HB yields represent preliminary data. In batch cultures of the hbd lipA mutant without extra aeration, the yields were significantly higher than those in the bioreactor cultures, demonstrating that more favorable R-3HB yields with methanol as a substrate are achievable.
Acknowledgments
We thank Benjamin Scheer for excellent technical assistance, Karin Lange and Elke Häusler for help with cultivations, and Martina Effenberger for some supporting chemical analysis. Vectors pCM80, pCM157, and pCM184 were a kind gift of Marina G. Kalyuzhnaya and Mary E. Lidstrom, University of Washington. We thank Dieter Jendrossek, University of Stuttgart, Stuttgart, Germany, and Claudia Einwich and Lars Braun, Umwelt- und Ingenieurtechnik GmbH Dresden, Dresden, Germany, for helpful discussions.
This work was supported by the German Federal Ministry of Economics and Technology (BMWi) via the AiF-German Federation of Industrial Research Associations Otto von Guericke (project no. KF001006UL6).
Footnotes
Published ahead of print on 25 June 2010.
REFERENCES
- 1.Ackermann, J. U., S. Müller, A. Lösche, T. Bley, and W. Babel. 1995. Methylobacterium rhodesianum cells tend to double the DNA content under growth limitations and accumulate PHB. J. Biotechnol. 39:9-20. [Google Scholar]
- 2.Aneja, P., and T. C. Charles. 1999. Poly-3-hydroxybutyrate degradation in Rhizobium (Sinorhizobium) meliloti: isolation and characterization of a gene encoding 3-hydroxybutyrate dehydrogenase. J. Bacteriol. 181:849-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babel, W., J. U. Ackermann, and U. Breuer. 2001. Physiology, regulation, and limits of the synthesis of poly(3HB). Adv. Biochem. Eng. Biotechnol. 71:125-157. [DOI] [PubMed] [Google Scholar]
- 4.Bergmeyer, H. U., K. Gawehn, H. Klotzsch, H. A. Krebs, and D. H. Williamson. 1967. Purification and properties of crystalline 3-hydroxybutyrate dehydrogenase from Rhodopseudomonas spheroides. Biochem. J. 102:423-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer, H. W., and D. Roulland-Dussoix. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41:459-472. [DOI] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Breuer, U., J. U. Ackermann, and W. Babel. 1995. Accumulation of poly(3-hydroxybutyric acid) and overproduction of exopolysaccharides in a mutant of a methylotrophic bacterium. Can. J. Microbiol. 41:55-59. [Google Scholar]
- 8.Charles, T. C., G. Q. Cai, and P. Aneja. 1997. Megaplasmid and chromosomal loci for the PHB degradation pathway in Rhizobium (Sinorhizobium) meliloti. Genetics 146:1211-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, G. Q., and Q. Wu. 2005. Microbial production and applications of chiral hydroxyalkanoates. Appl. Microbiol. Biotechnol. 67:592-599. [DOI] [PubMed] [Google Scholar]
- 10.Chistoserdova, L., S. W. Chen, A. Lapidus, and M. E. Lidstrom. 2003. Methylotrophy in Methylobacterium extorquens AM1 from a genomic point of view. J. Bacteriol. 185:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chistoserdova, L. V., and M. E. Lidstrom. 1991. Purification and characterization of hydroxypyruvate reductase from the facultative methylotroph Methylobacterium extorquens AM1. J. Bacteriol. 173:7228-7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi, Y. J., L. Morel, D. Bourque, A. Mullick, B. Massie, and C. B. Miguez. 2006. Bestowing inducibility on the cloned methanol dehydrogenase promoter (PmxaF) of Methylobacterium extorquens by applying regulatory elements of Pseudomonas putida F1. Appl. Environ. Microbiol. 72:7723-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chun, K. T., H. J. Edenberg, M. R. Kelley, and M. G. Goebl. 1997. Rapid amplification of uncharacterized transposon-tagged DNA sequences from genomic DNA. Yeast 13:233-240. [DOI] [PubMed] [Google Scholar]
- 14.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. U. S. A. 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao, H. J., Q. Wu, and G. Q. Chen. 2002. Enhanced production of D-(-)-3-hydroxybutyric acid by recombinant Escherichia coli. FEMS Microbiol. Lett. 213:59-65. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 17.Handrick, R., S. Reinhardt, and D. Jendrossek. 2000. Mobilization of poly(3-hydroxybutyrate) in Ralstonia eutropha. J. Bacteriol. 182:5916-5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilger, U., K. Sattler, and U. Littkowsky. 1991. Studies on the growth-associated accumulation of poly-β-hydroxybutyric acid with Methylobacterium rhodesianum Z. Zentralbl. Mikrobiol. 146:83-88. [Google Scholar]
- 19.Jordan, S. W., and J. E. Cronan, Jr. 2003. The Escherichia coli lipB gene encodes lipoyl (octanoyl)-acyl carrier protein:protein transferase. J. Bacteriol. 185:1582-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korotkova, N., L. Chistoserdova, V. Kuksa, and M. E. Lidstrom. 2002. Glyoxylate regeneration pathway in the methylotroph Methylobacterium extorquens AM1. J. Bacteriol. 184:1750-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korotkova, N., and M. E. Lidstrom. 2001. Connection between poly-β-hydroxybutyrate biosynthesis and growth on C1 and C2 compounds in the methylotroph Methylobacterium extorquens AM1. J. Bacteriol. 183:1038-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon, Y. M., and S. C. Ricke. 2000. Efficient amplification of multiple transposon-flanking sequences. J. Microbiol. Methods 41:195-199. [DOI] [PubMed] [Google Scholar]
- 23.Lee, S. Y., and Y. Lee. 2003. Metabolic engineering of Escherichia coli for production of enantiomerically pure (R)-(−)-hydroxycarboxylic acids. Appl. Environ. Microbiol. 69:3421-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, S. Y., Y. Lee, and F. Wang. 1999. Chiral compounds from bacterial polyesters: sugars to plastics to fine chemicals. Biotechnol. Bioeng. 65:363-368. [DOI] [PubMed] [Google Scholar]
- 25.Liu, Q., S. P. Ouyang, A. Chung, Q. Wu, and G. Q. Chen. 2007. Microbial production of R-3-hydroxybutyric acid by recombinant E. coli harboring genes of phbA, phbB, and tesB. Appl. Microbiol. Biotechnol. 76:811-818. [DOI] [PubMed] [Google Scholar]
- 26.Marx, C. J., and M. E. Lidstrom. 2002. Broad-host-range cre-lox system for antibiotic marker recycling in gram-negative bacteria. Biotechniques 33:1062-1067. [DOI] [PubMed] [Google Scholar]
- 27.Marx, C. J., and M. E. Lidstrom. 2001. Development of improved versatile broad-host-range vectors for use in methylotrophs and other Gram-negative bacteria. Microbiology 147:2065-2075. [DOI] [PubMed] [Google Scholar]
- 28.Morris, T. W., K. E. Reed, and J. E. Cronan, Jr. 1995. Lipoic acid metabolism in Escherichia coli: the lplA and lipB genes define redundant pathways for ligation of lipoyl groups to apoprotein. J. Bacteriol. 177:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa, H., T. Harada, T. Ichinose, K. Takeno, S. Matsumoto, M. Kobayashi, and M. Sakai. 2007. Biomethanol production and CO2 emission reduction from forage grasses, trees, and crop residues. Jpn. Agric. Res. Q. 41:173-180. [Google Scholar]
- 30.Nath, A., S. Bhat, J. Devle, and A. J. Desai. 2005. Enhanced production of 3-hydroxybutyric acid (3-HB) by in vivo depolymerization of polyhydroxybutyric acid in 3-HB dehydrogenase mutants of Methylobacterium sp. ZP24. Ann. Microbiol. 55:107-111. [Google Scholar]
- 31.Olah, G. A., A. Goeppert, and G. K. S. Prakash. 2006. Beyond oil and gas: the methanol economy. Wiley-VCH, Weinheim, Germany.
- 32.Reed, K. E., and J. E. Cronan, Jr. 1993. Lipoic acid metabolism in Escherichia coli: sequencing and functional characterization of the lipA and lipB genes. J. Bacteriol. 175:1325-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riis, V., and W. Mai. 1988. Gas chromatographic determination of poly-β-hydroxybutyric acid in microbial biomass after hydrochloric acid propanolysis. J. Chromatogr. 445:285-289. [Google Scholar]
- 34.Ruth, K., A. Grubelnik, R. Hartmann, T. Egli, M. Zinn, and Q. Ren. 2007. Efficient production of (R)-3-hydroxycarboxylic acids by biotechnological conversion of polyhydroxyalkanoates and their purification. Biomacromolecules 8:279-286. [DOI] [PubMed] [Google Scholar]
- 35.Schleinitz, K. M., S. Kleinsteuber, T. Vallaeys, and W. Babel. 2004. Localization and characterization of two novel genes encoding stereospecific dioxygenases catalyzing 2(2,4-dichlorophenoxy)propionate cleavage in Delftia acidovorans MC1. Appl. Environ. Microbiol. 70:5357-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seebach, D., H. F. Chow, R. F. W. Jackson, M. A. Sutter, S. Thaisrivongs, and J. Zimmermann. 1986. (+)-11,11′-Di-O-methylelaiophylidene: preparation from elaiophylin and total synthesis from (R)-3-hydroxybutyrate and (S)-malate. Liebigs Ann. Chem. 1986:1281-1308.
- 37.Shiraki, M., T. Endo, and T. Saito. 2006. Fermentative production of (R)-(−)-3-hydroxybutyrate using 3-hydroxybutyrate dehydrogenase null mutant of Ralstonia eutropha and recombinant Escherichia coli. J. Biosci. Bioeng. 102:529-534. [DOI] [PubMed] [Google Scholar]
- 38.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology 1:784-791. [Google Scholar]
- 39.Suarez, A., A. Güttler, M. Strätz, L. H. Staendner, K. N. Timmis, and C. A. Guzman. 1997. Green fluorescent protein-based reporter systems for genetic analysis of bacteria including monocopy applications. Gene 196:69-74. [DOI] [PubMed] [Google Scholar]
- 40.Taté, R., A. Riccio, M. Iaccarino, and E. J. Patriarca. 1997. Cloning and transcriptional analysis of the lipA (lipoic acid synthetase) gene from Rhizobium etli. FEMS Microbiol. Lett. 149:165-172. [DOI] [PubMed] [Google Scholar]
- 41.Vanden Boom, T. J., K. E. Reed, and J. E. Cronan, Jr. 1991. Lipoic acid metabolism in Escherichia coli: isolation of null mutants defective in lipoic acid biosynthesis, molecular cloning and characterization of the E. coli lip locus, and identification of the lipoylated protein of the glycine cleavage system. J. Bacteriol. 173:6411-6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Dien, S. J., Y. Okubo, M. T. Hough, N. Korotkova, T. Taitano, and M. E. Lidstrom. 2003. Reconstruction of C3 and C4 metabolism in Methylobacterium extorquens AM1 using transposon mutagenesis. Microbiology 149:601-609. [DOI] [PubMed] [Google Scholar]
- 43.Wang, C., D. J. Meek, P. Panchal, N. Boruvka, F. S. Archibald, B. T. Driscoll, and T. C. Charles. 2006. Isolation of poly-3-hydroxybutyrate metabolism genes from complex microbial communities by phenotypic complementation of bacterial mutants. Appl. Environ. Microbiol. 72:384-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamane, T. 1993. Yield of poly-D(-)-3-hydroxybutyrate from various carbon sources: a theoretical study. Biotechnol. Bioeng. 41:165-170. [DOI] [PubMed] [Google Scholar]
- 45.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]