Abstract
Viral infections spread based on the ability of viruses to overcome multiple barriers and move from cell to cell, tissue to tissue, and person to person and even across species. While there are fundamental differences between these types of transmissions, it has emerged that the ability of viruses to utilize and manipulate cell-cell contact contributes to the success of viral infections. Central to the excitement in the field of virus cell-to-cell transmission is the idea that cell-to-cell spread is more than the sum of the processes of virus release and entry. This implies that virus release and entry are efficiently coordinated to sites of cell-cell contact, resulting in a process that is distinct from its individual components. In this review, we will present support for this model, illustrate the ability of viruses to utilize and manipulate cell adhesion molecules, and discuss the mechanism and driving forces of directional spreading. An understanding of viral cell-to-cell spreading will enhance our ability to intervene in the efficient spreading of viral infections.
Viruses are small protein capsids that harbor genetic information. In the case of enveloped viruses, an additional lipid bilayer surrounds the capsid. In order to replicate, viruses are completely dependent on their host. They replicate their genetic information within cells, assemble and release viral progenies to infect additional cells, and spread the viral infection. The viruses discussed in this review are enveloped. They pinch off from the producer cell and enter the next cell by membrane fusion. Enveloped viruses can spread via two distinct routes, either through the cell-free aqueous environment or, alternatively, by remaining cell associated and being passed on by direct cell-cell contact. The latter mode of spread is often designated cell-to-cell transmission (for an excellent review, see reference 108). In our review, we will concentrate on lessons learned, particularly in the retrovirus field, and include cross references to other viruses.
Over the years, strong evidence has accumulated that many viruses can efficiently spread by direct cell-cell contact (57, 95, 108). An early striking observation was that viruses were able to spread in the presence of neutralizing antibodies that completely blocked the spread of cell-free virus (14, 39, 47, 82, 95). Herpes-, rhabdo-, and measles viruses were noted to spread along neuronal networks, which implied that these viruses can be transmitted via neurological synapses (54, 67). The ability of vaccinia and African swine fever viruses to induce actin tails in infected cells suggested a spreading mechanism that is related to that of the bacteria Listeria and Shigella (23, 36, 62). For many other viruses, the evidence that cell-cell contact plays a role was initially more indirect. The observed poor infectivity of cell-free virus often could not explain its rapid spreading in culture (9, 29). Early electron micrographs of HIV accumulating to high numbers at the interface between cells had a lasting impact (95). Finally, few could deny the persuasive power of time-lapse movies that directly displayed the movement of viruses from one cell to another (51, 56, 112).
Another influential observation in this field was the finding that the addition of a few dendritic cells (DC) to a culture of T cells could dramatically enhance HIV infection of T cells (18). DCs were later shown to capture and present HIV to T cells in a process that was reminiscent of how antigen-presenting cells (APC) present antigen (40, 79). Thus, following the early realization that neurotropic viruses spread along neurological synapses, a model in which immunotropic viruses such as HIV may utilize immunological synapses for efficient cell-to-cell spread began to emerge (79).
The next fundamental step in the understanding of virus cell-to-cell transmission came with the realization that HIV- and human T-lymphotropic virus (HTLV)-infected cells could establish similar cell-cell contacts between infected and uninfected T cells (53, 58, 60). T cells normally do not form long-lived immunological synapses with each other. Consequently, in analogy to immunological synapses, these cell-cell contacts were designated infectious, virological, or viral synapses (4, 53, 79). Similar observations were made for murine leukemia virus (MLV) in cultures of fibroblasts (56, 112). MLV infection established cell-cell adhesions with uninfected neighboring cells due to interactions between the viral envelope glycoprotein expressed in the infected cell and the viral receptor expressed in the target cell (112). Thus, it appears that viruses can either utilize existing cell-cell contacts or exploit basic cell adhesion biology to deliberately establish contact between infected and uninfected target cells for the purpose of efficient spreading.
VIRAL UTILIZATION OF EXISTING CELL-CELL CONTACTS
Cell-cell adhesion and specialized biological synapses are essential building blocks in tissues and organs of multicellular organisms (133). In order to overcome these barriers, many viruses have evolved into perfect insiders of cell-cell adhesion and biological synapses. The ability of neurotropic viruses to spread via neurological synapses was recognized many years ago and has been elegantly exploited to map neuronal circuits (27, 71, 104). Herpesviruses can spread in both directions along neurons, and their capsids can undergo anterograde and retrograde transport along microtubules (114). Spread via synapses requires that the chosen receptors specifically localize to the synapse. In fact, receptor choice allows first insights into the mechanism of virus spreading. Nectins, used as receptors by a number of herpesviruses, may localize to puncta adherens that provide a peripheral structural framework for neurological synapses (122). The discovery of tight junction components claudin-1 and occludin as hepatitis C virus (HCV) entry factors points to the use of tight junctions during HCV spread within the polarized liver epithelium (33, 98). Spread via tight junctions is common for viruses that infect epithelial layers (8, 11). Recently developed in vitro culture systems will allow experimental access to studying the mechanism by which these viruses spread from cell to cell (20, 61, 106).
Retroviruses and other immunotropic viruses often utilize immunological synapses for cell-to-cell spread. Contact between antigen-presenting cells and T cells is initiated by an interaction between intercellular adhesion molecules (ICAMs) expressed on APCs and LFA-1, the CD11α/CD18 αL/β2 integrin. Following the initial contact, major histocompatibility complex (MHC) molecules carrying antigenic peptides and the T-cell receptor move into the center of the contact zone, while the integrin forms an outer ring (44, 52). Talin binding to the cytoplasmic tail of integrins marks the outer rings. A center-directed flow driven by actomyosin lies behind these reorganizations (63, 90, 131). The central and peripheral zones are called the central and peripheral supramolecular activation clusters, cSMAC and pSMAC, respectively (84) (Fig. 1A). In addition to the actin-driven surface movement, cell-cell adhesion positions the PAR complex at the cytoplasmic face of the cell-cell contact zone to establish cellular polarity (72, 123). The microtubule organizing center (MTOC) moves toward the contact zone, and exocyst positioning polarizes vesicle release toward the contact zone (25, 65, 66, 75, 99, 119, 120). While these events have been described in great detail for the T-cell side, polarization is often observed on both sides of the synapse (10, 15). The duration, stability, and turnover of immunological synapses are under the control of many regulatory and inhibitory adhesion proteins (26, 123). Chemokine receptors, including HIV coreceptors CXCR4 and CCR5, serve as regulatory receptors and are recruited to immunological synapses (16, 21, 83). The concentration of both receptors in the cell-cell interface reduces the responsiveness of T cells to soluble chemokines, thereby preventing their migration and stabilizing the immunological synapse (83).
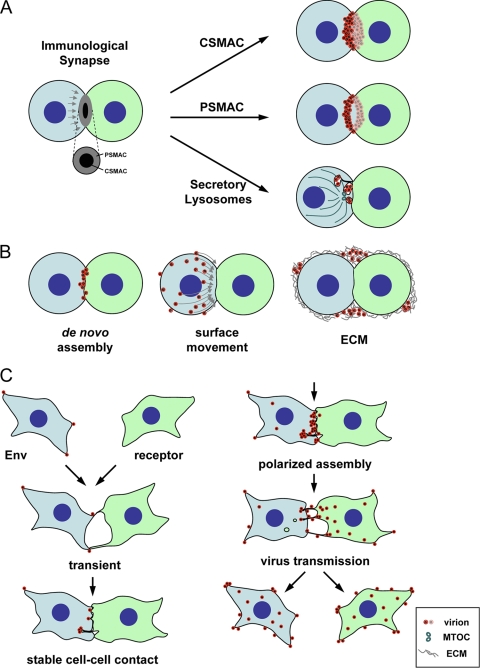
FIG. 1.
Viruses can either utilize existing synaptic contacts or establish contact between infected and uninfected cells to promote viral spreading. (A) Utilization of structural elements of immunological synapses for viral spreading. The architecture of the immunological synapse consists of central and peripheral supermolecular adhesion complexes, cSMAC and pSMAC, respectively (84). The antigen-presenting cell is shown in green, and the effector cell is shown in blue. Exocytosis of secretory lysosomes can be observed in the cSMAC zone. Interestingly, all three structural elements have been associated with the accumulation and transmission of viruses from infected to uninfected cells (43, 51, 102). (B) Several distinct mechanisms can contribute to the accumulation of viruses at the synapse. The association of viruses with the cell-cell interface could be the consequence of either de novo assembly or surface transmission (55, 56). Extracellular matrix components (ECM) have also been observed to play a role in a peripheral mode of transmission (92). Virus-infected cells are blue, and the receptor-expressing target cells are labeled as green. (C) Establishment of adhesion between infected and uninfected cells using MLV as the model system (56, 112). MLV-infected fibroblasts were observed to establish contact with uninfected cells in a reaction driven by Env-receptor interactions. Initial transient contacts are stabilized, and virus assembly was observed to be redirected toward sites of cell-cell contact after an ∼30-min delay. Following virus transmission, cell-cell contacts are downregulated. Virus-infected cells are blue, and the receptor-expressing target cells are green.
Amazingly, all three of these structural elements within the immunological synapse, cSMACs, exocytic sites within the central zone, and pSMACs, have been associated with the transmission of HIV (Fig. 1A). The relocation of HIV from vesicles or deep invaginations found in infected macrophages or dendritic cells to contact sites formed with neighboring lymphocytes was observed by time-lapse microscopy (12, 43, 136). Other HIV and HTLV accumulations between lymphocytes that appear either as buttons or outer rings are more consistent with the architecture of cSMACs and pSMACs, respectively (51, 53, 102). Mechanistically, the accumulation of viruses in the synapse could be the consequence of either de novo assembly in the cell-cell interface or actomyosin-driven surface movement of completely assembled particles from surrounding areas (55) (Fig. 1B). Moreover, a role for extracellular matrix components, such as galectin and collagens, has been described for the surface transmission of HTLV (92) (Fig. 1B).
While viruses utilize some features of cell-cell communication, they suppress others. For instance, HIV hijacks the cellular pathway for the presentation of antigen in order to spread the infection, but can prevent the actual presentation of antigens. This occurs through the expression of Nef, which downregulates MHC molecules (34). Nef alleles from various lentiviruses also downregulate CD4 and CD3 and remodel the actin cytoskeleton, which influences the duration and signaling events within the immunological synapse (5, 34, 103, 121).
Finally, while the exploitation of existing synapses allows efficient spreading from cell to cell, viruses also hijack the capacity of certain cell types to mediate long-range transport throughout an organism. The ability of neurotropic viruses to spread across neurons allows these viruses to enter peripheral neurons yet quickly reach the central nervous system (24, 105). Likewise, the infection of peripheral dendritic cells allows HIV to move with these cells into the lymph node for transfer to T cells (40, 76, 96). Thus, viral exploitation of existing forms of cell-cell communication extends beyond just the cellular transmission aspects and explains spreading within an organism.
CAMS AND VAMS: VIRAL MIMICRY OF CELL ADHESION MOLECULES
In addition to utilizing existing cell-cell contacts, viruses can deliberately establish cell-cell contact between infected cells and uninfected target cells (53, 58, 112). To understand the biology of this process, it is helpful to examine biological synaptogenesis. Despite the complexity of each biological synapse, synaptogenesis is initially driven by a few dominant cellular adhesion molecules (CAMs) (3, 133). These proteins alone, if expressed in cells that lack most other adhesion proteins, have the ability to form synapses and induce cellular polarity. In the case of neurosynaptogenesis, dominant adhesion activity has been attributed to the homotypic adhesion molecule SynCAM as well as the heterotypic adhesion molecule neuroligin/neurexin (13, 109). E-cadherin and ICAMs/LFA-1 are dominant homotypic and heterotypic cellular adhesion molecules that can drive the formation of epithelial and immunological synapses, respectively (63, 132). Synaptobiogenesis often proceeds through filopodial and dendritic intermediates (35, 64, 89, 113, 127, 137). Cytoplasmic signaling events downstream of cell adhesion allow the reorganization of the underlying cytoskeleton, e.g., by suppressing filopodial formation in order to organize a smooth and broad cell-cell interface (1, 118a). The establishment of cell-cell adhesion is often followed by an induction of cellular polarity that leads to the reorientation of the MTOC as already mentioned previously for the immunological synapse (31, 133).
Importantly, viruses use several mechanisms to exploit cellular adhesion biology to establish contact between the infected and uninfected target cell. First, HTLV-1 infection can upregulate endogenous adhesion proteins, such as ICAM-1, as well as components of the extracellular matrix (38, 92). Second, mouse mammary tumor virus expresses a superantigen that causes proliferation of infected B and T cells but may also stabilize and prolong contact between B cells and T cells (42, 48). Third, some viruses appear to express dominant adhesion proteins (20, 53, 58, 112). In the case of MLV, the viral protein that exhibits adhesion molecule features is the viral glycoprotein Env (112). Cells that express MLV Env can establish cell-cell contact with cells expressing the viral receptor (Fig. 1C). Env mutants that no longer interact with the viral receptor with high affinity fail to establish cell-cell contact (112). Moreover, in analogy to cellular adhesion and subsequent polarization, the establishment of adhesion between MLV-infected cells and uninfected target cells is followed by the polarized assembly of viruses at the cell-cell interface (56) (Fig. 1C). This polarized assembly process depends on signaling via the cytoplasmic tail of Env. Upon incorporation of MLV Env into budding virions, the cytoplasmic tail is cleaved off by the viral protease, thereby transforming an adhesion protein into a highly fusiogenic fusion protein that facilitates virus entry into the neighboring cells (22, 45, 101). Thus, the Env fusion protein initially functions as a viral adhesion molecule (VAM) mimicking the behavior of a cellular adhesion molecule. It will be important in the future to understand the specific adhesion biology utilized by various viruses.
While these MLV spreading experiments have been performed with transformed cancer cells that have lost many of their endogenous adhesion proteins, in lymphocytes and particularly in primary cells, VAMs likely synergize with endogenous CAMs. Indeed, the study of HIV and HTLV spreading suggests that Env from these viruses synergizes with LFA-1 and ICAM-1 but is not essential for the establishment of cell-cell contact (19, 43, 102, 118). In contrast, ICAM-1 and LFA-1 support efficient spreading, underscoring the synergy between VAMs and CAMs (49, 59, 102, 129).
Finally, virus spreading in primary cells can be distinct from that observed with tissue culture cells. In migrating primary T cells, which are already polarized prior to the establishment of cell-cell contact, HIV assembly is directed to the uropod (19) (Akira Ono, personal communication). Upon contact with uninfected cells at the leading edge, the cell turns around to establish prolonged contact through the uropod that also harbors many adhesion proteins and signaling components of the immunological synapse (6, 72, 107).
Thus, while viruses can utilize existing synapses for cell-to-cell spread, VAMs can also specifically promote adhesion between infected and uninfected cells in cultures that usually do not form synaptic contacts. To mention one more example in support of this model, while herpesviruses can spread through neurological synapses, they can also establish new cell-cell contacts outside the synapse to promote viral spread to neighboring cells (28, 108). The ability to use viral glycoprotein/receptor pairs with dominant adhesion features will also allow viruses to manipulate existing synapses (78, 126).
MECHANISM OF VIRUS CELL-TO- CELL TRANSMISSION
Viruses can spread via either a cell-free mode or a cell-associated mode involving direct cell-cell contact. Efficient virus spreading can be achieved by either route. For cell-free aqueous spreading to be efficient, a virally infected cell would have to release large numbers of viral particles and reach distant areas by diffusion. These particles must be sufficiently stable, not quickly cleared and, importantly, still able to efficiently bind and infect uninfected target cells. In contrast, if any of these criteria are not fulfilled, spreading via the cell-free mode is impaired. For instance, if viruses are not efficiently released into the extracellular milieu, spreading by the cell-free mode would obviously be blocked. However, virus retention on producer cells may not necessarily block cell-to-cell transmission. In fact, studies showing that surface retention on producer cells plays an important role in the spreading of viruses by cell-cell contact are emerging (92, 111). Intriguingly, it remains to be determined if tetherin, a recently identified antiviral factor that retains HIV particles on the cell, will promote or block cell-to-cell transmission (46, 86, 87, 94, 128). Second, released viruses may be too unstable to allow for cell-free spreading but be able to undergo rapid spreading via sites of cell-cell contact. As an alternative, released viruses could be captured and stabilized by cell surface or extracellular matrix components (85, 92, 93). Third, viral gene expression may be too low in certain cell types to allow efficient particle generation. However, inefficient virus assembly and release can be rescued at sites of cell-cell contact by locally enhancing virus assembly and release (56, 57). Fourth, cell-free viruses may not be able to efficiently bind to target cells, which likely represents one of the most commonly observed blocks to cell-free virus spreading (91, 97, 124). Single viruses, due to their small size and limited number of glycoproteins, may only weakly bind to target membranes. They may not recruit receptor to levels sufficient to induce uptake and/or conformational changes needed to infect cells. Moreover, single viruses may not be able to activate quiescent cells sufficiently to support viral replication. In contrast, Env highly enriched at broad cell-cell interfaces may recruit receptor and signaling proteins to levels that make cells susceptible to viral replication (2, 7, 125, 134, 135). It should be noted that certain extracellular components may similarly promote efficient virus binding and infection of cells (85).
These general considerations already point to a number of conditions under which viruses may not be fit enough for cell-free virus spreading. However, spread by direct cell-cell contact is likely more than a salvage pathway for the unfit. While researchers were forced to discover the importance of cell-to-cell spread for viruses with poor infectivity-to-particle ratios, it is worthwhile to consider the possibility that even the most stable viruses use cell-to-cell spread. There are several appealing advantages associated with direct cell-to-cell spread that could be exploited by many viruses. The first is speed: rather by going through all the steps of cell-free transmission, the entire extracellular replication cycle of release, transmission, and entry can proceed quickly at sites of cell-cell contact and exploit cytoskeletal forces for the purpose of spreading. Moreover, the observed enhancement of budding at sites of cell-cell contact can promote spreading at lower levels of gene expression (56). The second is immune evasion: limited exposure time to the extracellular space can allow evasion of neutralizing antibodies (39, 51). Third, exploiting cell-cell communication is an effective way to overcome the various physical and immunological barriers within an organism in order to spread the infection.
Finally, while cell-to-cell spread has its appeal, under some circumstances, cell-free virus spread might be more advantageous. Cell-free virus is not restricted to specific cell-cell interactions and may facilitate spread from person to person. As such, it is possible to imagine that some viruses, notably HIV, may have come up with mechanisms to switch between cell-free and cell contact-dependent modes of spreading.
DRIVING FORCES FOR DIRECTIONAL VIRAL SPREAD
The spread of viral infection depends on the directional transmission of virus particles from infected cells to uninfected target cells. The most critical prerequisite for virus spreading, irrespective of whether transmission occurs via the cell-free or cell-cell contact-dependent mode, is that the viral receptor is downregulated in infected cells. Downregulation of the viral receptor in the producer cell and high affinity for the receptor expressed on the target cell alone establish an affinity gradient that drives viral spreading. This simple model is modified in an interesting way by a frequently observed retention of viruses on the surface of the producer cell despite the completion of assembly (86, 92, 111). As mentioned above, this prevents release into the medium, but it does not necessarily prevent cell-to-cell spreading (92, 111). In fact, if diffusion coefficients are comparable, diffusion along a two-dimensional surface would promote viral spread more efficiently than diffusion in three dimensions. Interestingly, following downregulation of the high-affinity receptor, viruses can be retained on the surface of their own producer cell due to nonspecific virus-cell interactions (111). Over time and due to substrate and/or extracellular matrix trapping, this retention can result in an accumulation of viruses that are subsequently efficiently transferred to target cells (Fig. 2A).
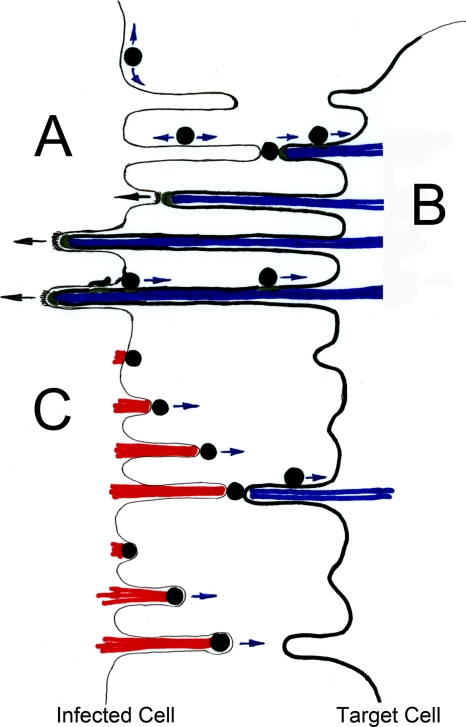
FIG. 2.
Driving forces for directional spreading of viruses (adapted from reference 113 with permission of the publisher). (A) Affinity-based model for directional spreading. Downregulation of the viral receptor in the infected cells allows the establishment of a general affinity gradient between the infected and uninfected cell. Additional virus interactions with the infected cell can retain completely budded viral particles at the surface of the infected cell. Low-affinity retention on the surface of producer cells allows for diffusive movement along the cell surface (111). This process can position viruses for subsequent high-affinity interactions with target cells expressing receptor, thereby driving directional spreading of surface associated viruses. (B) Viral transmission driven by retrograde F-actin flow of the target cell. High-affinity interactions with receptor (green) in the target cell allows surface-associated viruses to engage the underlying F-actin flow (blue) to move toward the cell body of the target cell (17, 68, 69, 110, 115). This process, originally designated “virus surfing,” can promote a driving force for cell-to-cell spreading for the surface-retained viruses presented in panel A. In addition, long-term Env-receptor interactions between infected and uninfected cells can anchor target cell membranes directly at the infected cells (112). Because virus assembly can be redirected toward these sites of cell-cell contact (56), viruses can immediately engage target cell F-actin flow (blue) to move toward the target cell upon completion of assembly. (C) Viral transmission driven by actin assembly in the infected cell. In contrast to the depictions in panel B, viruses can induce actin comet tail formation (red) inside the infected cell to propel themselves toward neighboring cells (23, 30, 62). While vaccinia virus induces actin tails (red) beneath the plasma membrane of completely released viruses, capsids of the African swine fever virus induce actin tails (red) while still topologically within the cytoplasm. Such actin-propelled motion may be sufficient to drive transmission, but a combination of actin-driven movements both away from the infected cell (red) as well as toward the target cell (blue) are also plausible (81).
Importantly, upon contact with the target cell, viruses bind to their viral receptor with specificity and high affinity. High-affinity interactions between ligands, including viruses and their receptors at the plasma membrane, can result in the establishment of a link from the surface to the underlying actin cytoskeleton (68, 69). Filamentous actin (F-actin) undergoes constant turnover that is driven by assembly “pushing” at the tip and myosin II-driven “pulling” at the base of the actin filament (80). Consequently, viruses end up “surfing” toward endocytic hot spots at the cell body (17, 68-70, 110, 115). As such, viruses do not recruit an individual myosin motor to each particle but, rather, utilize the general turnover of F-actin. Thus, viruses engage a high-affinity interaction with receptor-expressing cells that allows them to utilize actin-driven motion to move toward target cells (17, 111, 112) (Fig. 2B).
This affinity-based mode of transmission has recently been reported for transient interactions between fibroblasts chronically infected with MLV and uninfected target cells (111). In contrast to transient cell-cell interactions, prolonged interactions between infected and uninfected cells lead to the anchoring of target cell membranes, including filopodial membranes, in the infected cell (56, 112). The infected cell in turn redirects virus assembly directly to these sites of cell-cell contact (56). Consequently, as soon as viruses assemble, they can utilize retrograde F-actin flow to propel themselves toward target cells. Thus, both transmission pathways exploit actin dynamics from target cells for efficient cell-to-cell transmission (Fig. 2A and B).
In contrast to this process, other viruses induce actin tails within the infected cell to propel themselves toward target cells (23, 30, 62). Processes with two distinct membrane topologies have been observed. In the case of vaccinia virus, the virus is already completely released, but it uses the proteins A33 and A36 to induce the assembly of actin tails that propel viruses toward neighboring cells (17, 23, 37) (Fig. 2). In contrast to the case with vaccinia virus, African swine fever virus capsids form actin tails when they are still within the cytoplasm (62) (Fig. 2). Thus, viruses have evolved clever ways to utilize the actin cytoskeleton in either infected cells or target cells to propel themselves toward neighboring cells.
In addition to these fundamentally distinct mechanisms of cell-to-cell spread, viral spreading via the interior of nanotubes has been proposed but requires validation (32, 41, 113). Given that the transfer of capsid remains Env and receptor dependent, viruses such as HIV may move along the outside of nanotubes, not unlike MLV along filopodial bridges (118).
MIND THE GAP: THE EXPERIMENTAL APPROACH TO CELL-TO-CELL TRANSMISSION
Having discussed the mechanism of cell-to-cell transmission, we turn to the question of how a researcher can experimentally address whether a cell-free or cell-associated mode of virus spread is used by a given virus. This is more complicated than previously thought, because both modes usually involve extracellular virus. Most of the existing assays are not very convincing, and there is a need to combine functional and imaging approaches to collectively build an argument. The first experimental hint that cell-to-cell transmission may play a role is often that the infectivity produced by infected cells and released into the culture supernatant does not match the observed infectivity when the same number of infected cells is cocultured with uninfected cells (9). Second, fast kinetics of spreading in cocultures compared to that of cell-free virus has provided an argument for direct cell-to-cell spread (9, 29, 30). Third, viral growth in plaques, while potentially indicating direct cell-to-cell spread, may just reflect the decline of diffusion that is proportional to the square of the distance. Thus, several experimental conditions to suppress either diffusion or to prevent cell-cell contact have been explored. Diffusion has been slowed using viscous materials, such as methylcellulose (56, 130). Direct cell-cell contact has been prevented by coculturing infected and uninfected cells in Transwells, porous membranes that while preventing cell migration, allow virus diffusion (51, 74). Alternatively, shaking of cell cultures is used to prevent the formation of stable cell-cell contacts (74, 117).
Some of the most convincing experimental evidence in support of cell-to-cell transmission is resistance to neutralizing antibodies that completely block cell-free virus (14, 39, 47, 82, 95). These examples are most frequently observed for cell-cell contacts of a broad nature that are known to be tight (39). In contrast, many cell-cell contacts are less tight and remain sensitive to neutralizing antibodies (74, 112).
In the light of these experimental difficulties, morphological and imaging approaches have been very helpful. The accumulation of viruses, potentially even budding sites, specifically at sites of cell-cell contacts, can provide an intriguing snapshot that suggests the existence of a dynamic process (9, 58, 79, 95). The “seeing is believing” approach was most convincingly demonstrated in time-lapse microscopy videos that directly monitored the movement of viruses from one cell to another at sites of cell-cell contact (51, 56, 112). Moreover, the transfer of particles apparently correlated with the subsequent infection of these cells (51). Single-cell imaging that incorporates functional readouts into the imaging approach can generate convincing data. The correlation of live-cell imaging with scanning electron microscopy and/or tomography as well as super-resolution fluorescence can provide detailed mechanistic insights, such as whether viruses move along the surface or through the interior of cellular bridges (50, 73, 74, 92, 102, 112).
One of the critical challenges for the field of cell-to-cell transmission is to understand how viruses actually spread in vivo in living organisms. In addition, it will be critically important to identify cellular factors and small-molecule inhibitors that specifically affect one or the other mode of transmission. Early successes are the specific dependence of cell-cell contact-mediated spreading, but not that of cell-free virus on contributions from the cytoskeleton (51, 53, 58, 77). The identification of Zap70 and abl tyrosine kinases represent the first cellular factors required specifically for the contact-mediated spread of HIV and vaccinia virus (88, 100, 116). Hopefully over the next years, the field will identify several molecules specifically required for the contact-mediated transmission of viruses. Novel reporter constructs that specifically measure only contact-mediated spread will greatly facilitate this approach for retroviruses (77). The identification of such factors would also provide novel targets for antiviral therapies designed to block virus cell-to-cell transmission.
Acknowledgments
We thank Akira Ono for sharing unpublished results and are grateful to Pradeep Uchil, Xaver Sewald, and Shan Liu for critical readings of the manuscript.
Our work in the area of cell-to-cell transmission is supported by NIH R01 grants CA098727 and AI084096 to W.M., fellowships from the American Foundation for AIDS Research (amfAR) to J.J., and funds from the China Scholarship Council-Yale World Scholars in the Biomedical Sciences to P.Z.
Footnotes
Published ahead of print on 7 April 2010.
REFERENCES
- 1.Abe, K., O. Chisaka, F. Van Roy, and M. Takeichi. 2004. Stability of dendritic spines and synaptic contacts is controlled by alpha N-catenin. Nat. Neurosci. 7:357-363. [DOI] [PubMed] [Google Scholar]
- 2.Agosto, L. M., J. J. Yu, M. K. Liszewski, C. Baytop, N. Korokhov, L. M. Humeau, and U. O'Doherty. 2009. The CXCR4-tropic human immunodeficiency virus envelope promotes more-efficient gene delivery to resting CD4+ T cells than the vesicular stomatitis virus glycoprotein G envelope. J. Virol. 83:8153-8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akins, M. R., and T. Biederer. 2006. Cell-cell interactions in synaptogenesis. Curr. Opin. Neurobiol. 16:83-89. [DOI] [PubMed] [Google Scholar]
- 4.Alfsen, A., H. Yu, A. Magerus-Chatinet, A. Schmitt, and M. Bomsel. 2005. HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Mol. Biol. Cell 16:4267-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arhel, N., M. Lehmann, K. Clauss, G. U. Nienhaus, V. Piguet, and F. Kirchhoff. 2009. The inability to disrupt the immunological synapse between infected human T cells and APCs distinguishes HIV-1 from most other primate lentiviruses. J. Clin. Invest. 119:2965-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azar, G. A., F. Lemaitre, E. A. Robey, and P. Bousso. 2010. Subcellular dynamics of T cell immunological synapses and kinapses in lymph nodes. Proc. Natl. Acad. Sci. U. S. A. 107:3675-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balabanian, K., J. Harriague, C. Decrion, B. Lagane, S. Shorte, F. Baleux, J. L. Virelizier, F. Arenzana-Seisdedos, and L. A. Chakrabarti. 2004. CXCR4-tropic HIV-1 envelope glycoprotein functions as a viral chemokine in unstimulated primary CD4+ T lymphocytes. J. Immunol. 173:7150-7160. [DOI] [PubMed] [Google Scholar]
- 8.Balfe, P., and J. A. McKeating. 2009. The complexities of hepatitis C virus entry. J. Hepatol. 51:609-611. [DOI] [PubMed] [Google Scholar]
- 9.Bangham, C. R. 2003. The immune control and cell-to-cell spread of human T-lymphotropic virus type 1. J. Gen. Virol. 84:3177-3189. [DOI] [PubMed] [Google Scholar]
- 10.Barcia, C., N. S. Sanderson, R. J. Barrett, K. Wawrowsky, K. M. Kroeger, M. Puntel, C. Liu, M. G. Castro, and P. R. Lowenstein. 2008. T cells' immunological synapses induce polarization of brain astrocytes in vivo and in vitro: a novel astrocyte response mechanism to cellular injury. PLoS One 3:e2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. [DOI] [PubMed] [Google Scholar]
- 12.Bennett, A. E., K. Narayan, D. Shi, L. M. Hartnell, K. Gousset, H. He, B. C. Lowekamp, T. S. Yoo, D. Bliss, E. O. Freed, and S. Subramaniam. 2009. Ion-abrasion scanning electron microscopy reveals surface-connected tubular conduits in HIV-infected macrophages. PLoS Pathog. 5:e1000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biederer, T., Y. Sara, M. Mozhayeva, D. Atasoy, X. Liu, E. T. Kavalali, and T. C. Sudhof. 2002. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297:1525-1531. [DOI] [PubMed] [Google Scholar]
- 14.Black, F. L., and J. L. Melnick. 1955. Microepidemiology of poliomyelitis and herpes-B infections: spread of the viruses within tissue cultures. J. Immunol. 74:236-242. [PubMed] [Google Scholar]
- 15.Boes, M., J. Cerny, R. Massol, M. Op den Brouw, T. Kirchhausen, J. Chen, and H. L. Ploegh. 2002. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature 418:983-988. [DOI] [PubMed] [Google Scholar]
- 16.Bromley, S. K., D. A. Peterson, M. D. Gunn, and M. L. Dustin. 2000. Cutting edge: hierarchy of chemokine receptor and TCR signals regulating T cell migration and proliferation. J. Immunol. 165:15-19. [DOI] [PubMed] [Google Scholar]
- 17.Burckhardt, C. J., and U. F. Greber. 2009. Virus movements on the plasma membrane support infection and transmission between cells. PLoS Pathog. 5:e1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. [DOI] [PubMed] [Google Scholar]
- 19.Chen, P., W. Hubner, M. A. Spinelli, and B. K. Chen. 2007. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J. Virol. 81:12582-12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ch'ng, T. H., and L. W. Enquist. 2005. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J. Virol. 79:10875-10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contento, R. L., B. Molon, C. Boularan, T. Pozzan, S. Manes, S. Marullo, and A. Viola. 2008. CXCR4-CCR5: a couple modulating T cell functions. Proc. Natl. Acad. Sci. U. S. A. 105:10101-10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford, S., and S. P. Goff. 1985. A deletion mutation in the 5′ part of the pol gene of Moloney murine leukemia virus blocks proteolytic processing of the gag and pol polyproteins. J. Virol. 53:899-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cudmore, S., P. Cossart, G. Griffiths, and M. Way. 1995. Actin-based motility of vaccinia virus. Nature 378:636-638. [DOI] [PubMed] [Google Scholar]
- 24.Curanovic, D., and L. Enquist. 2009. Directional transneuronal spread of alpha-herpesvirus infection. Future Virol. 4:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das, V., B. Nal, A. Dujeancourt, M. I. Thoulouze, T. Galli, P. Roux, A. Dautry-Varsat, and A. Alcover. 2004. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity 20:577-588. [DOI] [PubMed] [Google Scholar]
- 26.Davis, D. M. 2009. Mechanisms and functions for the duration of intercellular contacts made by lymphocytes. Nat. Rev. Immunol. 9:543-555. [DOI] [PubMed] [Google Scholar]
- 27.DeFalco, J., M. Tomishima, H. Liu, C. Zhao, X. Cai, J. D. Marth, L. Enquist, and J. M. Friedman. 2001. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science 291:2608-2613. [DOI] [PubMed] [Google Scholar]
- 28.De Regge, N., H. J. Nauwynck, K. Geenen, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, T. C. Mettenleiter, and H. W. Favoreel. 2006. Alpha-herpesvirus glycoprotein D interaction with sensory neurons triggers formation of varicosities that serve as virus exit sites. J. Cell Biol. 174:267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimitrov, D. S., R. L. Willey, H. Sato, L. J. Chang, R. Blumenthal, and M. A. Martin. 1993. Quantitation of human immunodeficiency virus type 1 infection kinetics. J. Virol. 67:2182-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doceul, V., M. Hollinshead, L. van der Linden, and G. L. Smith. 2010. Repulsion of superinfecting virions: a mechanism for rapid virus spread. Science 327:873-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dupin, I., E. Camand, and S. Etienne-Manneville. 2009. Classical cadherins control nucleus and centrosome position and cell polarity. J. Cell Biol. 185:779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eugenin, E. A., P. J. Gaskill, and J. W. Berman. 2009. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell Immunol. 254:142-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans, M. J., T. von Hahn, D. M. Tscherne, A. J. Syder, M. Panis, B. Wolk, T. Hatziioannou, J. A. McKeating, P. D. Bieniasz, and C. M. Rice. 2007. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446:801-805. [DOI] [PubMed] [Google Scholar]
- 34.Fackler, O. T., A. Alcover, and O. Schwartz. 2007. Modulation of the immunological synapse: a key to HIV-1 pathogenesis? Nat. Rev. Immunol. 7:310-317. [DOI] [PubMed] [Google Scholar]
- 35.Fiala, J. C., M. Feinberg, V. Popov, and K. M. Harris. 1998. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J. Neurosci. 18:8900-8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finlay, B. B., and P. Cossart. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276:718-725. [DOI] [PubMed] [Google Scholar]
- 37.Frischknecht, F., V. Moreau, S. Rottger, S. Gonfloni, I. Reckmann, G. Superti-Furga, and M. Way. 1999. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature 401:926-929. [DOI] [PubMed] [Google Scholar]
- 38.Fukudome, K., M. Furuse, N. Fukuhara, S. Orita, T. Imai, S. Takagi, M. Nagira, Y. Hinuma, and O. Yoshie. 1992. Strong induction of ICAM-1 in human T cells transformed by human T-cell-leukemia virus type 1 and depression of ICAM-1 or LFA-1 in adult T-cell-leukemia-derived cell lines. Int. J. Cancer. 52:418-427. [DOI] [PubMed] [Google Scholar]
- 39.Ganesh, L., K. Leung, K. Lore, R. Levin, A. Panet, O. Schwartz, R. A. Koup, and G. J. Nabel. 2004. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J. Virol. 78:11980-11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 41.Gerdes, H. H., and R. N. Carvalho. 2008. Intercellular transfer mediated by tunneling nanotubes. Curr. Opin. Cell Biol. 20:470-475. [DOI] [PubMed] [Google Scholar]
- 42.Golovkina, T. V., A. Chervonsky, J. P. Dudley, and S. R. Ross. 1992. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell 69:637-645. [DOI] [PubMed] [Google Scholar]
- 43.Gousset, K., S. D. Ablan, L. V. Coren, A. Ono, F. Soheilian, K. Nagashima, D. E. Ott, and E. O. Freed. 2008. Real-time visualization of HIV-1 GAG trafficking in infected macrophages. PLoS Pathog. 4:e1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grakoui, A., S. K. Bromley, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science 285:221-227. [DOI] [PubMed] [Google Scholar]
- 45.Green, N., T. M. Shinnick, O. Witte, A. Ponticelli, J. G. Sutcliffe, and R. A. Lerner. 1981. Sequence-specific antibodies show that maturation of Moloney leukemia virus envelope polyprotein involves removal of a COOH-terminal peptide. Proc. Natl. Acad. Sci. U. S. A. 78:6023-6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gummuluru, S., C. M. Kinsey, and M. Emerman. 2000. An in vitro rapid-turnover assay for human immunodeficiency virus type 1 replication selects for cell-to-cell spread of virus. J. Virol. 74:10882-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta, P., R. Balachandran, M. Ho, A. Enrico, and C. Rinaldo. 1989. Cell-to-cell transmission of human immunodeficiency virus type 1 in the presence of azidothymidine and neutralizing antibody. J. Virol. 63:2361-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Held, W., G. A. Waanders, A. N. Shakhov, L. Scarpellino, H. Acha-Orbea, and H. R. MacDonald. 1993. Superantigen-induced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell 74:529-540. [DOI] [PubMed] [Google Scholar]
- 49.Hioe, C. E., P. C. Chien, Jr., C. Lu, T. A. Springer, X. H. Wang, J. Bandres, and M. Tuen. 2001. LFA-1 expression on target cells promotes human immunodeficiency virus type 1 infection and transmission. J. Virol. 75:1077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang, B., S. A. Jones, B. Brandenburg, and X. Zhuang. 2008. Whole-cell 3D STORM reveals interactions between cellular structures with nanometer-scale resolution. Nat. Methods 5:1047-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hubner, W., G. P. McNerney, P. Chen, B. M. Dale, R. E. Gordon, F. Y. Chuang, X. D. Li, D. M. Asmuth, T. Huser, and B. K. Chen. 2009. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science 323:1743-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huppa, J. B., and M. M. Davis. 2003. T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 3:973-983. [DOI] [PubMed] [Google Scholar]
- 53.Igakura, T., J. C. Stinchcombe, P. K. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. Tanaka, M. Osame, and C. R. Bangham. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713-1716. [DOI] [PubMed] [Google Scholar]
- 54.Iwasaki, Y., and H. F. Clark. 1975. Cell to cell transmission of virus in the central nervous system. II. Experimental rabies in mouse. Lab. Invest. 33:391-399. [PubMed] [Google Scholar]
- 55.Jin, J., N. Sherer, and W. Mothes. 2010. Surface transmission or polarized egress? Lessons learned from HTLV cell-to-cell transmission. Viruses 2:601-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin, J., N. M. Sherer, G. Heidecker, D. Derse, and W. Mothes. 2009. Assembly of the murine leukemia virus is directed towards sites of cell-cell contact. PLoS Biol. 7:e1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson, D. C., and M. T. Huber. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jolly, C., K. Kashefi, M. Hollinshead, and Q. J. Sattentau. 2004. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J. Exp. Med. 199:283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jolly, C., I. Mitar, and Q. J. Sattentau. 2007. Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J. Virol. 81:13916-13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jolly, C., and Q. J. Sattentau. 2004. Retroviral spread by induction of virological synapses. Traffic 5:643-650. [DOI] [PubMed] [Google Scholar]
- 61.Jones, C. T., M. T. Catanese, L. M. Law, S. R. Khetani, A. J. Syder, A. Ploss, T. S. Oh, J. W. Schoggins, M. R. Macdonald, S. N. Bhatia, and C. M. Rice. 2010. Real-time imaging of hepatitis C virus infection using a fluorescent cell-based reporter system. Nat. Biotechnol. 28:167-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jouvenet, N., M. Windsor, J. Rietdorf, P. Hawes, P. Monaghan, M. Way, and T. Wileman. 2006. African swine fever virus induces filopodia-like projections at the plasma membrane. Cell Microbiol. 8:1803-1811. [DOI] [PubMed] [Google Scholar]
- 63.Kaizuka, Y., A. D. Douglass, R. Varma, M. L. Dustin, and R. D. Vale. 2007. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc. Natl. Acad. Sci. U. S. A. 104:20296-20301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knott, G. W., A. Holtmaat, L. Wilbrecht, E. Welker, and K. Svoboda. 2006. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat. Neurosci. 9:1117-1124. [DOI] [PubMed] [Google Scholar]
- 65.Kuhn, J. R., and M. Poenie. 2002. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity 16:111-121. [DOI] [PubMed] [Google Scholar]
- 66.Kupfer, A., G. Dennert, and S. J. Singer. 1983. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc. Natl. Acad. Sci. U. S. A. 80:7224-7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lawrence, D. M., C. E. Patterson, T. L. Gales, J. L. D'Orazio, M. M. Vaughn, and G. F. Rall. 2000. Measles virus spread between neurons requires cell contact but not CD46 expression, syncytium formation, or extracellular virus production. J. Virol. 74:1908-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lehmann, M. J., N. M. Sherer, C. B. Marks, M. Pypaert, and W. Mothes. 2005. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J. Cell Biol. 170:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lidke, D. S., K. A. Lidke, B. Rieger, T. M. Jovin, and D. J. Arndt-Jovin. 2005. Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors. J. Cell Biol. 170:619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lidke, D. S., P. Nagy, R. Heintzmann, D. J. Arndt-Jovin, J. N. Post, H. E. Grecco, E. A. Jares-Erijman, and T. M. Jovin. 2004. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nat. Biotechnol. 22:198-203. [DOI] [PubMed] [Google Scholar]
- 71.Loewy, A. D. 1998. Viruses as transneuronal tracers for defining neural circuits. Neurosci. Biobehav Rev. 22:679-684. [DOI] [PubMed] [Google Scholar]
- 72.Ludford-Menting, M. J., J. Oliaro, F. Sacirbegovic, E. T. Cheah, N. Pedersen, S. J. Thomas, A. Pasam, R. Iazzolino, L. E. Dow, N. J. Waterhouse, A. Murphy, S. Ellis, M. J. Smyth, M. H. Kershaw, P. K. Darcy, P. O. Humbert, and S. M. Russell. 2005. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity 22:737-748. [DOI] [PubMed] [Google Scholar]
- 73.Majorovits, E., M. Nejmeddine, Y. Tanaka, G. P. Taylor, S. D. Fuller, and C. R. Bangham. 2008. Human T-lymphotropic virus-1 visualized at the virological synapse by electron tomography. PLoS One 3:e2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin, N., S. Welsch, C. Jolly, J. A. Briggs, D. Vaux, and Q. J. Sattentau. 2010. Virological synapse-mediated spread of human immunodeficiency virus type 1 between T cells is sensitive to entry inhibition. J. Virol. 84:3516-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin-Cofreces, N. B., J. Robles-Valero, J. R. Cabrero, M. Mittelbrunn, M. Gordon-Alonso, C. H. Sung, B. Alarcon, J. Vazquez, and F. Sanchez-Madrid. 2008. MTOC translocation modulates IS formation and controls sustained T cell signaling. J. Cell Biol. 182:951-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Masurier, C., B. Salomon, N. Guettari, C. Pioche, F. Lachapelle, M. Guigon, and D. Klatzmann. 1998. Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice. J. Virol. 72:7822-7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mazurov, D., A. Ilinskaya, G. Heidecker, P. A. Lloyed, and D. Derse. 2010. Quantitative comparison of HTLV-1 and HIV-1cell-to-cell infection with new replication dependent vectors. PLoS Pathog. 6:e1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCarthy, K. M., D. W. Tank, and L. W. Enquist. 2009. Pseudorabies virus infection alters neuronal activity and connectivity in vitro. PLoS Pathog. 5:e1000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 80.Medeiros, N. A., D. T. Burnette, and P. Forscher. 2006. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol. 8:215-226. [DOI] [PubMed] [Google Scholar]
- 81.Mercer, J., and A. Helenius. 2008. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 320:531-535. [DOI] [PubMed] [Google Scholar]
- 82.Merz, D. C., A. Scheid, and P. W. Choppin. 1980. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J. Exp. Med. 151:275-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Molon, B., G. Gri, M. Bettella, C. Gomez-Mouton, A. Lanzavecchia, A. C. Martinez, S. Manes, and A. Viola. 2005. T cell costimulation by chemokine receptors. Nat. Immunol. 6:465-471. [DOI] [PubMed] [Google Scholar]
- 84.Monks, C. R., B. A. Freiberg, H. Kupfer, N. Sciaky, and A. Kupfer. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395:82-86. [DOI] [PubMed] [Google Scholar]
- 85.Munch, J., E. Rucker, L. Standker, K. Adermann, C. Goffinet, M. Schindler, S. Wildum, R. Chinnadurai, D. Rajan, A. Specht, G. Gimenez-Gallego, P. C. Sanchez, D. M. Fowler, A. Koulov, J. W. Kelly, W. Mothes, J. C. Grivel, L. Margolis, O. T. Keppler, W. G. Forssmann, and F. Kirchhoff. 2007. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 131:1059-1071. [DOI] [PubMed] [Google Scholar]
- 86.Neil, S. J., S. W. Eastman, N. Jouvenet, and P. D. Bieniasz. 2006. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neil, S. J., T. Zang, and P. D. Bieniasz. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425-430. [DOI] [PubMed] [Google Scholar]
- 88.Newsome, T. P., I. Weisswange, F. Frischknecht, and M. Way. 2006. Abl collaborates with Src family kinases to stimulate actin-based motility of vaccinia virus. Cell Microbiol. 8:233-241. [DOI] [PubMed] [Google Scholar]
- 89.Niell, C. M., M. P. Meyer, and S. J. Smith. 2004. In vivo imaging of synapse formation on a growing dendritic arbor. Nat. Neurosci. 7:254-260. [DOI] [PubMed] [Google Scholar]
- 90.Oddos, S., C. Dunsby, M. A. Purbhoo, A. Chauveau, D. M. Owen, M. A. Neil, D. M. Davis, and P. M. French. 2008. High-speed high-resolution imaging of intercellular immune synapses using optical tweezers. Biophys. J. 95:L66-L68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pais-Correia, A. M., M. Sachse, S. Guadagnini, V. Robbiati, R. Lasserre, A. Gessain, O. Gout, A. Alcover, and M. I. Thoulouze. 2010. Biofilm-like extracellular viral assemblies mediate HTLV-1 cell-to-cell transmission at virological synapses. Nat. Med. 16:83-89. [DOI] [PubMed] [Google Scholar]
- 93.Pan, Y. W., J. M. Scarlett, T. T. Luoh, and P. Kurre. 2007. Prolonged adherence of human immunodeficiency virus-derived vector particles to hematopoietic target cells leads to secondary transduction in vitro and in vivo. J. Virol. 81:639-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perez-Caballero, D., T. Zang, A. Ebrahimi, M. W. McNatt, D. A. Gregory, M. C. Johnson, and P. D. Bieniasz. 2009. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Phillips, D. M. 1994. The role of cell-to-cell transmission in HIV infection. AIDS 8:719-731. [DOI] [PubMed] [Google Scholar]
- 96.Piguet, V., and R. M. Steinman. 2007. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 28:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Platt, E. J., S. L. Kozak, J. P. Durnin, T. J. Hope, and D. Kabat. 2010. Rapid dissociation of HIV-1 from cultured cells severely limits infectivity assays, causes the inactivation ascribed to entry inhibitors, and masks the inherently high level of infectivity of virions. J. Virol. 84:3106-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ploss, A., M. J. Evans, V. A. Gaysinskaya, M. Panis, H. You, Y. P. de Jong, and C. M. Rice. 2009. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 457:882-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poo, W. J., L. Conrad, and C. A. Janeway, Jr. 1988. Receptor-directed focusing of lymphokine release by helper T cells. Nature 332:378-380. [DOI] [PubMed] [Google Scholar]
- 100.Reeves, P. M., B. Bommarius, S. Lebeis, S. McNulty, J. Christensen, A. Swimm, A. Chahroudi, R. Chavan, M. B. Feinberg, D. Veach, W. Bornmann, M. Sherman, and D. Kalman. 2005. Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases. Nat. Med. 11:731-739. [DOI] [PubMed] [Google Scholar]
- 101.Rein, A., J. Mirro, J. G. Haynes, S. M. Ernst, and K. Nagashima. 1994. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J. Virol. 68:1773-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rudnicka, D., J. Feldman, F. Porrot, S. Wietgrefe, S. Guadagnini, M. C. Prevost, J. Estaquier, A. T. Haase, N. Sol-Foulon, and O. Schwartz. 2009. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J. Virol. 83:6234-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rudolph, J. M., N. Eickel, C. Haller, M. Schindler, and O. T. Fackler. 2009. Inhibition of T-cell receptor-induced actin remodeling and relocalization of Lck are evolutionarily conserved activities of lentiviral Nef proteins. J. Virol. 83:11528-11539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sabin, A. B. 1938. Progression of different nasally instilled viruses along different nervous pathways in the same host. Proc. Soc. Exp. Biol. Med. 38:270-275. [Google Scholar]
- 105.Sabin, A. B., and P. K. Olitsky. 1938. Influence of host factors on neuroinvasiveness of vesicular stomatitis virus. IV. Variations in neuroinvasiveness in different species. J. Exp. Med. 67:229-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sainz, B., Jr., V. TenCate, and S. L. Uprichard. 2009. Three-dimensional Huh7 cell culture system for the study of Hepatitis C virus infection. Virol. J. 6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sanchez-Madrid, F., and J. M. Serrador. 2009. Bringing up the rear: defining the roles of the uropod. Nat. Rev. Mol. Cell Biol. 10:353-359. [DOI] [PubMed] [Google Scholar]
- 108.Sattentau, Q. 2008. Avoiding the void: cell-to-cell spread of human viruses. Nat. Rev. Microbiol. 6:815-826. [DOI] [PubMed] [Google Scholar]
- 109.Scheiffele, P., J. Fan, J. Choih, R. Fetter, and T. Serafini. 2000. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101:657-669. [DOI] [PubMed] [Google Scholar]
- 110.Schelhaas, M., H. Ewers, M. L. Rajamaki, P. M. Day, J. T. Schiller, and A. Helenius. 2008. Human papillomavirus type 16 entry: retrograde cell surface transport along actin-rich protrusions. PLoS Pathog. 4:e1000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sherer, N. M., J. Jin, and W. Mothes. 2010. Directional spread of surface associated retroviruses regulated by differential virus-cell interactions. J. Virol. 84:3248-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sherer, N. M., M. J. Lehmann, L. F. Jimenez-Soto, C. Horensavitz, M. Pypaert, and W. Mothes. 2007. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat. Cell Biol. 9:310-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sherer, N. M., and W. Mothes. 2008. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends Cell Biol. 18:414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith, G. A., S. P. Gross, and L. W. Enquist. 2001. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc. Natl. Acad. Sci. U. S. A. 98:3466-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith, J. L., D. S. Lidke, and M. A. Ozbun. 2008. Virus activated filopodia promote human papillomavirus type 31 uptake from the extracellular matrix. Virology 381:16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sol-Foulon, N., M. Sourisseau, F. Porrot, M. I. Thoulouze, C. Trouillet, C. Nobile, F. Blanchet, V. di Bartolo, N. Noraz, N. Taylor, A. Alcover, C. Hivroz, and O. Schwartz. 2007. ZAP-70 kinase regulates HIV cell-to-cell spread and virological synapse formation. EMBO J. 26:516-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sourisseau, M., N. Sol-Foulon, F. Porrot, F. Blanchet, and O. Schwartz. 2007. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J. Virol. 81:1000-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sowinski, S., C. Jolly, O. Berninghausen, M. A. Purbhoo, A. Chauveau, K. Kohler, S. Oddos, P. Eissmann, F. M. Brodsky, C. Hopkins, B. Onfelt, Q. Sattentau, and D. M. Davis. 2008. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 10:211-219. [DOI] [PubMed] [Google Scholar]
- 118a.Stagi, M., A. I. Fogel, and T. Biederer. 2010. SynCAM 1 participates in axo-dendritic contact assembly and shapes neuronal growth concs. Proc. Natl. Acad. Sci. U. S. A. 107:7568-7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stinchcombe, J. C., G. Bossi, S. Booth, and G. M. Griffiths. 2001. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15:751-761. [DOI] [PubMed] [Google Scholar]
- 120.Stinchcombe, J. C., E. Majorovits, G. Bossi, S. Fuller, and G. M. Griffiths. 2006. Centrosome polarization delivers secretory granules to the immunological synapse. Nature 443:462-465. [DOI] [PubMed] [Google Scholar]
- 121.Stolp, B., M. Reichman-Fried, L. Abraham, X. Pan, S. I. Giese, S. Hannemann, P. Goulimari, E. Raz, R. Grosse, and O. T. Fackler. 2009. HIV-1 Nef interferes with host cell motility by deregulation of Cofilin. Cell Host Microbe 6:174-186. [DOI] [PubMed] [Google Scholar]
- 122.Togashi, H., J. Miyoshi, T. Honda, T. Sakisaka, Y. Takai, and M. Takeichi. 2006. Interneurite affinity is regulated by heterophilic nectin interactions in concert with the cadherin machinery. J. Cell Biol. 174:141-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Valitutti, S., and L. Dupre. 2010. Plasticity of immunological synapses. Curr. Top. Microbiol. Immunol. 340:209-228. [DOI] [PubMed] [Google Scholar]
- 124.van der Schaar, H. M., M. J. Rust, B. L. Waarts, H. van der Ende-Metselaar, R. J. Kuhn, J. Wilschut, X. Zhuang, and J. M. Smit. 2007. Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J. Virol. 81:12019-12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vasiliver-Shamis, G., M. W. Cho, C. E. Hioe, and M. L. Dustin. 2009. Human immunodeficiency virus type 1 envelope gp120-induced partial T-cell receptor signaling creates an F-actin-depleted zone in the virological synapse. J. Virol. 83:11341-11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vasiliver-Shamis, G., M. Tuen, T. W. Wu, T. Starr, T. O. Cameron, R. Thomson, G. Kaur, J. Liu, M. L. Visciano, H. Li, R. Kumar, R. Ansari, D. P. Han, M. W. Cho, M. L. Dustin, and C. E. Hioe. 2008. Human immunodeficiency virus type 1 envelope gp120 induces a stop signal and virological synapse formation in noninfected CD4+ T cells. J. Virol. 82:9445-9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vasioukhin, V., C. Bauer, M. Yin, and E. Fuchs. 2000. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 100:209-219. [DOI] [PubMed] [Google Scholar]
- 128.Vendrame, D., M. Sourisseau, V. Perrin, O. Schwartz, and F. Mammano. 2009. Partial inhibition of human immunodeficiency virus replication by type I interferons: impact of cell-to-cell viral transfer. J. Virol. 83:10527-10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang, J. H., C. Kwas, and L. Wu. 2009. Intercellular adhesion molecule 1 (ICAM-1), but not ICAM-2 and -3, is important for dendritic cell-mediated human immunodeficiency virus type 1 transmission. J. Virol. 83:4195-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang, Y. L., F. Lanni, P. L. McNeil, B. R. Ware, and D. L. Taylor. 1982. Mobility of cytoplasmic and membrane-associated actin in living cells. Proc. Natl. Acad. Sci. U. S. A. 79:4660-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wulfing, C., and M. M. Davis. 1998. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science 282:2266-2269. [DOI] [PubMed] [Google Scholar]
- 132.Yamada, S., and W. J. Nelson. 2007. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J. Cell Biol. 178:517-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yamada, S., and W. J. Nelson. 2007. Synapses: sites of cell recognition, adhesion, and functional specification. Annu. Rev. Biochem. 76:267-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yoder, A., D. Yu, L. Dong, S. R. Iyer, X. Xu, J. Kelly, J. Liu, W. Wang, P. J. Vorster, L. Agulto, D. A. Stephany, J. N. Cooper, J. W. Marsh, and Y. Wu. 2008. HIV envelope-CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell 134:782-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yu, D., W. Wang, A. Yoder, M. Spear, and Y. Wu. 2009. The HIV envelope but not VSV glycoprotein is capable of mediating HIV latent infection of resting CD4 T cells. PLoS Pathog. 5:e1000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu, H. J., M. A. Reuter, and D. McDonald. 2008. HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog. 4:e1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ziv, N. E., and S. J. Smith. 1996. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 17:91-102. [DOI] [PubMed] [Google Scholar]