Abstract
The human cytomegalovirus (HCMV) UL112-113 region encodes four phosphoproteins with common amino termini (p34, p43, p50, and p84) via alternative splicing and is thought to be required for efficient viral DNA replication. We have previously shown that interactions among the four UL112-113 proteins regulate their intranuclear targeting and enable the recruitment of the UL44 DNA polymerase processivity factor to viral prereplication foci. Here, we show that in virus-infected cells, the UL112-113 proteins form a complex with UL44 and other replication proteins, such as UL84 and IE2. In vitro assays showed that all four phosphoproteins interacted with UL44. Interestingly, p84 required both the shared amino-terminal region and the specific near-carboxy-terminal region for UL44 binding. UL44 required both the carboxy-terminal region and the central region, including the dimerization domain for p84 binding. The production of recombinant virus from mutant Towne bacterial artificial chromosome (BAC) DNA, which encodes intact p34, p43, and p50 and a carboxy-terminally truncated p84 defective in UL44 binding, was severely impaired compared to wild-type BAC DNA. A similar defect was observed when mutant BAC DNA encoded a carboxy-terminally truncated UL44 defective in p84 binding. In cotransfection replication assays using six replication core proteins, UL84, IE2, and UL112-113, the efficient replication of an HCMV oriLyt-containing plasmid required the regions of p84 and UL44 necessary for their interaction. Our data suggest that the UL112-113 proteins form a complex with other replication proteins such as UL44, UL84, and IE2 and that the specific interaction of UL112-113 p84 with UL44 is necessary for efficient viral DNA replication.
In human cytomegalovirus (HCMV), viral DNA replication requires six replication core proteins, such as DNA polymerase (UL54) and its associated polymerase processivity factor (UL44), single-stranded DNA-binding protein (UL57), and the heterotrimer consisting of DNA helicase (UL105), primase (UL70), and primase-associated factor (UL102) subunits. Their genes are initially predicted by their homology to essential herpes simplex virus type 1 (HSV-1) replication genes (8, 9) and are highly conserved among all herpesviruses. In cotransfection replication assays using oriLyt-containing plasmid DNA, five additional genetic loci (UL36-38 loci, TRS1/IRS1, IE2, UL84, and UL112-113) have also been found to contribute to effective viral DNA replication (24, 25, 32, 33, 41, 42).
The UL112-113 region encodes four nuclear phosphoproteins (p34, p43, p50, and p84) with common amino termini of 252 amino acids via alternative splicing (35, 39, 40). The UL112-113 proteins were previously shown to have DNA-binding activity (14) and enhance the IE2-mediated transactivation of the viral polymerase (UL54) promoter (13, 15, 19). Interestingly, the UL112-113 gene was recently found to activate the lytic cycle of Kaposi's sarcoma-associated herpesvirus (38). A more direct role of the UL112-113 proteins in viral DNA replication was suggested by the observation that the replication of oriLyt-containing plasmid DNA in cells cotransfected with the six replication core proteins plus IE2 and UL84 was significantly enhanced by the additional expression of the UL112-113 proteins (32). The notion that the UL112-113 locus is required for efficient viral DNA replication is also supported by genetic studies. When the expression of the UL112-113 proteins was blocked by specific antisense RNAs, viral DNA replication was suppressed (43). The deletion of a UL112 or UL113 open reading frame (ORF) from HCMV bacterial artificial chromosomes (BACs) significantly impaired their ability to produce progeny virus in transfected permissive cells (10, 44).
The localization patterns of the UL112-113 proteins are temporally regulated throughout the virus replication cycle. The UL112-113 proteins were found together with IE2 at the periphery of promyelocytic leukemia protein (PML)-nuclear bodies (NBs) in the early stages of infection and in viral prereplication foci and replication compartments in later stages of infection (4, 28, 43). We recently demonstrated that when coexpressed with the six replication core proteins, transfected UL112-113 proteins cause the relocalization of UL44 to PML-NB-associated sites (27). Furthermore, the UL112-113 proteins self-interacted and interacted with each other, and interactions among the four UL112-113 proteins were required for their targeting and recruiting of UL44 to viral prereplication sites (27). These studies suggest that the UL112-113 proteins may play an important role in the recruitment of viral replication core proteins to viral replication sites.
In the present study, we investigated whether the UL112-113 proteins interact with other replication proteins. We show that UL112-113 p84 is associated with UL44, UL84, and IE2 in virus-infected cells. We also provide evidence that although the p34, p43, p50, and p84 UL112-113 proteins all physically interact with UL44 in vitro, the specific interaction between p84 and UL44 may be necessary for efficient viral growth in permissive cells transfected with HCMV BAC DNA and efficient oriLyt-dependent DNA replication in cotransfection replication assays.
MATERIALS AND METHODS
Cell culture and virus infection.
Human foreskin diploid fibroblast (HF), Vero, and 293T cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. The HCMV Towne virus stocks used in this study were prepared as previously described (17). HF cells were infected with virus at the specified multiplicities of infection (MOIs). Two days after infection, total cell lysates were prepared for coimmunoprecipitation (CoIP) assays.
Transient DNA transfection.
293T cells were transfected via the N,N-bis-(2-hydroxyethyl)-2-aminoethanesulfonic acid-buffered saline (BBS) (Calbiochem) version of the calcium phosphate method (27). A mixture of plasmid DNA and sterile H2O was mixed with CaCl2 (to a final concentration of 0.25 M) and with an equal volume of 2× BBS (50 mM BBS [pH 7.0], 280 mM NaCl, 1.5 mM Na2HPO4). The mixture was kept at room temperature for 20 min and then added dropwise to cells. HF cells were transfected by electroporation.
Plasmids.
Expression plasmids for the p34, p40, p50, and p84 proteins encoded from the UL112-113 region and p84(ΔN252) [previously named 84(ΔExon-1)] were described previously (27). The original p84 cDNA expression plasmid expresses both p84 and, to a lesser extent, p43 (27). A cDNA encoding only p84 was produced on the pENTR-p84 cDNA template (27) by PCR using primers LMV491 (top) and LMV492 (bottom) according to the Stratagene QuikChange site-directed mutagenesis protocol (all of the primers used in this study are listed in Table 1). In this only-p84-encoding cDNA background, the carboxy-terminal truncation mutants of p84 were generated by PCR. The common 5′ primer for PCR was LMV8 (containing a BglII site). The 3′ primers (containing a BglII site) were LMV314 (for ΔC620), LMV315 (for ΔC560), LMV316 (for ΔC500), LMV317 (for ΔC440), and LMV318 (for ΔC380). An amino-terminal truncation mutant of p84, ΔN346 (pRYK134), was also produced by PCR using LMV320 (5′ primer with a BglII site) and LMV9 (3′ primer with a BglII site). All of the PCR-amplified DNA fragments were inserted into the BamHI site of the pENTR vector (Invitrogen). The entire UL112-113 genomic gene was cloned into the pENTR vector and resulted in pMY8. The ΔC620, ΔC560, and ΔC347 mutations, which were introduced on the p84 expression plasmid, were also produced on this pMY8 background. All mutants were verified by direct sequencing with LMV11 as a sequencing primer. Plasmid p84(ΔC347) was previously described (27).
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequence |
|---|---|
| LMV8 | 5′-GAAGATCTATGGATCTCCCTACTACCGTC-3′ |
| LMV9 | 5′-GAAGATCTTTAATCGTCGAAAAACGCCGCGAT-3′ |
| LMV11 | 5′-GAAGATCTATGGGCTCTCCTCCCCTCCGGGAG-3′ |
| LMV255 | 5′-GACGGATCCATGGATCGCAAGACGCGC-3′ |
| LMV256 | 5′-GACGATATCCTAGCCGCACTTTTGCTT-3′ |
| LMV307 | 5′-GACGGATCCACCTTCTTGCCCACGCCG-3′ |
| LMV308 | 5′-GACGGATCCAACAATTCCACGCCGTG-3′ |
| LMV309 | 5′-GACGGATCCTTCAATATGGAGTTCAGC-3′ |
| LMV310 | 5′-GACGGATCCGTGGTGGCGGCAGCCTCT-3′ |
| LMV311 | 5′-GACGATATCCTAGCCGCCGCCCGATCC-3′ |
| LMV313 | 5′-GACGATATCCTACGCCGCCGCCACGCG-3′ |
| LMV314 | 5′-GAAGATCTTTAGGAGGAGGACGACGACGA-3′ |
| LMV315 | 5′-GAAGATCTTTAGGGGTTTACCACGTAGGG-3′ |
| LMV316 | 5′GAAGATCTTTAGGTCCTCGCGACGCTGCT-3′ |
| LMV317 | 5′-GAAGATCTTTATTCCTGCGAATTCGAAGG-3′ |
| LMV318 | 5′GAAGATCTTTAGCTTTGGGCGCTGCTGGG-3′ |
| LMV319 | 5′-GACGATATCCTAGCACGGCAGTTTGGT-3′ |
| LMV320 | 5′-GAAGATCTGGTGCGTTACTTCTACCCATT-3′ |
| LMV391 | 5′-AGCGGGAAATCGTGCGTG-3′ |
| LMV392 | 5′-CAGGGTACATGGTGGTGCC-3′ |
| LMV491 | 5′-GCCGGCACCGACGGCGCGTTACTTCTA-3′ |
| LMV492 | 5′-TAGAAGTAACGCGCCGTCGGTGCCGGC-3′ |
| LMV707 | 5′-ATGGATCGCAAGACGCGCCTCTCGGAGCCGCCGACGCTGGCGCTGCGGCTGGCCTGGTGATTGAGGCGGGATCG-3′ |
| LMV708 | 5′-CTAGCCGCACTTTTGCTTCTTGGTGTTAGGGACGAACTCGAACGTTACAGTCAGAAGAACTCGTCAAGAAGGCG-3′ |
| LMV772 | 5′-ATTCCACTAGATGTGCGC-3′ |
| LMV773 | 5′-CAGATGCCCCGCCTAGGT-3′ |
| LMV774 | 5′-ATGGATCGCAAGACGCGC-3′ |
| LMV775 | 5′-CTAGCCGCACTTTTGCTT-3′ |
| LMV863 | 5′-GCAGCGTCGCGAGGACCGCCGCAGCTGTCTCCGCAGCCGGCGTTGGCCCCGGCCTGGTGATGACGGGATCG-3′ |
| LMV864 | 5′TTTCTCCGTCGCGATCTCCGAGAGGAGGAGGACGACGATGCAGCCTGTCAGAAGAACTCGTCAAGAAGGCG-3′ |
| LMV865 | 5′-GCAGCGGGATCGCGACCGCCG-3′ |
| LMV866 | 5′-TTTCTCCGTCGCGATCTCCGA-3′ |
| LMV1099 | 5′-ATAAGCGGGAGATGTGGATG-3′ |
| LMV1100 | 5′-TTCATCCTTTTTAGCACGGG-3′ |
| LMV1101 | 5′-ACCATGCAGGTGAACAACAA-3′ |
| LMV1102 | 5′-CATGAGGAAGGGAGTGGAGA-3′ |
| LMV1278 | 5′-GGTGCGTTACTTCTACCCATT-3′ |
| LMV1279 | 5′-TTAGGTCCTCGCGACGCTGCT-3′ |
| LMV1280 | 5′-TTCAATATGGAGTTCAGC-3′ |
| LMV1281 | 5′-CTAGCACGGCAGTTTGGT-3′ |
To produce UL44 truncation mutants, pENTR-UL44 (pJHA367) was used as a PCR template. For the construction of the carboxy-terminal truncation mutants, the common 5′ primer was LMV255 (with a BamHI site), and the 3′ primers (with an EcoRV site) were LMV311 (for ΔC390), LMV313 (for ΔC290), and LMV319 (for ΔC240). For the amino-terminal truncation mutants, the common 3′ primer was LMV256 (with an EcoRV site), and the 5′ primers (with a BamHI site) were LMV307 (for ΔN40), LMV308 (for ΔN80), LMV309 (for ΔN120), and LMV310 (for ΔN290). These PCR fragments were inserted into the BamHI/EcoRV sites of the pENTR vector.
The mammalian expression plasmids for the amino-terminal hemagglutinin (HA)- or Flag-tagged proteins were generated by moving the cDNAs on pENTR vectors into a pSG5 (12) background by using Gateway technology (Invitrogen). To construct the template plasmids for the in vitro transcription/translation reactions, wild-type or mutant cDNAs on pENTR vectors were moved into pSPUTK (without a tag) (Stratagene) or pCS3-MT (with a 6-Myc tag) plasmids (31) by using Gateway technology. These plasmids were also used to express proteins in cultured cells via DNA transfection.
The plasmid harboring HCMV replication-origin DNA (pSP38) and plasmids expressing the six replication core proteins (UL54, UL44, UL57, UL105, UL70, and UL102) and auxiliary proteins (IE2 and UL84) were previously described (32). Saccharomyces cerevisiae expression plasmids for GAL4-DNA-binding (GAL4-DB) domain fusions and GAL4-activation (GAL4-A) domain fusions were produced on the pAS1-CYH2 and pACTII backgrounds (5), respectively, by using Gateway technology.
BAC mutagenesis.
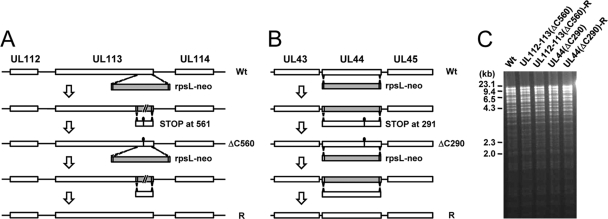
The Towne bacterial artificial chromosome (BAC) (T-BAC) clones encoding the UL112-113(ΔC560) and UL44(ΔC290) genes were generated by using a counterselection BAC modification kit (Gene Bridges, Germany). Briefly, rpsL-neo cassettes were PCR amplified by using primers containing homology arms consisting of 50 nucleotides upstream and downstream of the target gene plus 24 nucleotides homologous to the rpsL-neo cassette. The primer sets used were LMV863/LMV864 for the rpsL-neo cassette targeting UL112-113 and LMV707/LMV708 for the rpsL-neo cassette targeting UL44. The amplified DNA fragments were purified and introduced into Escherichia coli GS243 cells containing wild-type T-BAC (20) for recombination by electroporation using Gene Pulser II (Bio-Rad). The intermediate T-BAC construct containing the rpsL-neo cassette was selected on Luria broth (LB) plates containing kanamycin. Next, the mutated DNA fragments for replacing the rpsL-neo cassette were amplified by PCR using the following primer sets and templates: LMV865/LMV866 for UL112-113(ΔC560) with the pRYK322 template, which contains a ΔC560 mutation in a pMY8 background, and LMV774/LMV775 for UL44(ΔC290) with the pRYK324 template, which contains a ΔC290 mutation in a pJHA367 background. The amplified fragments were recombined into T-BAC DNAs containing the rpsL-neo cassette, and the UL112-113(ΔC560) and UL44(ΔC290) T-BACs were selected on LB plates containing streptomycin. The mutated regions were amplified and sequenced to verify the desired mutations. Revertant T-BACs were also generated from the mutant T-BACs. pMY8 (for UL112-113) and pMR102 (for UL44) were used as templates to produce DNA fragments containing wild-type target genes. These fragments were inserted into the mutant T-BACs by homologous recombination.
Electroporation.
T-BAC DNAs were introduced into HF cells by electroporation. For each reaction, HF cells (2 × 106) in 200 μl of resuspension buffer were mixed with 3 μg of T-BAC DNA, 4 μg of plasmid pCMV71 encoding transactivator pp71, and 1 μg of pEGFP-C1 for monitoring the electroporation efficiency. After electroporation at 1,300 V and 40 ms using a Microporator MP-100 instrument (Digital Bio Technology), the cells were plated into T-25 flasks. When they reached confluence, the cells were split into new flasks at a ratio of 1:2.
Yeast two-hybrid assay for protein interaction.
Yeast Y190 (MATa) cells were transformed with plasmids (TRP1) expressing GAL4-DB fusion proteins. Y187 (MATα) cells were transformed with plasmids (LEU2) expressing GAL4-A fusion proteins. The transformants were selected on synthetic medium plates lacking tryptophan or leucine. Y190 and Y187 cells were mated on yeast complete medium plates with overnight incubation. Diploid cells (MATa/α) were selected on synthetic medium plates lacking both tryptophan and leucine and were examined for lacZ expression by using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) filter assays as described previously (1). Yeast strains, media for yeast growth, and methods for yeast transformation were described previously (3, 18).
In vitro binding assays with GST fusion proteins.
Glutathione S-transferase (GST) and GST-UL44 fusion proteins were produced in E. coli BL21 cells as previously described (26). These proteins were incubated with in vitro-translated (IVT) [35S]Met-labeled UL112-113 proteins in 0.5 ml of EBC buffer (50 mM Tris-Cl [pH 7.0], 120 mM NaCl, 0.5% NP-40, 0.2 mM Na2VO4, 100 mM NaF) at 4°C for 2 h. The bead pellet was washed 10 times with 1 ml of NETN buffer (100 mM NaCl, 20 mM Tris-Cl [pH 8.0], 0.5% Triton X-100, and 1 mM EDTA). Boiled samples were then subjected to SDS-PAGE and autoradiography.
Cotransfection replication assay.
HF cells (2 × 106) were cotransfected with the replication protein plasmids (1 μg each) expressing the six replication core proteins plus IE2, UL84, and UL112-113 (wild type or mutant, as indicated) and a plasmid containing the HCMV replication origin (pSP38) by electroporation and plated onto 100-mm dishes. Total cellular DNAs were harvested 5 days posttransfection by using QIAamp DNA minikits (Qiagen). DNAs (10 μg each) were first digested with XbaI and then treated with DpnI. The digested DNA fragments were resolved by electrophoresis on a 1% agarose gel at 4°C at 100 V for 3 h. Replication products were analyzed by Southern blotting with α-32P-labeled KpnI-digested pSP38 DNA as a probe.
Southern blot analysis.
DNA fragments separated on a 1% agarose gel by electrophoresis were denatured and transferred onto uncharged nylon membranes (Hybond-N). The transferred DNAs were immobilized by UV cross-linking for 2 min at 1,600 J/cm2. DNA probes were radiolabeled with [α-32P]dCTP (NEN) by using the Random-Primed DNA labeling kit (Roche). Prehybridization was performed at 68°C for 1 h in 10 ml of prehybridization buffer (6× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 5× Denhardt's reagent [USB], 0.5× sodium dodecyl sulfate [SDS], and 100 μg/ml of salmon sperm DNA). DNA blots were hybridized with radiolabeled probes in the same solution at 68°C for 16 h. The blots were washed at room temperature for 5 min in 2× SSC-0.5% SDS and for 15 min in 2× SSC-0.1% SDS and at 65°C for 30 min to 4 h in 0.1× SSC-0.1% SDS. The blots were briefly washed at room temperature in 0.1× SSC and exposed to X-ray film (Kodak).
Reverse transcription (RT)-PCR.
Total RNAs were isolated from HF cells electroporated with BAC DNAs by using Trizol reagent (Invitrogen) and MaXtract high density (Qiagen). First-strand cDNA was synthesized by using the random hexamer primers in the SuperScript III system (Invitrogen). PCR was performed by using the following primer sets: LMV1099/LMV1100 (for IE1), LMV1101/LMV1102 (for IE2), LMV1278/LMV1279 (for UL112-113), LMV1280/LMV1281 (for UL44), and LMV391/LMV392 (for β-actin).
ChIP assay.
Chromatin immunoprecipitation (ChIP) assays were performed by using a kit (Upstate Biotechnology, Inc.), with minor modifications. In brief, DNA-cotransfected HF cells (4 × 106) were fixed with 1% formaldehyde for 10 min 5 days after transfection and then lysed with a lysis buffer provided with the kit. ChIP assays were performed with appropriate antibodies (Abs) or control immunoglobulin G (IgG). One-sixth of the lysate was reserved to facilitate the quantitation of the amount of DNA present in different samples prior to immunoprecipitation. For the detection of the HCMV oriLyt region, DNAs purified from immunocomplexes were amplified by PCR using primers LMV772 and LMV773. The PCR program used was 94°C for 5 min, 30 amplification cycles (94°C for 30 s, 57°C for 30 s, and 68°C for 45 s), and a final step at 72°C for 10 min.
Antibodies.
Rabbit anti-peptide polyclonal antibody (PAb) specific to UL112-113 p84 and monoclonal antibody (MAb) M23, which recognizes the shared N-terminal region of the four UL112-113 proteins, were previously described (14, 27). The anti-HA rat MAb (3F10) either conjugated with peroxidase or labeled with fluorescein, and anti-Myc mouse MAb 9E10 were purchased from Roche. The anti-Flag mouse MAb M2 was obtained from Sigma. Anti-UL44 (p52) mouse MAb and anti-p28 mouse MAb were purchased from Advanced Biotechnologies, Inc. The anti-UL84 mouse MAb was purchased from Virusys. Mouse MAb 810R, which detects epitopes present in both IE1 and IE2, and mouse MAb 12E2, specific to IE2, were purchased from Chemicon and Vancouver Biotech, respectively. Anti-proliferating cell nuclear antigen (PCNA) mouse MAb PC10 was obtained from Santa Cruz Biotechnology, Inc. The rabbit anti-PML PAb, referred to as PML(C), was previously described (2). Secondary Abs such as fluorescein isothiocyanate (FITC)-labeled donkey anti-mouse IgG, FITC-labeled goat anti-rabbit IgG, and rhodamine/redX-coupled donkey anti-mouse IgG were obtained from Jackson ImmunoResearch Laboratories, Inc.
Indirect immunofluorescence assay (IFA).
Cells were washed in phosphate-buffered saline (PBS), fixed with 1% paraformaldehyde in PBS at room temperature for 5 min, and then permeabilized on ice with 0.2% Triton X-100 for 20 min. Cells were incubated with rabbit PAbs at 1:800 dilutions, with anti-Flag Ab at a 1:2,000 dilution, and with anti-HA Ab at a 1:80 dilution in PBS at 37°C for 1 h, followed by incubation with appropriate secondary Abs at 1:100 dilutions at 37°C for 1 h. For double labeling, Abs were incubated together. All slides were examined and photographed with a Carl Zeiss Axiophot microscope.
CoIP assays.
293T cells (8 × 105) were harvested 2 days after transfection and sonicated in 0.7 ml coimmunoprecipitation (CoIP) buffer (50 mM Tris-Cl [pH 7.4], 50 mM NaF, 5 mM sodium phosphate, and 0.1% Triton X-100 containing protease inhibitors [Sigma]) with a microtip probe (Vibra-Cell; Sonics and Materials, Inc.) for 10 s (pulse on for l s and pulse off for 3 s). Cell lysates were incubated with anti-Myc Ab (Roche). After incubation for 16 h at 4°C, 30 μl of a 50% slurry of protein A- and protein G-Sepharose (Amersham) was added, and the mixture was then incubated for 2 h at 4°C to allow adsorption. The mixture was then pelleted and washed seven times with CoIP buffer. The beads were resuspended and boiled for 5 min in loading buffer. Each sample was analyzed by SDS-PAGE and immunoblotting with anti-HA Ab (Roche). For CoIP assays of virus-infected cells, HF cells (4 × 106) were infected with virus for 2 days, and CoIP assays were carried out with appropriate Abs.
Immunoblot analysis.
Samples were prepared by boiling in loading buffer, separated by SDS-PAGE, and then transferred onto a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). The membrane was blocked for 1 h in PBST (PBS plus 0.1% Tween 20 [Sigma]) containing 5% skim milk and then washed with PBST. After incubation with appropriate Abs, the proteins were visualized by standard procedures using an enhanced chemiluminescence system (Roche) and Kodak X-ray film.
RESULTS
The UL112-113 proteins form a complex with UL44, IE2, and UL84 in virus-infected cells.
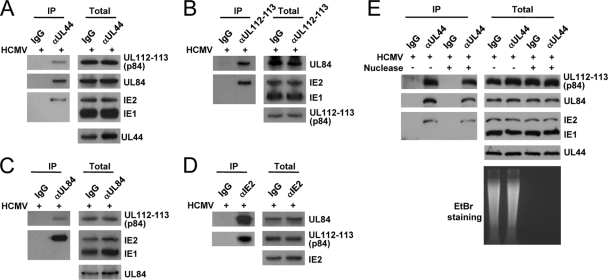
To investigate whether the UL112-113 proteins interact with other replication proteins such as UL44, UL84, and IE2, HF cells were infected with HCMV, and CoIP assays were performed. The results of CoIP assays with anti-UL44 Ab showed that UL44 coprecipitated with UL112-113 p84, UL84, and IE2 (Fig. 1 A). We could not determine whether UL112-113 p34, p43, and p50 are also present in the UL44 immune complex, because the Abs that detect these UL112-113 proteins in immunoblot assays are not available. Immunoprecipitation with an anti-UL112-113 Ab (M23), which detects all of the proteins p34, p43, p50, and p84 UL112-113 (14, 27), efficiently pulled down UL84 and IE2 (Fig. 1B). In similar CoIP assays using anti-UL84 and anti-IE2 Abs, UL84 coprecipitated with UL112-113 p84 and IE2 (Fig. 1C), and IE2 coprecipitated with UL84 and UL12-113 p84 (Fig. 1D). In CoIP assays with M23, anti-UL84, and anti-IE2 Abs, we could not address whether UL44 is coprecipitated, because UL44 comigrated with immunoglobulin heavy chains on SDS-PAGE gels. In control experiments, anti-UL44, M23, and anti-UL84 did not coprecipitate with IE1, and immunoprecipitation with control IgG did not pull down any viral proteins tested. Overall, these results suggest that the UL112-113 proteins may form a complex with UL44, IE2, and UL84 in virus-infected cells.
FIG. 1.
CoIP assays demonstrating the formation of a complex containing UL112-113, UL44, UL84, and IE2 in virus-infected cells. (A to D) HF cells were infected with HCMV (Towne) at an MOI of 2.0. At 48 h, total cell lysates were prepared and immunoprecipitated with anti-UL44 (αUL44) (A), anti-UL112-113 (M23) (B), anti-UL84 (C), and anti-IE2 (D) Abs. Immunoprecipitation with mouse IgG was used as a negative control. The immunoprecipitated samples were subjected to SDS-PAGE, followed by immunoblotting with Abs specific for UL112-113 p84, UL84, IE1/IE2, and UL44. Total cell lysates were also subjected to SDS-PAGE, and immunoblot analysis was performed to confirm the protein expression levels. (E) Immunoprecipitation was performed, as described above (A), with total cell lysate untreated or treated with 100 U/ml DNase (Roche) plus 10 μg/ml RNase (Sigma) at 4°C for 12 h. To confirm the removal of nucleic acids, DNA was recovered from total cell lysates by phenol and chloroform extraction and then analyzed on a 1% agarose gel containing ethidium bromide (EtBr).
Both IE2 and UL84 associate with DNA in the HCMV oriLyt region (23), and the UL112-113 proteins were also reported previously to have DNA-binding activity (14). To test whether the interactions among UL112-113, IE2, UL84, and UL44 are dependent on the presence of nucleic acids, we also performed separate CoIP assays in the presence or absence of nucleases. The results of CoIP assays with anti-UL44 Ab showed that UL44 still associated with UL112-113 p84, UL84, and IE2 after treatment with both DNase and RNase (Fig. 1E). The degradation of nucleic acids by nucleases was confirmed by analyzing the amounts of nucleic acids in cell lysates on an ethidium bromide-containing agarose gel (Fig. 1E). This result indicates that the association of UL112-113 p84 with UL44, IE2, and UL84 occurs in a nucleic acid-independent manner.
The UL112-113 proteins interact with UL44 but not with other replication core proteins.
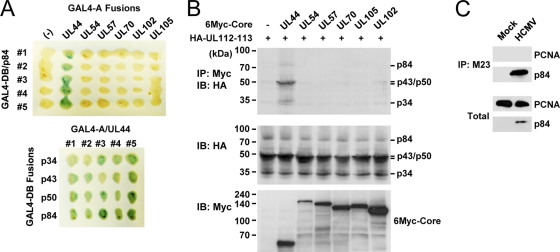
We next asked whether the UL112-113 proteins interact with other viral replication core proteins. First, in the yeast two-hybrid protein interaction assay, UL112-113 p84 was shown to specifically interact with UL44 but not with other replication core proteins such as UL54, UL57, UL70, UL102, and UL105 (Fig. 2 A, top). In similar assays with different UL112-113 proteins, p34, p43, p50, and p84 all interacted with UL44 to similar degrees (Fig. 2A, bottom). Therefore, all of the UL112-113 proteins were found to interact with UL44 in yeast. The specific interaction between the UL112-113 proteins and UL44 was also investigated by cotransfection assays. When 293T cells were cotransfected with plasmids encoding the UL112-113 proteins and one of the six replication core proteins, the results of CoIP assays showed that the UL112-113 proteins interact with UL44 but not with other replication core proteins (Fig. 2B).
FIG. 2.
Specific interaction of UL112-113 p84 with UL44 among the six replication core proteins. (A) X-Gal filter assays of yeast cells expressing both the GAL4-DB/UL112-113 (p34, p43, p50, and p84) fusion proteins and the GAL4-A/replication core (UL44, UL54, UL57, UL70, UL102, and UL105) fusion proteins. The cells expressing the GAL4-A proteins alone were used as a control. Green indicates a positive interaction. (B) 293T cells were cotransfected with plasmids encoding HA-tagged UL112-113 proteins and Myc-tagged replication core proteins (UL44, UL54, UL57, UL70, UL102, or UL105). (Top) At 48 h, total cell lysates were prepared and immunoprecipitated with anti-Myc Ab, followed by immunoblotting with anti-HA Ab. (Middle and bottom) Total cell lysates were also immunoblotted with anti-Myc or anti-HA Abs. (C) HF cells were infected with HCMV Towne at an MOI of 2.0. Total cell lysates were prepared 2 days postinfection and immunoprecipitated with M23 Ab specific for the UL112-113 proteins, followed by immunoblotting with anti-PCNA or anti-p84 Abs. Total cell lysates were also immunoblotted with anti-p84 or anti-PCNA Abs to show the protein expression levels.
The structure of UL44 is similar to that of PCNA, a cellular processivity factor (45). Therefore, using CoIP assays, we also investigated whether the UL112-113 proteins interact with PCNA. The virus-infected cells were immunoprecipitated with M23 Ab, which was followed by an immunoblot assay with anti-PCNA Ab. However, no interaction between the UL112-113 proteins and PCNA was detected under the conditions that we used (Fig. 2C). This result suggests that the UL112-113 proteins specifically interact with the viral polymerase processivity factor UL44 but not with the cellular processivity factor PCNA.
Characterization of the interaction between the UL112-113 proteins and UL44 in vitro.
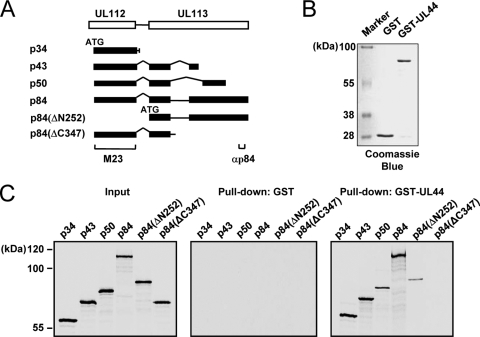
We further investigated the interaction between the UL112-113 proteins and UL44 in vitro. All of the proteins p34, p43, p50, and p84 as well as two amino- and carboxy-terminally truncated versions of p84 (ΔN252 and ΔC347) were produced labeled with [35S]Met by in vitro transcription and translation reactions (Fig. 3 A). The GST and GST-UL44 fusion proteins were produced in E. coli cells (Fig. 3B). We performed in vitro GST pulldown assays with these proteins (Fig. 3C). The results showed that p34, p43, p50, and p84 were all bound to GST-UL44 but not to GST alone, suggesting that the shared 252 amino acids may be involved in binding UL44. However, p84(ΔN252), which does not share the amino-terminal 252 amino acids, still weakly interacted with UL44, implying that another p84 region beyond the amino-terminal 252 amino acids may also contribute to UL44 binding. Interestingly, p84(ΔC347), which lacks the p84-specific carboxy-terminal region but retains the regions encoded by exons 1 and 2, completely lost UL44-binding activity. It is likely that the structure of the amino-terminal UL112-encoded region of p84(ΔC347) is quite different from the amino-terminal structures of p34, p43, and p50, with a resultant masking of the UL44-binding interface. Taken together, our results from in vitro binding assays suggest that all of the UL112-113 proteins physically interact with UL44 in vitro and that p84 requires both the shared amino-terminal region and the specific carboxy-terminal region for efficient UL44 binding.
FIG. 3.
Interaction of the UL112-113 proteins with UL44 in vitro. (A) Structures of the p34, p43, p50, and p84 proteins and the ΔΝ252 and ΔC347 mutants. The ATG start codons of the cDNA are indicated. The 35S-labeled p34, p43, p50, and p84 proteins and ΔN25 and ΔC347 mutants were synthesized individually by in vitro transcription/translation reactions as described in Materials and Methods. (B and C) In vitro binding assays of the in vitro-translated UL112-113 proteins with bacterially produced GST or GST-UL44. One-sixth of the GST or GST-UL44 proteins used in each reaction was visualized with Coomassie blue staining (B). The GST or GST-UL44 proteins immobilized to glutathione-Sepharose beads were incubated with 35S-labeled UL112-113 proteins (p34, p43, p50, p84, ΔN252, or ΔC347). One-tenth of the labeled UL112-113 proteins used in each binding reaction was loaded as input controls. The bound proteins were fractionated on an SDS-8% PAGE gel and visualized by autoradiography (C).
UL112-113 p84 domains required for UL44 binding.
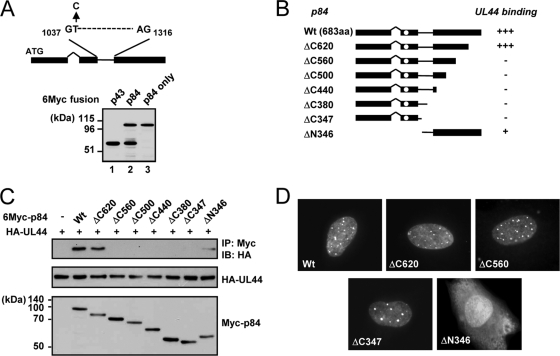
The expression plasmid containing the cDNA for UL112-113 p84 also expressed a small amount of p43 because of an internal splicing event (27). When the same cDNA was placed into a pCS3-MT background (with a 6-Myc tag), significant amounts of p43 were produced (Fig. 4 A, lane 2). To construct an expression plasmid encoding only p84, we generated an expression plasmid containing p84-specific cDNA, in which the internal splicing donor sequence is disrupted by changing thymine (T) at nucleotide position 1038 of the p84 open reading frame to cytosine (C). This nucleotide change did not alter the codon. When examined in transfected 293T cells, this p84-specific cDNA completely abolished the expression of p43 (Fig. 4A, lane 3).
FIG. 4.
CoIP assays to determine the region of UL112-113 p84 required for UL44 binding. (A) Diagram indicating the splice donor and splice acceptor sites used for the production of p43 from p84 cDNA. A cDNA expressing only p84 was generated by a thymine-to-cytosine substitution at nucleotide 1038 (top). 293T cells were transfected with the indicated plasmids encoding Myc-tagged p43, p84 plus p43 (from the original p84 cDNA), or p84 only. At 48 h, total cell lysates were prepared and subjected to SDS-PAGE, followed by immunoblotting with anti-Myc Ab (bottom). (B) Structures of intact p84 and its truncation mutants that were used. The location of the reported NLS (13, 15, 19) is indicated by open circles. The results (shown in C) are summarized (+++, strong positive interaction; +, positive interaction; −, negative interaction). aa, amino acids. (C) Interaction of UL44 with p84 (wild type or mutants). 293T cells were cotransfected with HA-tagged UL44 and Myc-tagged p84 (wild-type or mutant versions). At 48 h, total cell lysates were prepared and immunoprecipitated with anti-Myc Ab, followed by immunoblotting (IB) with anti-HA Ab (top). The expression levels of each protein are shown by immunoblotting of total cell lysates with anti-HA Ab (middle) or anti-Myc Ab (bottom). (D) Localization patterns of p84 and its mutants. HF cells were transfected with plasmids encoding Myc-tagged wild-type or indicated mutant p84. After 48 h, the cells were fixed with methanol, followed by IFA with anti-Myc Ab and rhodamine/redX-labeled anti-mouse IgG.
To map the region of p84 necessary for UL44 binding, we constructed a series of p84 truncation mutants in the p84-specific cDNA background (Fig. 4B). We coexpressed 6-Myc-tagged wild-type and mutant p84 and HA-UL44 in 293T cells and analyzed their interactions by CoIP assays. We found that both wild-type p84 and the ΔC620 mutant efficiently interacted with UL44, whereas the ΔC560, ΔC500, ΔC440, ΔC380, and ΔC347 mutants did not do so (Fig. 4C). The ΔN346 mutant, which contains only the carboxy-terminal region unique to p84, interacted with UL44 very weakly (Fig. 4C), consistent with the observation that p84(ΔN252) only weakly interacted with UL44 in vitro (Fig. 3). When the localization patterns of mutant proteins were examined by IFA, all carboxy-terminal truncation mutants tested were distributed in nuclear punctuate patterns similar to that of intact p84, whereas the ΔN346 mutant, which lacks the reported nuclear localization signal (NLS) (19), was diffusely distributed in both the nucleus and cytoplasm (Fig. 4D). These data suggest that p84 requires both the amino-terminal 252-amino-acid region shared with other UL112-113 proteins and the specific near-carboxy-terminal region for efficient UL44 binding in cotransfected cells.
Domains of UL44 required for UL112-113 p84 binding.
To identify the regions of UL44 required for UL112-113 p84 binding, a series of UL44 truncation mutants was generated (Fig. 5 C). 293T cells were cotransfected with plasmids expressing 6-Myc-UL44 (wild type and mutant) and HA-p84, and CoIP assays were performed. The results showed that wild-type UL44 and the ΔN40 mutant interacted with p84, whereas all of the C-terminal truncation mutants, such as ΔC390, ΔC290, and ΔC240, did not bind to p84 at similar levels (Fig. 5A), indicating that the carboxy-terminal region of UL44 is critical for p84 binding. We also found that amino-terminal truncation mutants such as ΔN80, ΔN120, and ΔN290 did not bind to p84 (Fig. 5A). Therefore, all mutants except ΔN40 failed to bind to p84 (Fig. 5C).
FIG. 5.
CoIP assays to map the region of UL44 required for dimerization and UL112-113 p84 binding. (A) Interaction of p84 with UL44 (wild type or mutants). 293T cells were cotransfected with the indicated plasmids, and total cell lysates were prepared and immunoprecipitated with anti-Myc Ab. (Top) The precipitated proteins were subjected to SDS-PAGE and immunoblotting with anti-HA Ab. (Middle and bottom) The expression levels of the HA-p84 and Myc-UL44 proteins are shown by immunoblotting with anti-HA Ab (middle) and anti-Myc Ab (bottom). Note that the 6-Myc-UL44(ΔN290) level in total cell lysates is very low compared to those of other proteins. (B) Dimerization of UL44 proteins. 293T cells were cotransfected with the plasmids encoding HA-UL44 or Myc-UL44 (wild type or mutants), as indicated, and CoIP assays were performed as described above (A). (C) Diagram showing the structure of the wild-type and mutant UL44 proteins used and summary of the results in A and B. The abilities of the wild type and UL44 truncation mutants to dimerize with each other and interact with p84 are indicated as + (positive) or − (negative). The positions of the reported two potential NLSs (6) are indicated by open circles. (D) Localization patterns of wild-type and mutant UL44 are shown by IFA as described in the legend of Fig. 4D.
UL44 was shown previously to form a head-to-head homodimer in the shape of a C clamp (7). In similar CoIP assays with plasmids expressing 6-Myc-UL44 (wild type and mutant) and HA-UL44, we found that 6-Myc-UL44 and its mutant versions, such as the ΔC390, ΔC290, and ΔN40 mutants, interacted with HA-UL44, whereas the ΔC240, ΔN80, ΔN120, and ΔN290 mutants did not (Fig. 5B). Therefore, the results of UL44 mapping experiments revealed that the self-interaction of UL44 requires the central region from amino acids 40 to 290, whereas UL44 requires both the dimerization domain and the carboxy-terminal region for p84 binding (Fig. 5C). Two potential NLSs of UL44 have been identified at amino acids 162 to 168 (NLS1) and 425 to 431 (NLS2), and NLS2 was necessary for its nuclear localization in COS7 and U373-MG cells (6). We also found that NLS2 is necessary for the efficient nuclear targeting of the green fluorescent protein (GFP)-UL44 fusion protein in HF cells; however, it was not absolutely required for the nuclear localization of Flag- or Myc-tagged UL44 in HF cells, suggesting that NLS1 may also be effective in HF cells (data not shown). When we looked at the localization patterns of Myc-tagged wild-type and mutant UL44 used for mapping studies of 293T or HF cells, like wild-type UL44, the ΔC390, ΔC290, ΔN40, and ΔN80 mutants were all localized to the nucleus, suggesting that the failure of p84 binding or self-interaction in UL44 mutants in this experiment is not due to the lack of nuclear distribution (Fig. 5D).
Colocalization interaction between the UL112-113 proteins and UL44.
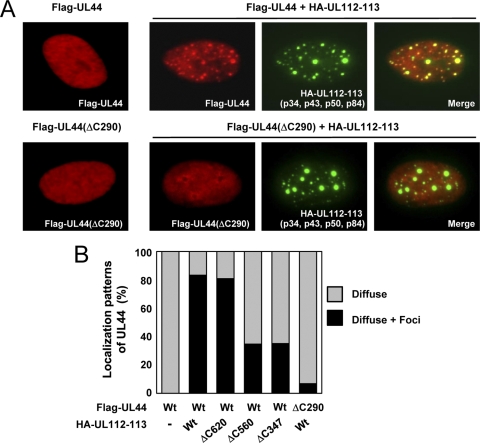
We previously demonstrated that the expression of the UL112-113 proteins is necessary for the efficient recruitment of UL44 to viral prereplication sites (27). We examined whether the colocalization interaction between the UL112-113 proteins and UL44 requires the specific interaction of p84 with UL44. HF cells were cotransfected with a plasmid expressing UL44 and a plasmid containing the UL112-113 gene, which expressed p34, p43, p50, and p84. The IFA results showed that the localization pattern of UL44 was changed from the nuclear diffuse pattern into a pattern showing both nuclear diffuse and focus forms, with the latter being colocalized with the UL112-113 proteins (Fig. 6 A, top). We used the mutant versions of UL112-113 plasmids containing the ΔC620, ΔC560, or ΔC347 mutations, which express intact p34, p43, and p50 but a carboxy-terminally truncated form of p84 (see Fig. 9A for immunoblots). The results showed that, unlike the UL112-113(ΔC620) gene, the UL112-113(ΔC560) and UL112-113(ΔC347) genes less efficiently caused the relocalization of UL44 into foci (Fig. 6B). In similar assays, UL44(ΔC290) was distributed mostly as a nuclear diffuse form and was not redistributed by the UL112-113 proteins (Fig. 6A, bottom, and B). These results suggest that the specific interaction between UL112-113 p84 and UL44 may be responsible for the redistribution of UL44 into the UL112-113 foci in cotransfected cells.
FIG. 6.
Colocalization interactions between the UL112-113 and UL44 proteins. (A) HF cells were transfected with a plasmid encoding Flag-UL44 or Flag-UL44(ΔC290) alone or cotransfected with a plasmid carrying the UL112-113 genomic gene, which encodes the p34, p43, p50, and p84 proteins (as HA-tagged forms). After 48 h, the cells were fixed with paraformaldehyde, followed by double-label IFA with anti-Flag (red) and anti-HA (green) Abs. For cotransfected cells, two side-by-side panels of single-labeled IFA images and a third panel of merged images are shown. (B) Quantitation of the relocalization of UL44 by the UL112-113 proteins. HF cells were cotransfected with plasmids encoding wild-type or mutant forms of Flag-UL44 and HA-UL112-113 proteins as indicated. Double-label IFA was performed as described above (A). The percentages of cotransfected cells exhibiting only the nuclear diffuse pattern of UL44 are indicated as gray bars, whereas those displaying both the nuclear diffuse and nuclear focus patterns of UL44, which represents the relocalization of UL44 to the PML-NB-associated sites by the UL112-113 proteins, are indicated as black bars. For each cotransfection, more than 100 cotransfected cells were counted.
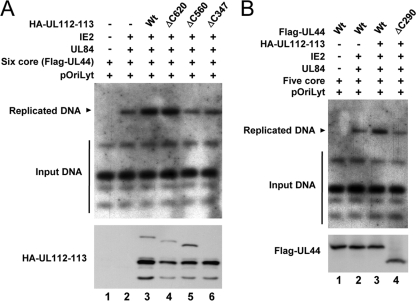
FIG. 9.
Requirements of the regions of UL112-113 p84 and UL44 responsible for their interaction for oriLyt-dependent DNA replication. (A) HF cells were cotransfected with plasmids encoding the HCMV replication origin (pSP38), six replication core proteins (UL54, UL44, UL57, UL105, UL70, and UL102), UL84, IE2, or UL112-113 (wild type or mutant), as indicated. At 5 days, total cellular DNAs were isolated and digested with XbaI and DpnI. DNA fragments were separated by electrophoresis, and Southern blot analysis was performed with the 32P-labeled KpnI-digested pSP38 DNA as a probe. (B) HF cells were cotransfected with plasmids encoding the HCMV replication origin (pSP38), five replication core proteins (UL54, UL57, UL105, UL70, and UL102), UL84, IE2, UL112-113, and UL44 (wild type or mutant), as indicated. The replicated oriLyt-containing plasmid DNAs were detected as described above (A).
The interaction of UL112-113 p84 with UL44 promotes viral growth in cells transfected with HCMV BAC clones.
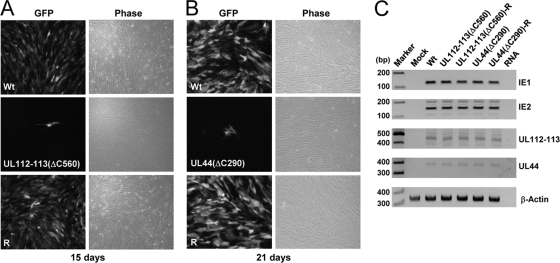
To determine whether the interaction of UL112-113 p84 with UL44 promotes viral growth, we generated two mutant T-BAC clones carrying the UL112-113(ΔC560) gene or the UL44(ΔC290) gene (Fig. 7). The UL112-113(ΔC560) gene expresses intact p34, p43, and p50 and p84(ΔC560), which is defective in UL44 binding. The UL44(ΔC290) gene expresses a carboxy-terminally truncated form of UL44(1-290), which retains DNA-binding and polymerase-binding activities and processivity activity but lacks the carboxy-terminal region required for p84 binding. We also produced their revertant T-BAC clones. When wild-type, mutant, and revertant T-BAC clones were introduced into permissive HF cells, the mutant UL112-113(ΔC560) and UL44(ΔC290) BAC clones did not produce progeny virions under conditions where the wild-type and revertant BAC clones efficiently produced viruses (Fig. 8 A and B). In control experiments, we examined the amounts of viral immediate-early (IE) (IE1 and IE2) and early (UL112-113 and UL44) transcripts produced at 9 days in cells electroporated with wild-type, mutant, and revertant BAC DNAs. The results of RT-PCR assays showed that the levels of these viral mRNAs were comparable, suggesting that the expression of viral IE and early genes is not affected by the UL112-113 or UL44 mutations introduced (Fig. 8C). These data provide genetic evidence demonstrating that the regions required for the interaction between UL112-113 p84 and UL44 are necessary for efficient viral growth.
FIG. 7.
Generation of recombinant T-BAC UL112-113(ΔC560) and UL44(ΔC290) clones. (A) Construction of the mutant T-BAC UL112-113(ΔC560) clone. The C-terminal region of UL113 was replaced by a marker cassette (rpsL-neo) containing the 50-nucleotide homologous arm, which increases sensitivity to streptomycin, and the kanamycin resistance marker. Intermediate BAC clones were isolated based on resistance to kanamycin (see Materials and Methods). In a second round of homologous recombination, the rpsL-neo cassette was replaced by the mutated DNA fragment containing a stop codon at amino acid position 561 of UL112-113 p84. To construct the revertant T-BAC clone, the mutant gene was replaced with the rpsL-neo cassette, and the wild-type T-BAC clone was then rescued by homologous recombination with the wild-type DNA fragment. (B) Construction of the mutant T-BAC UL44(ΔC290) clone. Insertion of a marker cassette and replacement by the mutant or wild-type DNA fragment were performed as described above (A). (C) Restriction fragment DNA patterns obtained following EcoRI/BamHI digestion of the wild-type, mutant, and revertant T-BAC DNAs were analyzed by agarose gel electrophoresis. The sizes of λ-HindIII are shown.
FIG. 8.
Infectivity of the mutant T-BAC clones carrying the UL112-113(ΔC560) or UL44(ΔC290) gene. (A) HF cells were transfected with wild-type, UL112-113(ΔC560), or revertant T-BAC clones via electroporation (see Materials and Methods) and monitored for the propagation of green fluorescent protein (GFP) signals. The GFP and phase-contrast images were taken 15 days after electroporation. (B) HF cells were transfected with wild-type, UL44(ΔC290), or revertant T-BAC clones via electroporation, and the images were taken 21 days later. (C) Total RNAs were prepared in cells at 9 days after electroporation, and the amounts of IE1, IE2, UL112-113, and UL44 mRNAs were measured by RT-PCR.
The interaction between UL112-113 p84 and UL44 promotes oriLyt-dependent DNA replication.
To investigate whether the interaction of UL112-113 p84 with UL44 is required for efficient DNA replication of an oriLyt-containing plasmid (pSP38), HF cells were cotransfected with an HCMV oriLyt-containing plasmid and plasmids encoding the six replication core proteins plus UL84, IE2, and UL112-113 (wild type and mutant). The newly synthesized oriLyt-containing plasmid DNAs, which were resistant to DpnI digestion, were detected by Southern blot analysis using the KpnI-digested oriLyt-containing plasmid DNA as a probe. Consistent with data from a previous report (32), the coexpression of six replication core proteins, UL84, and IE2 produced the replicated oriLyt-containing plasmid DNAs, and the addition of the UL112-113 plasmid expressing p34, p43, p50, and p84 significantly enhanced replication levels (Fig. 9 A, lanes 1 to 3). When plasmids carrying the UL112-113(ΔC620), UL112-113(ΔC560), or UL112-113(ΔC347) genes were used, the UL112-113(ΔC620) plasmid enhanced replication levels, like the wild-type UL112-113 plasmid, but both the UL112-113(ΔC560) and UL112-113(ΔC347) plasmids, which expressed p84(ΔC560) and p84(ΔC347), defective in UL44 binding, did not enhance replication levels (Fig. 9A, lanes 4 to 6). We also found that the replication-promoting effect of the UL112-113 proteins was not observed if a plasmid expressing UL44(ΔC290), which is defective in p84 binding, was used (Fig. 9B). The results of these cotransfection replication assays demonstrate that the specific interaction of UL112-113 p84 with UL44 may be required for efficient oriLyt-dependent DNA replication.
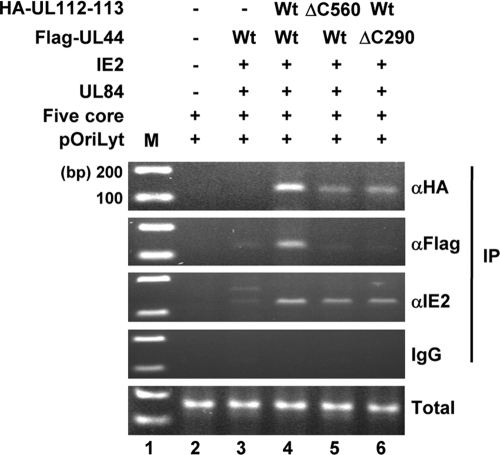
We finally performed ChIP assays with similar cotransfected cells to address whether the interaction between UL112-113 p84 and UL44 affects the association of UL112-113 and UL44 on the viral oriLyt region. The PCR primers were selected to amplify about 150 bp within the essential region of oriLyt (23). The results showed that the UL112-113 proteins are also associated with the oriLyt region in cells expressing the six replication core proteins plus UL84 and IE2 (Fig. 10, lane 4). We also found that the levels of UL44 and, to a lesser extent, UL112-113, which are associated with the oriLyt region, are markedly reduced if either the UL112-113(ΔC560) or the UL44(ΔC290) plasmid is used (Fig. 10, lanes 5 and 6). However, in a control experiment, the level of IE2 associated with the oriLyt region was not affected by the absence of the p84-UL44 interaction (Fig. 10). These results suggest that the interaction between UL112-113 p84 and UL44 specifically promotes the loading of UL44 onto the viral oriLyt region.
FIG. 10.
Requirements of the interaction between UL112-113 p84 and UL44 for their efficient association with the oriLyt region. HF cells were cotransfected with plasmids containing the HCMV replication origin (pSP38), five replication core proteins (UL54, UL57, UL105, UL70, and UL102), UL84, IE2, Flag-UL44 (wild type or mutant), and HA-UL112-113 (wild type or mutant), as indicated. At 5 days, the cells were harvested and analyzed by ChIP assays using anti-HA, anti-Flag, and anti-IE2 Abs or using a control IgG. The immunoprecipitated oriLyt-containing DNAs were amplified by PCR. The total amounts of input plasmid DNAs were also amplified by PCR.
DISCUSSION
In this study, we demonstrated that UL112-113 p84 forms a complex with UL44, UL84, and IE2 in virus-infected cells. We could not determine whether other UL112-113 proteins such as p34, p43, and p50 are also present in this complex because of the lack of specific Abs that recognize each of these proteins. Although the M23 Ab that we used did immunoprecipitate all of the UL112-113 proteins, it did not detect the UL112-113 proteins well in immunoblot analyses (data not shown; K. Hirai, personal communication). However, the findings that the UL112-113 proteins self-interact and interact with each other (27) and that all of the UL112-113 proteins are capable of binding to UL44 in vitro (in this study) strongly suggest that all of the UL112-113 proteins may be present in this virus-induced protein complex. Using in vitro binding assays, we found that, like UL44, IE2 also physically interacts with the UL112-113 proteins, whereas no direct interaction was observed between UL84 and the UL112-113 proteins (data not shown). Considering that UL84 was found to interact directly with both IE2 and UL44 (11, 34, 37), the interaction between UL84 and the UL112-113 proteins appears to be indirect.
Whether the different UL112-113 proteins produced via alternative splicing have a specific role has long remained unclear. We previously proposed that the expression of p84 may influence the intranuclear targeting of p34, p40, and p50 to PML-NB-associated sites (27). In this study, we provide evidence that the specific interaction of p84 with UL44 plays a key role in promoting viral DNA replication. Although the p34, p43, p50, and p84 UL112-113 proteins all physically interacted with UL44, suggesting a role for the shared amino-terminal region of UL112-113 in UL44 binding, our mapping experiments revealed that p84 required both the amino-terminal region and the specific near-carboxy-terminal region for UL44 binding. It is evident that the p84-specific carboxy-terminal region is necessary for efficient viral growth, since when the carboxy-terminally truncated forms of p84 were expressed together with intact p34, p40, and p50, the ability of the UL112-113 proteins to promote both oriLyt-dependent DNA replication in cotransfected cells and viral growth in HCMV BAC DNA-transfected cells was greatly impaired. Although other activities of the p84-specific carboxy-terminal region, in addition to contributing to UL44 binding, cannot be excluded, it is most likely that the specific interaction of UL112-113 p84 and UL44 plays a critical role in viral growth.
A possible role for the UL112-113 proteins in orchestrating the formation of viral prereplication sites was proposed previously (4, 27, 28). Our results are very supportive of this role for the UL112-113 proteins. First, the UL112-113 proteins formed a complex with UL44, UL84, and IE2 in virus-infected cells. Second, in cotransfected cells, the relocalization of UL44 to the UL112-113 foci relied on the interaction between UL112-113 p84 and UL44. Third, like UL84 and IE2, the UL112-113 proteins were also detected on the HCMV oriLyt region, and in ChIP assays, the specific interaction between UL112-113 p84 and UL44 was necessary to enhance levels of oriLyt-associated UL44 and UL112-113 proteins. The complex containing UL112-113, UL44, UL84, and IE2 was formed in the absence of nucleic acids. Therefore, it is likely that the UL112-113 proteins may act as a scaffold to stabilize the initiator complex at the replication origin.
We found that UL112-113 p84 recognizes both the dimerization domain and the carboxy-terminal region of UL44. A structural analysis using the amino-terminal 290 amino acids of UL44, which corresponds to UL44(ΔC290) in this study, showed that UL44 has a structural fold remarkably similar to those of other processivity factors, including herpesvirus simplex virus type 1 (HSV-1) UL42, a PCNA monomer (45), and the β-subunit of DNA polymerase (Pol) III (22), despite no apparent sequence homology. Further study using the same UL44 protein showed that UL44 forms a head-to-head C-clamp-shaped homodimer and may act as a hybrid processivity factor, since it binds to DNA directly like UL42 but forms a C clamp like PCNA, which is a head-to-tail toroidal homotrimer (7). Therefore, it is conceivable that UL112-113 p84 interacts with UL44 and forms a ring-shaped structure, which surrounds DNA like PCNA. Whether this interaction contributes to the processivity activity of UL44 needs to be addressed.
UL44(ΔC290) has been shown in vitro to retain all known biochemical activities of wild-type UL44, i.e., DNA- and DNA polymerase (UL54)-binding activities, and activity as a processivity factor. Interestingly, our results demonstrate that the carboxy-terminal region (amino acids 290 to 330) of UL44 is also necessary for both efficient DNA replication in cotransfected cells and viral growth in cells transfected with HCMV BAC DNAs. Since this carboxy-terminal region of UL44 was required for UL112-113 p84 binding, the defects of the UL44(ΔC290) gene may be at least partly due to its failure to interact with UL112-113 p84. However, it is also possible that other activities of UL44 that are critical for viral growth are also mediated through its carboxy-terminal region. It was reported previously that UL44 is phosphorylated by viral kinase UL97 (16, 21) and protein kinase CK2 (6). In particular, CK2 was shown to phosphorylate UL44 at a Ser413 residue upstream of NLS2 and then enhance the nuclear transport of UL44 in COS7 cells (6). Since UL44(ΔC290), which lacks NLS2, was efficiently localized in the nucleus in HF cells, it is unlikely that the inefficient oriLyt-dependent DNA replication and reduced viral growth in cells expressing UL44(ΔC290) are due to its failure to accumulate in the nucleus. However, it is possible that the phosphorylation event at the carboxy-terminal region of intact UL44 may have an important regulatory function in viral DNA replication. In this regard, it is notable that a viral uracil DNA glycosylase (UNG) encoded by UL114 interacts with the C-terminal region of UL44 and accumulates in viral replication foci, accelerating the accumulation of viral DNA (29, 30). A recent report also demonstrated that nucleolin interacts with UL44 and is necessary for efficient viral replication (36), although whether the carboxy-terminal region is involved in this interaction is not known. Further studies of the role of the carboxy-terminal region of UL44, using both genetic and biochemical approaches, are warranted.
Acknowledgments
We thank Gary S. Hayward (Johns Hopkins University School of Medicine, Baltimore, MD), Debora H. Spector (University of California, San Diego, CA), and Kanji Hirai (Tokyo Medical and Dental University, Tokyo, Japan) for plasmids and antibodies.
This work was supported by the Basic Science Research Program and the Mid-Career Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (KRF-2006-311-C00463 and 2009-0078805).
Footnotes
Published ahead of print on 10 June 2010.
REFERENCES
- 1.Ahn, J. H., E. J. Brignole III, and G. S. Hayward. 1998. Disruption of PML subnuclear domains by the acidic IE1 protein of human cytomegalovirus is mediated through interaction with PML and may modulate a RING finger-dependent cryptic transactivator function of PML. Mol. Cell. Biol. 18:4899-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, J. H., and G. S. Hayward. 2000. Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection. Virology 274:39-55. [DOI] [PubMed] [Google Scholar]
- 3.Ahn, J. H., and G. S. Hayward. 1997. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J. Virol. 71:4599-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn, J. H., W. J. Jang, and G. S. Hayward. 1999. The human cytomegalovirus IE2 and UL112-113 proteins accumulate in viral DNA replication compartments that initiate from the periphery of promyelocytic leukemia protein-associated nuclear bodies (PODs or ND10). J. Virol. 73:10458-10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn, J. H., Y. Xu, W. J. Jang, M. J. Matunis, and G. S. Hayward. 2001. Evaluation of interactions of human cytomegalovirus immediate-early IE2 regulatory protein with small ubiquitin-like modifiers and their conjugation enzyme Ubc9. J. Virol. 75:3859-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvisi, G., D. A. Jans, J. Guo, L. A. Pinna, and A. Ripalti. 2005. A protein kinase CK2 site flanking the nuclear targeting signal enhances nuclear transport of human cytomegalovirus ppUL44. Traffic 6:1002-1013. [DOI] [PubMed] [Google Scholar]
- 7.Appleton, B. A., A. Loregian, D. J. Filman, D. M. Coen, and J. M. Hogle. 2004. The cytomegalovirus DNA polymerase subunit UL44 forms a C clamp-shaped dimer. Mol. Cell 15:233-244. [DOI] [PubMed] [Google Scholar]
- 8.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. Hutchison III, T. Kouzarides, J. A. Martignetti, et al. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125-169. [DOI] [PubMed] [Google Scholar]
- 9.Davison, A. J., A. Dolan, P. Akter, C. Addison, D. J. Dargan, D. J. Alcendor, D. J. McGeoch, and G. S. Hayward. 2003. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J. Gen. Virol. 84:17-28. [DOI] [PubMed] [Google Scholar]
- 10.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. U. S. A. 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao, Y., K. Colletti, and G. S. Pari. 2008. Identification of human cytomegalovirus UL84 virus- and cell-encoded binding partners by using proteomics analysis. J. Virol. 82:96-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green, S., I. Issemann, and E. Sheer. 1988. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 16:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iskenderian, A. C., L. Huang, A. Reilly, R. M. Stenberg, and D. G. Anders. 1996. Four of eleven loci required for transient complementation of human cytomegalovirus DNA replication cooperate to activate expression of replication genes. J. Virol. 70:383-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwayama, S., T. Yamamoto, T. Furuya, R. Kobayashi, K. Ikuta, and K. Hirai. 1994. Intracellular localization and DNA-binding activity of a class of viral early phosphoproteins in human fibroblasts infected with human cytomegalovirus (Towne strain). J. Gen. Virol. 75(Pt. 12):3309-3318. [DOI] [PubMed] [Google Scholar]
- 15.Kerry, J. A., M. A. Priddy, T. Y. Jervey, C. P. Kohler, T. L. Staley, C. D. Vanson, T. R. Jones, A. C. Iskenderian, D. G. Anders, and R. M. Stenberg. 1996. Multiple regulatory events influence human cytomegalovirus DNA polymerase (UL54) expression during viral infection. J. Virol. 70:373-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krosky, P. M., M. C. Baek, W. J. Jahng, I. Barrera, R. J. Harvey, K. K. Biron, D. M. Coen, and P. B. Sethna. 2003. The human cytomegalovirus UL44 protein is a substrate for the UL97 protein kinase. J. Virol. 77:7720-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, H. R., D. J. Kim, J. M. Lee, C. Y. Choi, B. Y. Ahn, G. S. Hayward, and J. H. Ahn. 2004. Ability of the human cytomegalovirus IE1 protein to modulate sumoylation of PML correlates with its functional activities in transcriptional regulation and infectivity in cultured fibroblast cells. J. Virol. 78:6527-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, J. M., H. J. Kang, H. R. Lee, C. Y. Choi, W. J. Jang, and J. H. Ahn. 2003. PIAS1 enhances SUMO-1 modification and the transactivation activity of the major immediate-early IE2 protein of human cytomegalovirus. FEBS Lett. 555:322-328. [DOI] [PubMed] [Google Scholar]
- 19.Li, J., T. Yamamoto, K. Ohtsubo, M. Shirakata, and K. Hirai. 1999. Major product pp43 of human cytomegalovirus U(L)112-113 gene is a transcriptional coactivator with two functionally distinct domains. Virology 260:89-97. [DOI] [PubMed] [Google Scholar]
- 20.Marchini, A., H. Liu, and H. Zhu. 2001. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 75:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marschall, M., M. Freitag, P. Suchy, D. Romaker, R. Kupfer, M. Hanke, and T. Stamminger. 2003. The protein kinase pUL97 of human cytomegalovirus interacts with and phosphorylates the DNA polymerase processivity factor pUL44. Virology 311:60-71. [DOI] [PubMed] [Google Scholar]
- 22.Novotny, J., I. Rigoutsos, D. Coleman, and T. Shenk. 2001. In silico structural and functional analysis of the human cytomegalovirus (HHV5) genome. J. Mol. Biol. 310:1151-1166. [DOI] [PubMed] [Google Scholar]
- 23.Pari, G. S. 2008. Nuts and bolts of human cytomegalovirus lytic DNA replication. Curr. Top. Microbiol. Immunol. 325:153-166. [DOI] [PubMed] [Google Scholar]
- 24.Pari, G. S., and D. G. Anders. 1993. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J. Virol. 67:6979-6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pari, G. S., M. A. Kacica, and D. G. Anders. 1993. Open reading frames UL44, IRS1/TRS1, and UL36-38 are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA synthesis. J. Virol. 67:2575-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park, J. J., Y. E. Kim, H. T. Pham, E. T. Kim, Y. H. Chung, and J. H. Ahn. 2007. Functional interaction of the human cytomegalovirus IE2 protein with histone deacetylase 2 in infected human fibroblasts. J. Gen. Virol. 88:3214-3223. [DOI] [PubMed] [Google Scholar]
- 27.Park, M. Y., Y. E. Kim, M. R. Seo, J. R. Lee, C. H. Lee, and J. H. Ahn. 2006. Interactions among four proteins encoded by the human cytomegalovirus UL112-113 region regulate their intranuclear targeting and the recruitment of UL44 to prereplication foci. J. Virol. 80:2718-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penfold, M. E., and E. S. Mocarski. 1997. Formation of cytomegalovirus DNA replication compartments defined by localization of viral proteins and DNA synthesis. Virology 239:46-61. [DOI] [PubMed] [Google Scholar]
- 29.Prichard, M. N., H. Lawlor, G. M. Duke, C. Mo, Z. Wang, M. Dixon, G. Kemble, and E. R. Kern. 2005. Human cytomegalovirus uracil DNA glycosylase associates with ppUL44 and accelerates the accumulation of viral DNA. Virol. J. 2:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ranneberg-Nilsen, T., H. A. Dale, L. Luna, R. Slettebakk, O. Sundheim, H. Rollag, and M. Bjoras. 2008. Characterization of human cytomegalovirus uracil DNA glycosylase (UL114) and its interaction with polymerase processivity factor (UL44). J. Mol. Biol. 381:276-288. [DOI] [PubMed] [Google Scholar]
- 31.Roth, M. B., A. M. Zahler, and J. A. Stolk. 1991. A conserved family of nuclear phosphoproteins localized to sites of polymerase II transcription. J. Cell Biol. 115:587-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarisky, R. T., and G. S. Hayward. 1996. Evidence that the UL84 gene product of human cytomegalovirus is essential for promoting oriLyt-dependent DNA replication and formation of replication compartments in cotransfection assays. J. Virol. 70:7398-7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, J. A., and G. S. Pari. 1995. Expression of human cytomegalovirus UL36 and UL37 genes is required for viral DNA replication. J. Virol. 69:1925-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spector, D. J., and M. J. Tevethia. 1994. Protein-protein interactions between human cytomegalovirus IE2-580aa and pUL84 in lytically infected cells. J. Virol. 68:7549-7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staprans, S. I., D. K. Rabert, and D. H. Spector. 1988. Identification of sequence requirements and trans-acting functions necessary for regulated expression of a human cytomegalovirus early gene. J. Virol. 62:3463-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strang, B. L., S. Boulant, and D. M. Coen. 2010. Nucleolin associates with the human cytomegalovirus DNA polymerase accessory subunit UL44 and is necessary for efficient viral replication. J. Virol. 84:1771-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strang, B. L., E. Sinigalia, L. A. Silva, D. M. Coen, and A. Loregian. 2009. Analysis of the association of the human cytomegalovirus DNA polymerase subunit UL44 with the viral DNA replication factor UL84. J. Virol. 83:7581-7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wells, R., L. Stensland, and J. Vieira. 2009. The human cytomegalovirus UL112-113 locus can activate the full Kaposi's sarcoma-associated herpesvirus lytic replication cycle. J. Virol. 83:4695-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright, D. A., and D. H. Spector. 1989. Posttranscriptional regulation of a class of human cytomegalovirus phosphoproteins encoded by an early transcription unit. J. Virol. 63:3117-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wright, D. A., S. I. Staprans, and D. H. Spector. 1988. Four phosphoproteins with common amino termini are encoded by human cytomegalovirus AD169. J. Virol. 62:331-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu, Y., S. A. Cei, A. R. Huete, and G. S. Pari. 2004. Human cytomegalovirus UL84 insertion mutant defective for viral DNA synthesis and growth. J. Virol. 78:10360-10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu, Y., S. A. Cei, A. Rodriguez Huete, K. S. Colletti, and G. S. Pari. 2004. Human cytomegalovirus DNA replication requires transcriptional activation via an IE2- and UL84-responsive bidirectional promoter element within oriLyt. J. Virol. 78:11664-11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto, T., S. Suzuki, K. Radsak, and K. Hirai. 1998. The UL112/113 gene products of human cytomegalovirus which colocalize with viral DNA in infected cell nuclei are related to efficient viral DNA replication. Virus Res. 56:107-114. [DOI] [PubMed] [Google Scholar]
- 44.Yu, D., M. C. Silva, and T. Shenk. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. U. S. A. 100:12396-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuccola, H. J., D. J. Filman, D. M. Coen, and J. M. Hogle. 2000. The crystal structure of an unusual processivity factor, herpes simplex virus UL42, bound to the C terminus of its cognate polymerase. Mol. Cell 5:267-278. [DOI] [PubMed] [Google Scholar]