Abstract
Myc is deregulated by Kaposi's sarcoma-associated herpesvirus (KSHV) latent proteins, but its role in KSHV latency is not clear. We found that Myc knockdown with RNA interference (RNAi) induced KSHV reactivation and increased the protein and mRNA levels of RTA, a key viral regulator of KSHV reactivation. Myc knockdown increased, whereas Myc overexpression inhibited, RTA promoter activity. KSHV reactivation and the activation of the RTA promoter induced by Myc depletion were inhibited by c-Jun N-terminal kinase (JNK) and p38 inhibitors but not by a MEK1 inhibitor. Myc knockdown inhibited primary effusion lymphoma (PEL) cell proliferation through inducing apoptosis and G1 cell cycle arrest. Thus, Myc may be a key cellular node coupling cellular transformation and KSHV latency.
Kaposi's sarcoma-associated herpesvirus (KSHV) is the etiological agent of Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and a plasmablastic variant of multicentric Castleman's disease (5, 6, 10). KSHV has two phases in its life cycle, i.e., latency and lytic replication. Latent virus can be reactivated into lytic replication. PEL cells are infected predominantly with latent KSHV (5), expressing only a few genes (8, 20). Latency is a barrier to the elimination of KSHV and to the treatment of KSHV-associated tumors because (i) latent genes, such as those for LANA, v-cyclin, vFLIP, and vIRF-3, regulate oncogenic pathways and promote tumorigenesis (4, 9, 13, 16, 17, 19) and (ii) latency helps virus evade detection and elimination by the host immune system (2).
Induction of KSHV reactivation presents an opportunity to target KSHV-associated tumor cells for destruction (1, 15, 23). KSHV RTA is the key viral regulator of KSHV reactivation (18, 22). However, which cellular genes are essential for maintaining KSHV latency is not very clear (3).
The proto-oncogene myc encodes the c-Myc (herein termed Myc) transcription factor, which regulates cell growth, proliferation, differentiation, and apoptosis (11). Myc is deregulated in ∼30% of human cancers, including B- and T-cell lymphomas (7, 12). Two KSHV latent proteins, LANA and vIRF-3/LANA2, deregulate Myc through enhancing the stability of Myc protein and enhancing its ability to transactivate the expression of its target genes, respectively (4, 16, 17), suggesting an important role of Myc in KSHV biology. We undertook the current study to address the role of Myc in the maintenance of KSHV latency.
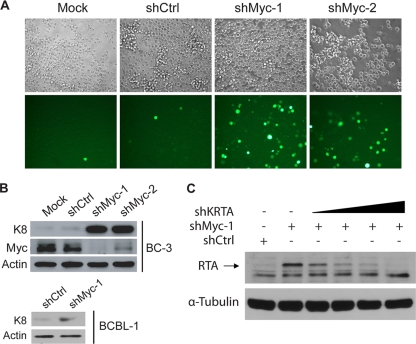
To examine whether Myc is required for the maintenance of KSHV latency, we used a reporter cell line, BC-3-G, which was derived from the PEL cell line BC-3, to monitor KSHV reactivation (25). In BC-3-G cells, the expression of enhanced green fluorescence protein (EGFP) is driven by the minimal promoter of a KSHV lytic gene, polyadenylated nuclear RNA (PAN) (21), which responds specifically to RTA and is expressed in high abundance, enabling the use of EGFP expression as a specific and sensitive indicator of the level of KSHV reactivation. To deplete Myc, we transduced BC-3-G cells with short hairpin RNA (shRNA) lentiviral vectors in the presence of 4 μg/ml of Polybrene to enhance transduction efficiency. At day 3 posttransduction, two shRNA lentiviral vectors against Myc, shMyc-1(targeting sequence, GAT GAG GAA GAA ATC GAT G) and shMyc-2 (targeting sequence, GGT CAG AGT CTG GAT CAA C), greatly increased the percentage of EGFP-positive cells, indicative of enhanced KSHV reactivation (Fig. 1 A). A control shRNA vector, shCtrl (targeting sequence, CAA CAA GAT GAA GAG CAC CAA), which does not target any human gene, did not induce reactivation compared with mock-transduced cells (Fig. 1A). shMyc-1 and shMyc-2 were able to significantly decrease Myc protein levels in BC-3 at day 3 posttransduction in a Western blotting assay (Fig. 1B). shMyc-1 was more effective than shMyc-2 in knocking down Myc (Fig. 1B). The low percentage of EGFP-positive cells in mock- or shCtrl-transduced cells represents spontaneous reactivation. shMyc-1 or shMyc-2 also significantly increased the protein level of the viral lytic gene K8 in BC-3 cells (Fig. 1B), further confirming that Myc depletion induces KSHV reactivation. Transduction of BCBL-1 cells with the more effective shMyc-1 also increased the K8 protein level (Fig. 1B), indicating that the requirement of Myc for the maintenance of KSHV latency is not unique to BC-3 cells.
FIG. 1.
Myc knockdown induces KSHV reactivation. (A) BC-3-G cells that were mock transduced or transduced with the indicated shRNA vectors were imaged with fluorescence microscopy for enhanced green fluorescence protein (EGFP) expression at day 3 posttransduction. Magnification, ×200. (B) BC-3 or BCBL-1 cells were mock transduced or transduced with the indicated shRNA vectors. At day 3 posttransduction, whole-cell lysates were collected and analyzed for KSHV K8 and/or Myc expression by Western blotting. An actin immunoblot is shown as a loading control. (C) BC-3 cells were cotransfected with the indicated shRNA plasmids. At day 3 posttransduction, whole-cell lysates were collected and analyzed for KSHV RTA expression by Western blotting. A tubulin immunoblot is shown as a loading control. Data for all panels are representative of three independent experiments.
RTA is both required and sufficient for the induction of KSHV reactivation (18, 22). We thus examined whether depletion of Myc by shMyc-1 increases the protein level of RTA. We cotransfected BC-3 cells with shCtrl, shMyc-1, or shMyc-1 together with increasing amounts of shKRTA, an shRNA plasmid targeting the KSHV RTA gene (Fig. 1C). At day 3 posttransduction, shMyc-1 significantly increased the protein level of RTA in Western blotting (Fig. 1C). Although the RTA antibody detected multiple protein bands, we were able to confirm the identity of the RTA band indicated by an arrow because the intensity of the band was decreased by increasing amounts of the transfected shKRTA plasmid (Fig. 1C).
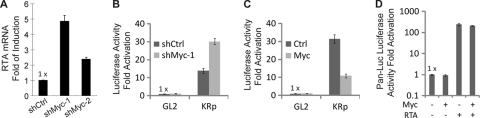
We then asked whether Myc depletion increases KSHV RTA expression at the transcription level. We transduced BC-3 cells with shMyc-1, shMyc-2, or shCtrl and measured the mRNA levels of RTA at day 2 posttransduction. shMyc-1 or shMyc-2 upregulated RTA mRNA levels (Fig. 2 A). shMyc-1 upregulated RTA expression to a greater extent than shMyc-2 did (Fig. 2A), consistent with our results showing that shMyc-1 was more effective than shMyc-2 in depleting Myc and reactivating KSHV (Fig. 1A and B). We also examined the effects of Myc depletion or Myc overexpression on the promoter activity of RTA using luciferase reporter assays (Fig. 2B and C). Myc depletion also increased KSHV RTA promoter activity (Fig. 2B). Conversely, Myc overexpression inhibited RTA promoter activity (Fig. 2C). Myc overexpression was not able to inhibit the activation of the PAN promoter by RTA (Fig. 2D), suggesting that Myc does not affect the ability of RTA to transactivate its target genes such as PAN. We concluded that Myc suppresses KSHV reactivation through repressing KSHV RTA expression transcriptionally.
FIG. 2.
Myc represses RTA transcription. (A) BC-3 cells were transduced with the indicated shRNA vectors. At day 2 posttransduction, the relative RTA mRNA levels were measured by reverse transcription-quantitative PCR (RT-Q-PCR), normalized to the actin mRNA levels, and displayed as fold induction over the shCtrl control. (B) 293T cells were cotransfected with pKRp (RTA promoter luciferase reporter plasmid) or pGL2 (control reporter plasmid) plus the indicated shRNA plasmids. At 48 h posttransfection, the luciferase activities were measured and are presented as relative fold induction over the luciferase activity of the pGL2-plus-shCtrl sample. (C) 293T cells were cotransfected with the indicated luciferase reporter and expression plasmids. At 48 h posttransfection, the luciferase activities were determined and are presented as relative fold induction over the luciferase activity of the pGL2-plus-Ctrl sample. (D) 293T cells were cotransfected with pPAN-Luc (PAN promoter luciferase reporter plasmid) and the indicated expression plasmids. At 48 h posttransfection, the luciferase activities were determined and are presented as relative fold induction over the luciferase activity of the sample without Myc and RTA. Data are the means ± standard deviations from three independent experiments.
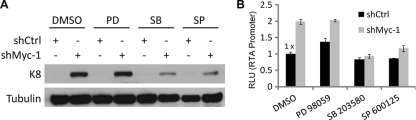
To gain insights into the mechanism underlying the role of Myc in the maintenance of KSHV latency, we explored cellular signaling pathways that may mediate KSHV reactivation downstream of Myc depletion. Previous studies showed that the mitogen-activated protein kinase (MAPK) signaling pathways play important roles in KSHV reactivation induced by phorbol esters or Ras (24, 25). We therefore examined whether MAPK signaling is required for KSHV reactivation induced by Myc depletion. SP 600125, a specific inhibitor of c-Jun N-terminal kinases (JNKs), and SB 203580, a specific inhibitor of p38 MAPK, significantly inhibited KSHV reactivation induced by Myc depletion, as indicated by K8 protein levels (Fig. 3 A). In contrast, the MEK1 inhibitor PD 98059 did not significantly affect KSHV reactivation induced by Myc depletion (Fig. 3A). Since Myc depletion upregulated RTA transcription (Fig. 2A and B), we asked whether JNK or p38 signaling is required for the upregulation of RTA transcription by Myc depletion. SP 600125 or SB 203580, but not PD 98059, inhibited the upregulation of RTA promoter activity by Myc depletion (Fig. 3B). Thus, JNK and p38 signaling is required for the upregulation of RTA transcription and KSHV reactivation downstream of Myc depletion.
FIG. 3.
Requirement of JNK and p38 signaling for KSHV reactivation induced by Myc knockdown. (A) BC-3 cells were transfected with shCtrl or shMyc-1. At day 2 posttransfection, cells were treated with 20 μM PD 98059, SB 203580, SP 600125, or the dimethyl sulfoxide (DMSO) vehicle control. At day 3 posttreatment, cells were collected and analyzed by Western blotting. A tubulin immunoblot is shown as a loading control. Data are representative of two independent experiments. (B) 293T cells were cotransfected with pKRp and the shCtrl or shMyc-1 plasmid. At 12 h posttransfection, cells were treated with 20 μM PD 98059, SB 203580, SP 600125, or the DMSO vehicle control. At 60 h posttransfection, the luciferase activities were measured and are presented as relative fold induction over the luciferase activity of the shCtrl-plus-DMSO sample. Data are the means ± standard deviations from three independent experiments.
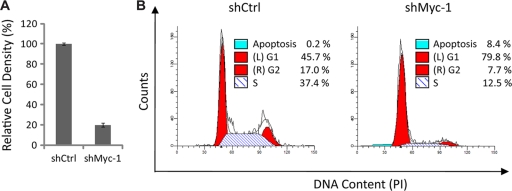
Lastly, we determined the effects of Myc knockdown on PEL cell proliferation and survival. We transduced BC-3 cells with the shCtrl or shMyc-1 vector and measured the number of live cells using the trypan blue exclusion assay at day 4 posttransduction (Fig. 4 A). shMyc-1 significantly decreased the number of cells (Fig. 4A), suggesting that Myc knockdown inhibits cell proliferation and/or cell survival. To determine the effects of Myc knockdown on PEL cell cycle progression, we analyzed the DNA content of BC-3 cells transduced with shCtrl or shMyc-1 by flow cytometry at day 3 posttransduction (Fig. 4B). The percentage of cells in the G1 phase (left red area), S phase (blue striped area), or G2 phase (right red area) or the apoptotic fraction (blue area) was determined with Modfit LT software (Fig. 4B). Myc knockdown increased the percentage of cells in the G1 phase and at the same time decreased the percentage of cells in the S or G2 phase (Fig. 4B), suggesting that Myc knockdown induces G1 cell cycle arrest. In addition, the percentage of apoptotic cells was also increased by Myc knockdown (Fig. 4B). Thus, Myc depletion inhibits both cell proliferation and cell survival, indicating a critical role of Myc in PEL cell transformation.
FIG. 4.
Myc knockdown induces G1 cell cycle arrest and apoptosis. (A) BC-3 cells were transduced with the shCtrl or shMyc-1 lentiviral vector. Cell numbers were determined by the trypan blue exclusion assay at day 4 posttransduction and are displayed as relative cell densities normalized to the number of shCtrl-transduced cells. Data are the means ± standard deviations from three independent experiments. (B) Flow cytometry evaluation of the cell cycle in shCtrl- and shMyc-1-transduced BC-3 cells at day 4 posttransduction. The percentage of cells in each phase of the cell cycle was determined by analysis performed with Modfit LT software. Data are representative of two independent experiments.
Of note, upregulation of RTA expression induced by Myc knockdown may not necessarily lead to enhanced virus production, since Myc knockdown might restrict steps of virus lytic replication downstream of RTA expression. Nonetheless, our data established an essential role of Myc in maintaining KSHV latency in PEL cells. Previous studies demonstrated that NF-κB, which is activated by the KSHV latent gene product vFlip (14), inhibits KSHV reactivation (3). Therefore, our results support an emerging theme that oncogenic pathways activated by KSHV latent gene products promote KSHV latency, reflecting on one hand the ability of KSHV to promote cellular homeostasis through manipulating key oncogenic pathways and on the other hand the ability to sense disruption of cellular homeostasis as signals for KSHV reactivation, both of which are essential for virus persistence in the host.
We also provide evidence that JNK and p38 signaling is required for KSHV reactivation upon Myc depletion. Given the well-established role of JNK and p38 signaling pathways in the stress response, our results suggest that the cellular stress response is a key component in the regulation of KSHV reactivation by Myc. Taken together, our results demonstrate a tight coupling between cellular transformation and the regulation of KSHV latency through Myc and point to JNK and p38 stress signaling as an important mechanistic link. Our data also provide a basis for exploring virus-activated oncogenic pathways to identify novel targets for inducing KSHV reactivation in clinical settings.
Acknowledgments
This work was supported by grants from the NIH (DE0190858 and CA091791).
X.L. and R.S. designed the study; X.L., S.C., and J.F. performed the research; H.D. constructed the pKRp plasmid; X.L. wrote the manuscript; and R.S. revised the manuscript.
Footnotes
Published ahead of print on 23 June 2010.
REFERENCES
- 1.Ambinder, R. F., K. D. Robertson, S. M. Moore, and J. Yang. 1996. Epstein-Barr virus as a therapeutic target in Hodgkin's disease and nasopharyngeal carcinoma. Semin. Cancer Biol. 7:217-226. [DOI] [PubMed] [Google Scholar]
- 2.Brander, C., T. Suscovich, Y. Lee, P. T. Nguyen, P. O'Connor, J. Seebach, N. G. Jones, M. van Gorder, B. D. Walker, and D. T. Scadden. 2000. Impaired CTL recognition of cells latently infected with Kaposi's sarcoma-associated herpes virus. J. Immunol. 165:2077-2083. [DOI] [PubMed] [Google Scholar]
- 3.Brown, H. J., M. J. Song, H. Deng, T. T. Wu, G. Cheng, and R. Sun. 2003. NF-kappaB inhibits gammaherpesvirus lytic replication. J. Virol. 77:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bubman, D., I. Guasparri, and E. Cesarman. 2007. Deregulation of c-Myc in primary effusion lymphoma by Kaposi's sarcoma herpesvirus latency-associated nuclear antigen. Oncogene 26:4979-4986. [DOI] [PubMed] [Google Scholar]
- 5.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 6.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 7.Dang, C. V., J. W. Kim, P. Gao, and J. Yustein. 2008. The interplay between MYC and HIF in cancer. Nat. Rev. Cancer 8:51-56. [DOI] [PubMed] [Google Scholar]
- 8.Dittmer, D., M. Lagunoff, R. Renne, K. Staskus, A. Haase, and D. Ganem. 1998. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J. Virol. 72:8309-8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Djerbi, M., V. Screpanti, A. I. Catrina, B. Bogen, P. Biberfeld, and A. Grandien. 1999. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J. Exp. Med. 190:1025-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupin, N., T. L. Diss, P. Kellam, M. Tulliez, M. Q. Du, D. Sicard, R. A. Weiss, P. G. Isaacson, and C. Boshoff. 2000. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 95:1406-1412. [PubMed] [Google Scholar]
- 11.Eisenman, R. N. 2001. Deconstructing myc. Genes Dev. 15:2023-2030. [DOI] [PubMed] [Google Scholar]
- 12.Finger, L. R., R. C. Harvey, R. C. Moore, L. C. Showe, and C. M. Croce. 1986. A common mechanism of chromosomal translocation in T- and B-cell neoplasia. Science 234:982-985. [DOI] [PubMed] [Google Scholar]
- 13.Friborg, J., Jr., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 14.Guasparri, I., S. A. Keller, and E. Cesarman. 2004. KSHV vFLIP is essential for the survival of infected lymphoma cells. J. Exp. Med. 199:993-1003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Gutierrez, M. I., J. G. Judde, I. T. Magrath, and K. G. Bhatia. 1996. Switching viral latency to viral lysis: a novel therapeutic approach for Epstein-Barr virus-associated neoplasia. Cancer Res. 56:969-972. [PubMed] [Google Scholar]
- 16.Liu, J., H. J. Martin, G. Liao, and S. D. Hayward. 2007. The Kaposi's sarcoma-associated herpesvirus LANA protein stabilizes and activates c-Myc. J. Virol. 81:10451-10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubyova, B., M. J. Kellum, J. A. Frisancho, and P. M. Pitha. 2007. Stimulation of c-Myc transcriptional activity by vIRF-3 of Kaposi sarcoma-associated herpesvirus. J. Biol. Chem. 282:31944-31953. [DOI] [PubMed] [Google Scholar]
- 18.Lukac, D. M., R. Renne, J. R. Kirshner, and D. Ganem. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252:304-312. [DOI] [PubMed] [Google Scholar]
- 19.Mann, D. J., E. S. Child, C. Swanton, H. Laman, and N. Jones. 1999. Modulation of p27(Kip1) levels by the cyclin encoded by Kaposi's sarcoma-associated herpesvirus. EMBO J. 18:654-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarid, R., O. Flore, R. A. Bohenzky, Y. Chang, and P. S. Moore. 1998. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1). J. Virol. 72:1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song, M. J., H. J. Brown, T. T. Wu, and R. Sun. 2001. Transcription activation of polyadenylated nuclear RNA by RTA in human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:3129-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. U. S. A. 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westphal, E. M., A. Mauser, J. Swenson, M. G. Davis, C. L. Talarico, and S. C. Kenney. 1999. Induction of lytic Epstein-Barr virus (EBV) infection in EBV-associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 59:1485-1491. [PubMed] [Google Scholar]
- 24.Xie, J., A. O. Ajibade, F. Ye, K. Kuhne, and S. J. Gao. 2008. Reactivation of Kaposi's sarcoma-associated herpesvirus from latency requires MEK/ERK, JNK and p38 multiple mitogen-activated protein kinase pathways. Virology 371:139-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu, F., J. N. Harada, H. J. Brown, H. Deng, M. J. Song, T. T. Wu, J. Kato-Stankiewicz, C. G. Nelson, J. Vieira, F. Tamanoi, S. K. Chanda, and R. Sun. 2007. Systematic identification of cellular signals reactivating Kaposi sarcoma-associated herpesvirus. PLoS Pathog. 3:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]