Abstract
Intramuscular administration of inactivated influenza virus vaccine is the main vaccine platform used for the prevention of seasonal influenza virus infection. In clinical trials, inactivated H5N1 vaccines have been shown to be safe and capable of eliciting immune correlates of protection. However, the H5N1 vaccines are poorly immunogenic compared to seasonal influenza virus vaccines. Needle-free vaccination would be more efficient and economical in a pandemic, and the development of an effective and safe mucosal adjuvant will be an important milestone. A stabilized chemical analog of double-stranded RNA, PIKA, was previously reported to be a potent mucosal adjuvant in a murine model. While PIKA stimulates dendritic cells in vitro, little was known about its receptor and adjuvanting mechanism in vivo. In this study, we demonstrated that the immunostimulatory effect of PIKA resulted in an increased number of mature antigen-presenting cells, with the induction of proinflammatory cytokines at the inoculation site. In addition, coadministration of PIKA with a poorly immunogenic H5N1 subunit vaccine led to antigen sparing and quantitative and qualitative improvements of the immune responses over those achieved with an unadjuvanted vaccine in mice. The adjuvanted vaccine provided protection against lethal challenge with homologous and heterologous H5N1 wild-type viruses. Mice lacking functional TLR3 showed diminished cytokine production with PIKA stimulation, diminished antibody responses, and reduced protective efficacy against wild-type virus challenge following vaccination. These data suggest that TLR3 is important for the optimal performance of PIKA as an adjuvant. With its good safety profile and antigen-sparing effect, PIKA could be an attractive adjuvant for use in future pandemics.
Influenza is an acute respiratory disease associated with significant morbidity and mortality worldwide. The newly emerged swine-origin H1N1 virus has caused the first influenza pandemic of this century (4). Since its appearance in April 2009, the virus has spread to every continent and caused significant morbidity and mortality (WHO website, http://gamapserver.who.int/h1n1/cases-deaths/h1n1_casesdeaths.html). The sporadic transmission of highly pathogenic avian influenza (HPAI) viruses (H5N1 influenza A viruses) from poultry to humans in Asia also raises concerns about a possible pandemic (2, 28).
Although vaccination is the most effective tool for the control of influenza (7, 33), the combined production capacity of global vaccine suppliers is not sufficient to meet the demand during a pandemic, so a vaccine shortage is expected. Any strategy that can maximize vaccine coverage will be valuable in a pandemic.
Inactivated seasonal influenza virus vaccines are administered mainly by the intramuscular (i.m.) route; however, it has been demonstrated that intranasal (i.n.) administration of inactivated influenza virus vaccines is more effective at inducing nasal IgA responses and protecting the respiratory epithelium (1, 47). Induction of immunity by the intranasal route often requires a high dose of vaccine or the inclusion of an adjuvant. Although a number of compounds have been identified as promising mucosal adjuvants, there is a need to continue to develop safe mucosal adjuvants, because some compounds, such as Escherichia coli heat-labile toxin and poly(I:C), are associated with significant side effects (27, 37).
We previously demonstrated the potency of a stabilized chemical analog of double-stranded RNA (dsRNA), PIKA, as an adjuvant for a seasonal influenza virus vaccine with a substantial antigen-sparing effect in mice (25). While we and others have shown that PIKA activates dendritic cells (DC) in culture (25, 38), there are no reports on this effect in vivo, and the protective efficacy of PIKA-adjuvanted vaccine against wild-type (wt) virus challenge has not been demonstrated. The current study was designed to evaluate changes in the number and phenotypic expression of local antigen-presenting cells (APC) and in cytokine expression at the inoculation site and to evaluate the adjuvanting potency of PIKA in a lethal-challenge model using a wt influenza virus with pandemic potential. The A/Vietnam/1203/2004 (H5N1) virus was chosen over the A/California/04/2009 (H1N1) virus as the challenge virus for two reasons. First, the H5N1 virus is more virulent than the 2009 H1N1 pandemic virus in mice (the 50% mouse lethal doses [MLD50] of the H5N1 and the H1N1 viruses are 100.4 and 105.8 50% tissue culture infective doses [TCID50], respectively [20, 41]), which allows a higher lethal-challenge dose to be used in the experiments. Second, the unadjuvanted split-virion H5N1 vaccine was poorly immunogenic in humans, requiring 12 times more antigen (two doses of 90 μg) than the typical seasonal influenza virus vaccine (15 μg) in order to generate immunity associated with protection against influenza in humans (42), while data from the H1N1 human vaccine trial show that the unadjuvanted H1N1 vaccine is able to elicit robust immune responses after a single dose (14, 51). Our results show that administration of PIKA with inactivated H5N1 vaccine elicited a rapid production of proinflammatory cytokines with infiltration of mature DC at the site of administration. This vaccine formulation allowed significant antigen sparing and provided protection against lethal challenge with the wt HPAI viruses A/Vietnam/1203/2004 and A/Indonesia/05/2005 (H5N1).
MATERIALS AND METHODS
Mice.
Six- to 8-week-old female BALB/c mice (Taconic Farms, Inc., Germantown, NY) were used in all mouse experiments. B6;129S1-Tlr3tm1Flv/J (stock no. 005217) and B6129SF2/J (stock no. 101045) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The animal study protocols were approved by the National Institutes of Health (NIH) Animal Care and Use Committee, and the experiments were conducted at the NIH.
Viruses.
The wt H5N1 viruses used in this study were kindly provided by Alexander Klimov, Influenza Division, Centers for Disease Control and Prevention, Atlanta, GA. Virus stocks were propagated in the allantoic cavities of 9-day-old embryonated specific-pathogen-free hen's eggs (Charles River Laboratories, Wilmington, MA) at 37°C. Clarified allantoic fluids were aliquoted and stored at −80°C.
Vaccines and administration of PIKA.
The monovalent influenza virus subvirion vaccine (NR-4143) was obtained from the Biodefense & Emerging Infections Research Resources Repository, NIAID, NIH (BEI Resources, Manassas, VA). PIKA was obtained from NewBiomed PIKA Pte Ltd. (Singapore). PIKA contains dsRNA that is longer than 100 bp. PIKA was used at a concentration of 100 μg per mouse. The endotoxin level of the lot of PIKA used in this study was determined by an independent accredited laboratory and was <1 endotoxin unit/mg of PIKA. The shelf life of PIKA is as long as 3 years in freeze-dried form (P. Brazier, unpublished data).
Immunization protocol.
For intranasal (i.n.) immunization, mice were lightly anesthetized with 4% isoflurane, followed by i.n. administration of immunogens in 50 μl. For immunization by the subcutaneous (s.c.) route, the immunogens were administered in two injections at the base of the tail, 50 μl at each side of the tail. For intramuscular (i.m.) vaccination, 50 μl was injected into the tibialis anterior muscle of each leg.
Preparation of single-cell suspensions and fluorescence-activated cell sorter (FACS) analysis.
Lungs were finely cut and treated with 4 mg of collagenase A (from Clostridium histolyticum; Roche Diagnostics GmbH, Mannheim, Germany) in 2 ml of RPMI medium for 30 min at 37°C. The digested tissues were then homogenized by pressing against a sieve with a plastic plunger. Erythrocytes were removed by osmotic shock, and the cell pellets were resuspended in RPMI 1640 medium. Cell suspensions were treated with mouse Fc Block (BD PharMingen, CA) on ice for 15 min before staining with monoclonal antibodies (MAbs) against CD11b (M1/70), CD11c (HL3), and CD86 (GL1) for 30 min on ice. Cells were washed twice with phosphate-buffered saline (PBS) with 1% fetal calf serum (FCS) before analysis on a FACSCalibur (BD Biosciences, San Jose, CA). A total of 80,000 events were acquired for each lung sample. The data were analyzed by FlowJo (Tree Star, Inc., Ashland, OR).
ELISA.
Influenza virus-specific antibody (Ab) levels in sera were determined by enzyme-linked immunosorbent assays (ELISA) (21) using plates coated with a beta-propiolactone (BPL)-inactivated whole-virion preparation of the cold-adapted (ca) influenza virus vaccine A/Vietnam/1203/2004 ca (in which the hemagglutinin [HA] and neuraminidase [NA] genes were derived from A/Vietnam/1203/04 and the internal protein genes from A/Ann Arbor/6/60 ca; 50 HA units/well). Other than the removal of the multiple-basic amino acid motif, the sequence of the HA molecule of the H5N1 ca virus is identical to that of the wild-type H5N1 virus. The sera were serially diluted in half-log dilutions and incubated on plates at 4°C overnight. Bound Ab was detected with polyclonal goat anti-mouse immunoglobulin conjugated with horseradish peroxidase (Dako, Glostrup, Denmark). 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS; Sigma-Aldrich Pty Ltd., St. Louis, MO) was used as a substrate; the reaction was stopped with 1% sodium dodecyl sulfate (SDS) solution after 15 min, and the color intensity was measured with a microplate reader (SpectraMax M5; Molecular Devices, Sunnyvale, CA) at a wavelength of 405 nm and a reference wavelength of 450 nm. An optical density (OD) of >0.2 was considered to be positive. For isotyping experiments, isotype-specific MAbs (IgG1, LO-MG-1-2; IgG2a, LO-MG2a-7; IgG2b, LO-MG2b-2) were used; all were purchased from Abcam Inc., Cambridge, MA. The IgA-specific MAb (C10-1) was from BD Biosciences.
Virus titration assay.
Lungs, nasal turbinates, and brain were harvested, weighed, and homogenized in L-15 medium to prepare a 10% (wt/vol) tissue homogenate. The homogenates were clarified by low-speed centrifugation, and viral titers determined on MDCK cell monolayers were expressed as log10 TCID50/g of tissue as previously described (12).
Microneutralization (MN) assay.
The neutralizing antibody titers against homologous virus in serum samples were determined as previously described (22). In brief, serial 2-fold dilutions of heat-inactivated serum were prepared starting at a 1:10 dilution. Equal volumes of serum and wt A/Vietnam/1203/2004 virus (100 TCID50) were mixed and incubated at room temperature for 1 h before inoculation onto MDCK cell monolayers in four replicates. The plates were scored at day 4 for cytopathic effect (CPE), and the neutralizing antibody titer was defined as the reciprocal of the highest dilution of serum that completely neutralized the infectivity of 100 TCID50 of the virus.
Cytokine analysis.
Lungs were homogenized in 1 ml of Leibovitz's (L-15) medium or RMPI 1640 medium (Invitrogen), and the concentrations of various cytokines or chemokines in clarified samples were measured by the Bio-plex protein array system using the manufacturer's protocol (Bio-Rad, Hercules, CA).
Statistical analysis.
The significance of the difference between any two different groups was assessed by the Mann-Whitney test using Prism 5 (GraphPad Software, CA). In selected experiments where the sample size of each group was less than 5, the unpaired t test was used. P values of <0.05 are considered significantly different.
RESULTS
Intranasal administration of PIKA led to functional maturation of antigen-presenting cells (APC) and proinflammatory cytokines in the lungs of mice.
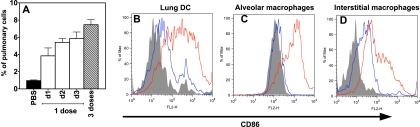
Previously, we and others demonstrated that PIKA is able to activate DC, resulting in the production of proinflammatory cytokines in vitro (25, 38). In this study, we examined the effect of PIKA in vivo. Groups of three mice were given 1 or 3 doses of PIKA in a volume of 50 μl i.n. The mice were sacrificed at the indicated time points after the final dose, and single-cell suspensions were prepared from lungs. As shown in Fig. 1 A, when mice received a single dose of PIKA, there was an influx of DC (CD11bint, CD11cint [5]) into the lungs, starting on day 1 postadministration and continuing to increase over the next 2 days. Mice that received 3 daily doses of PIKA had the highest number of DC in the lungs, making up approximately 7.5% of the cell population in the lungs. Apart from an increase in cell number, the DC also underwent maturation associated with an upregulation of the costimulatory molecule CD86 (48). As shown in Fig. 1B, the majority of the DC were CD86dim in mice that received PBS, with only a small population of mature DC (CD86 hi). On the other hand, the DC in the lungs of mice that received PIKA showed a mature phenotype, with high expression of CD86. This upregulation of CD86 expression continued even after 3 days following a single dose of PIKA (data not shown). In addition to lung DC, other cell types, such as alveolar macrophages (CD11blow CD11chigh) and interstitial macrophages (CD11bhigh CD11c−), also upregulated CD86 expression in response to PIKA stimulation (Fig. 1C and D).
FIG. 1.
Infiltration and maturation of antigen-presenting cells in the lungs of mice after intranasal administration of PIKA. Groups of three mice were given 100 μg of PIKA in a volume of 50 μl or 50 μl of PBS intranasally. One group continued to receive daily dosing of PIKA for two additional days. Lungs were harvested 24 h after the final dose. Single-cell suspensions were prepared, and different cell populations were identified by their differential surface expression of CD11b and CD11c. (A) Lung dendritic cells (lung DC [CD11bint and CD11cint]) expressed as a percentage of total cells in the lungs. The bars and error bars represent the means and standard errors for the group. d1, day 1. (B to D) Lung DC, alveolar macrophages (CD11blo and CD11chi), and interstitial macrophages (CD11bhi and CD11−) were identified, and surface expression of CD86 was determined. Gray, isotype control; blue, PBS control; red, PIKA treated. The diagrams are representative of three individual animals from each group from a single experiment.
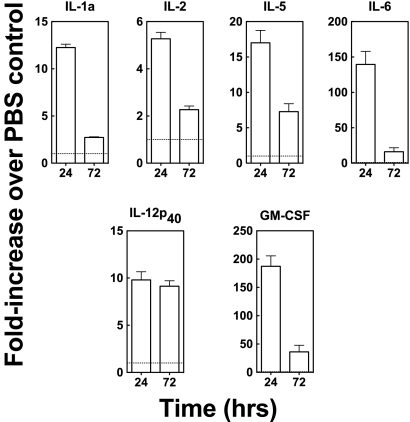
To evaluate the cytokine production induced by PIKA, mice were given PIKA or PBS i.n. in a volume of 50 μl, and lung homogenates from day 1 and day 3 postadministration were tested for cytokine/chemokine levels. As shown in Fig. 2, there were increases in the levels of proinflammatory cytokines, such as interleukin 1a (IL-1a) and IL-12p40, in the lungs of mice that received a single dose of PIKA, and the levels were still above the baseline 3 days later. Taken together, these data are consistent with our previous in vitro observations that PIKA is able to activate the innate immune system, leading to functional maturation of professional APCs.
FIG. 2.
Intranasal administration of PIKA induced the production of proinflammatory cytokines. Groups of three BALB/c mice each were given either 100 μg of PIKA i.n. in PBS or 50 μl of PBS alone and were sacrificed 24 or 72 h later. The lungs were stored at −80°C until all samples were collected and homogenized in 1 ml of RPMI 1640 medium. To measure the concentrations of various cytokines, 50 μl of clarified samples was tested in duplicate using the Bio-plex protein array system. The concentration of each cytokine detected in the PIKA-treated group is expressed as the fold increase over the concentration detected in the PBS-treated group. The bars and error bars represent the means and standard errors for each group. Note that, due to the differences in the expression levels of the various cytokines, the scales of the y axes are different. These data are representative of two independent experiments. The absolute values of the cytokines in this experiment can be found in Table S1 in the supplemental material.
Coadministration of PIKA with a subvirion H5N1 vaccine potentiates the immunogenicity and has a significant antigen-sparing effect.
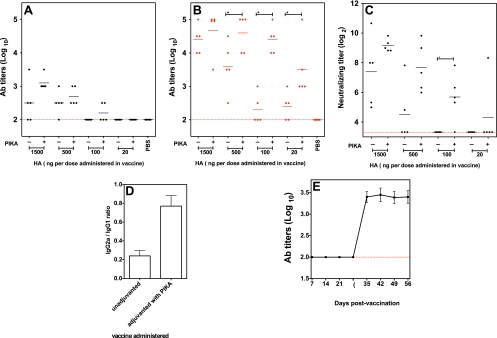
Mosca et al. showed that the injury produced by an injection can activate cells at the injection site (30). To minimize such nonspecific activation, a noninvasive intranasal route was selected to demonstrate the potency of PIKA as an adjuvant. Groups of five mice received various amounts of a subvirion vaccine (from 1,500 ng to 20 ng) with or without PIKA by the i.n. route. Four weeks after the first dose, sera were collected, and the mice received a second dose of vaccine. Sera were collected again 4 weeks later. Influenza virus-specific antibody titers in sera were determined by ELISA. As shown in Fig. 3 A, after a single dose of vaccine, only 3/5 and 4/5 mice that received the vaccine alone (1,500 ng and 500 ng, respectively) had detectable antibody responses in the sera, whereas in the corresponding groups that received the vaccine with PIKA, all mice had influenza virus-specific antibodies. With 100 ng of the vaccine, 2/5 mice that received the adjuvanted vaccine showed detectable antibody responses, compared to 0/5 in the unadjuvanted group. At 20 ng, none of the mice mounted antibody responses. There was a significant rise in the antibody titer after the second dose (Fig. 3B). With 1,500 ng of HA, all mice had high antibody titers, even in the absence of an adjuvant, and the titers in the groups of mice that received adjuvanted versus unadjuvanted vaccine were not significantly different. The antibody titers in the unadjuvanted group were lower with lower doses of vaccine. For the 100-ng and 20-ng groups, only 2/5 and 3/5 mice showed detectable serum antibody after two doses of the unadjuvanted vaccine. The addition of PIKA to the vaccine improved the immunogenicity of the vaccine; the antibody titers achieved with PIKA (at 500, 100, and 20 ng of HA) were significantly higher than those in mice that received unadjuvanted vaccine (P, <0.05). Furthermore, the antibody titer achieved with 100 ng of the vaccine with PIKA was comparable to the titer achieved with 1,500 ng of unadjuvanted vaccine (P, 0.91), proving that coadministration of PIKA with the vaccine can provide significant antigen sparing. Enhancement of serum neutralizing antibody titers was observed when lower doses of the immunogen were used (Fig. 3C). Apart from the quantitative difference, there was also a qualitative difference in the response elicited by the adjuvanted vaccine compared with the unadjuvanted vaccine. At a dose of 1,500 ng of HA, although the antibody titers were comparable between the two groups, the group that received the unadjuvanted vaccine showed a bias toward a Th2 response, and most of the influenza virus-specific antibodies belonged to the IgG1 isotype (Fig. 3D). For the adjuvanted group, the isotype distribution was more balanced, with approximately 4-fold more IgG2a antibodies compared to the unadjuvanted group.
FIG. 3.
Coadministration of PIKA improved the immunogenicity of an H5N1 subunit vaccine, with an antigen-sparing effect. Groups of 5 mice were given two doses of the indicated amount of the H5N1 subunit vaccine, with or without PIKA, intranasally. (A and B) Influenza virus-specific serum antibody titers were determined on day 28 (A) and day 56 (B) by ELISA by using a BPL-inactivated H5N1 vaccine as the coating antigen. Each filled circle represents the antibody titer of an individual mouse, and each line represents the mean titer of the group of mice. The dashed lines represent the lower limit of detection. The asterisk indicates that the difference between the two groups was statistically significant (P < 0.05). (C) Serum neutralizing (MN) antibody titers on day 56 after vaccination against the wt A/Vietnam/1203/2004 virus. Each filled circle represents the MN titer of an individual mouse, and each line represents the geometric mean for the group of mice. (D) The IgG1 and IgG2a titers in day 56 sera from mice that received 1,500 ng of unadjuvanted or adjuvanted vaccine were determined by ELISA, and the ratios of IgG2a to IgG1 were calculated. (E) A group of 5 mice received 15 ng of adjuvanted vaccine i.n. and received a second dose on day 28. Serum samples were collected every 7 days after the initial vaccination to monitor the development of influenza virus-specific antibody responses by using an ELISA. The bars and error bars represent the means and standard errors for each group. The data were obtained from a single experiment.
In the previous experiment, mice that received a single dose of 20 ng of the adjuvanted vaccine showed no significant antibody response, but the antibody titers increased after the second dose of vaccine. To examine the kinetics of the antibody response, mice were given 15 ng of the adjuvanted vaccine i.n. on day 0 and day 28, and serum samples were collected weekly. As shown in Fig. 3E, no antibody response was detected following the first dose of vaccine, but there was a sharp increase in the serum antibody titer within 7 days of the second dose, and the antibody responses were maintained at a high level.
The immune responses elicited by the adjuvanted vaccine were sufficient to protect mice from wild-type A/Vietnam/1203/2004 challenge.
To determine the efficacy of this vaccine strategy, the vaccinated mice were anesthetized and challenged with 105 TCID50 of the wt A/Vietnam/1203/2004 (VN04) H5N1 virus. The VN04 (H5N1) wt virus is highly virulent in mice, and the challenge dose represents 4 × 104 MLD50. On day 4 following challenge, mice were sacrificed, and viral titers in various organs were determined. As shown in Fig. 4 A, for the 1,500-ng and 500-ng groups, all vaccinated mice showed significant reductions in viral titers in lungs, compared to the PBS control group, ranging from a 100-fold to a 1 million-fold reduction. Interestingly, despite the presence of comparable antibody titers in the two groups that received 1,500 ng of HA (Fig. 3B; summarized in Table 1), only 2/5 mice in the unadjuvanted group were fully protected from replication of virus in the lungs, whereas all mice (5/5) in the adjuvanted group were protected from pulmonary virus replication. A similar trend was observed in the groups that received 500 ng of the vaccine antigen. At a vaccine dose of 100 ng, the viral titers in the lungs of mice that received the unadjuvanted vaccine were not statistically different from those in the PBS control group (P, 0.14), but the immunity induced by the adjuvanted vaccine was sufficient to protect the mice from viral replication in the lungs; 3/5 mice had no virus detected in the lungs, and the titers were significantly lower than those in the unadjuvanted group (P, 0.012). Finally, for the 20-ng groups, having the adjuvant in the vaccine still led to a significant reduction in the viral titer compared with the unadjuvanted group (P, 0.041). As for viral replication in the upper respiratory tract, 2/5 mice that received 1,500 ng of unadjuvanted vaccine had no virus detected in the nasal turbinates (Fig. 4B), and overall, higher challenge virus titers were observed as the dose of vaccine was reduced. Mice that received 100 ng or 20 ng of unadjuvanted vaccine had viral titers in the nasal turbinates that were comparable to those of mice that received PBS (P, >0.05). On the other hand, none of the mice that received 1,500 ng, 500 ng, or 100 ng of the adjuvanted vaccine had virus detected in their nasal turbinates. For the 20-ng group, although virus was detected in the nasal turbinates, the titers were still statistically lower than those for the unadjuvanted group and the PBS control group (P, <0.05). Finally, viral titers in the brain were evaluated, because the H5N1 virus can disseminate and replicate in the brain (Fig. 4C). For mice that received 1,500 ng of HA or 500 ng of HA, virus was not detected in the brain on day 4 postinfection (p.i.), regardless of whether the vaccine was adjuvanted or not. At lower doses of vaccine, such as 100 ng or 20 ng, more mice in the unadjuvanted groups had virus detected in the brain (2/5 and 4/5, respectively) compared with the adjuvanted group (0/5 and 1/5, respectively). In summary, our data showed that the quantitative and qualitative improvements in the humoral responses induced by the H5N1 vaccine with the incorporation of PIKA as an adjuvant were associated with better protection from wt virus challenge, even when a low dose of immunogen was used.
FIG. 4.
The enhanced immunogenicity of the adjuvanted H5N1 subunit vaccine was associated with better protection against lethal challenge with wt H5N1 viruses. (A to C) Groups of 5 mice were primed and boosted with the indicated vaccine doses as described for Fig. 3. On day 56, the mice were challenged intranasally with 105 TCID50 of wt VN04 (H5N1) virus. Viral titers on day 4 p.i. in the lung (A), nasal turbinates (B), and brain (C) are expressed as log10 TCID50/g of tissue. Each filled circle represents the titer of an individual mouse, and each line represents the mean for the group of mice. An asterisk indicates that the difference between two groups was statistically significant (P < 0.05). An asterisk shown above a single column of data indicates that the difference between this group and the PBS group was statistically significant (P < 0.05). (D and E) Groups of 5 mice were primed and boosted with 100 ng of vaccine as described for Fig. 3 and were challenged with 105 TCID50 of wt A/Indonesia/05/2005 (H5N1) virus (clade 2.1) on day 56. The viral titers on day 4 p.i. in nasal turbinates (D) and the lung (E) are expressed as log10 TCID50/g of tissue. The data are representative of two independent experiments.
TABLE 1.
Serological parameters and pulmonary viral titers achieved by mice that received i.n. administration of 1,500 ng of an unadjuvanted or PIKA-adjuvanted H5N1 subunit vaccine
| Amt of PIKA (μg) | Mice | Serum ELISA Ab titer (log10) | Serum MN Aba titer (log2) | IgG2a/IgG1 ratio | Pulmonary viral titer (TCID50/g) |
|---|---|---|---|---|---|
| 0 | M1 | 4.0 | 5.3 | 1:7.1 | 4.5 |
| M2 | 4.0 | 5.0 | 1:8.3 | 5.2 | |
| M3 | 4.5 | 10.7 | 1:4.5 | 5.2 | |
| M4 | 5.0 | 8.0 | 1:3.3 | 1.5 | |
| M5 | 4.5 | 8.0 | 1:2.4 | 1.5 | |
| 100 | M1 | 5.0 | 9.3 | 1:1.5 | 1.5 |
| M2 | 5.0 | 9.8 | 1:1.4 | 1.5 | |
| M3 | 3.5 | 8.8 | 1:0.9 | 1.5 | |
| M4 | 5.0 | 9.0 | 1:1.2 | 1.5 | |
| M5 | 5.0 | 8.8 | 1:2.0 | 1.5 |
MN Ab, microneutralizing antibody.
The immunity induced by the adjuvanted vaccine provides protection against heterologous H5N1 virus challenge.
In order to determine whether the adjuvanted vaccine with the VN04 H5N1 virus antigen can protect mice from heterologous H5N1 virus challenge, vaccinated mice were challenged intranasally with 1 × 105 TCID50 of A/Indonesia/05/2005 (clade 2.1). Viral titers in the lungs and nasal turbinates were determined on day 4 p.i. As shown in Fig. 4D and E, mice that received the adjuvanted vaccine showed significant reductions in viral titers at both sites (P, <0.05) compared to the unadjuvanted group. The unadjuvanted vaccine provided no significant protection; the viral titers were similar to those for the PBS group. Therefore, the breadth of the immune responses induced by the adjuvanted vaccine was sufficient to protect mice from a heterologous virus challenge.
The adjuvanting effect of PIKA is compatible with other routes of inoculation.
Since the i.m. route is the commonest route for administering seasonal influenza virus vaccine, it was of interest to determine if the benefits described so far with the addition of PIKA as an adjuvant for the H5N1 vaccine delivered intranasally would be observed when the vaccine was administered by other routes. Mice were given two doses of 100 ng of the subvirion vaccine with or without PIKA by 3 different routes: i.n., s.c., and i.m. This dose was chosen because it was the dose at which the adjuvanted vaccine showed the most significant improvement in immunogenicity compared with the unadjuvanted vaccine (Fig. 3B). Following the first dose, mice that received the adjuvanted vaccine had significantly higher antibody titers than those that received the unadjuvanted vaccine by all 3 routes (Fig. 5 A). Following the second dose, the groups that received the adjuvanted vaccine continued to show higher antibody titers, and the difference reached statistical significance for the groups vaccinated by the i.n. and i.m. routes (Fig. 5B) (P, <0.05). It is also worth mentioning that the immunogenicity of the unadjuvanted vaccine given s.c. and i.m. was greater than that observed when it was administered i.n. However, i.n. administration was more effective than s.c. administration at inducing IgA responses in lungs (Fig. 5C). Irrespective of the route of vaccination, the responses induced by the unadjuvanted vaccine maintained a bias toward a Th2 response (Fig. 5D). With the addition of PIKA, there was an increase in IgG2a subclass antibodies, resulting in a more balanced IgG2a/IgG1 ratio.
FIG. 5.
The adjuvanting effect of PIKA can be achieved by multiple routes of administration. (A and B) Groups of 5 mice were vaccinated with two doses of 100 ng of the unadjuvanted or adjuvanted vaccine by the indicated routes. Influenza virus-specific serum antibody titers were determined on day 28 (A) and day 56 (B) by ELISA using a BPL-inactivated H5N1 vaccine as the coating antigen. (C) Groups of 5 mice were vaccinated with two doses of 500 ng of PIKA-adjuvanted vaccines by the indicated routes, and pulmonary IgA titers were determined by ELISA on day 56. (D) The IgG1 and IgG2a titers in day 56 sera from mice that received 100 ng of unadjuvanted or adjuvanted vaccine were determined by ELISA, and the ratios of IgG2a to IgG1 were calculated. The IgG2a/IgG1 ratio for the group that received the unadjuvanted vaccine through the i.n. route could not be determined, because the ELISA antibody titer was below the lower detection limit of the assay, and is denoted as N.A. (E and F) Groups of 5 mice were vaccinated as described for panel B and were challenged with 105 TCID50 of wt VN04 (H5N1) virus. Viral titers on day 4 p.i. in nasal turbinates (E) and the lung (F) are expressed as log10 TCID50/g of tissue. Each filled circle represents the titer of an individual mouse, and each line represents the mean for the group of mice. The asterisk indicates that the difference between two groups was statistically significant (P < 0.05). The data were obtained from a single experiment. A separate experiment showed that 100% of the mice that received 500 ng of the adjuvanted vaccine survived lethal challenge.
To determine the efficacy of the vaccine in protecting mice from wt virus infection, vaccinated mice were challenged with 105 TCID50 of the wt VN04 (H5N1) virus, and viral titers in the lungs and nasal turbinates were determined on day 4 p.i. As shown in Fig. 5E, when mice were vaccinated by the s.c. route, although the difference between the unadjuvanted and adjuvanted groups did not reach statistical significance, more mice that received the unadjuvanted vaccine had virus detected in the nasal turbinates compared with the adjuvanted vaccine group (2/5 versus 0/5). When mice were vaccinated by i.m. immunization, mice that received the unadjuvanted vaccine had significantly higher viral titers in the nasal turbinates than mice that received the adjuvanted vaccine (P, 0.011). The results in the lungs mirrored the viral titers in the nasal turbinates, in that fewer mice that received the adjuvanted vaccine had virus detected in the lungs, and the titers were significantly lower than in mice that received the unadjuvanted vaccine (Fig. 5F). These results suggest that the adjuvanting property of PIKA and enhanced protection against wt H5N1 viruses are observed when the adjuvant is administered through different routes.
The importance of TLR3 in mediating the adjuvanting effect of PIKA.
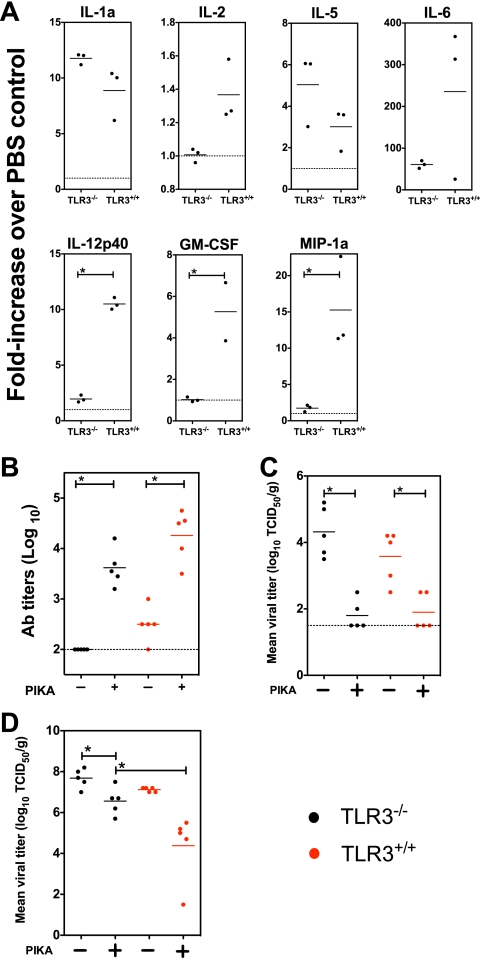
To determine the importance of TLR3 in mediating the adjuvanting effect of PIKA, TLR3−/− mice were given 100 μg of PIKA i.n., and the production of various cytokines and chemokines in the lungs was examined. The results showed that the production of some cytokines, such as IL-1a and IL-5, was TLR3 independent, because mice lacking functional TLR3 produced comparable levels of these cytokines (Fig. 6A), whereas TLR3−/− mice showed diminished production of other cytokines, such as IL-2, IL-6, IL-12p40, granulocyte-macrophage colony-stimulating factor (GM-CSF), and MIP-1a, with IL-12p40, GM-CSF, and MIP-1a differences reaching statistical significance (P, <0.05 by an unpaired t test). TLR3−/− and TLR3+/+ mice were primed and boosted with 100 ng of the unadjuvanted or adjuvanted vaccine i.n. as previously described, and the serum antibody titers on day 56 were determined. As shown in Fig. 6B, the addition of PIKA as an adjuvant improved the immunogenicity of the vaccine in both strains of mice; however, the antibody titers achieved in TLR3−/− mice were lower than those in TLR3+/+ mice, though the difference did not reach statistical significance (P, 0.095). To determine the biological significance of the diminished antibody responses, the mice were challenged with the wt VN04 (H5N1) virus, and the viral titers in nasal turbinates and lungs were determined on day 4 p.i. As shown in Fig. 6C, TLR3−/− mice that received the adjuvanted vaccine showed significant reductions in the viral titers in the nasal turbinates compared with those for mice that received the unadjuvanted vaccine. In addition, the level of clearance was comparable with that achieved with the adjuvanted vaccine in TLR3+/+ mice. In the lungs (Fig. 6D), although the TLR3−/− mice that received the adjuvanted vaccine showed a significant reduction in viral titers over TLR3−/− mice that received the unadjuvanted vaccine, TLR3+/+ mice that received the adjuvanted vaccine had the lowest viral titers. The difference in viral titers between TLR3−/− and TLR3+/+ mice that received the adjuvanted vaccine was about 100-fold and was statistically significant (P, 0.012). In summary, our results suggest that TLR3 is important for the optimal performance of PIKA as an adjuvant.
FIG. 6.
TLR3 is important for the adjuvanting property of PIKA in mice. (A) Groups of three TLR3−/− or TLR3+/+ mice each were given either 100 μg of PIKA i.n. in PBS or 50 μl of PBS alone and were sacrificed 24 h later. The lungs were homogenized in 1 ml of RPMI-1640 medium and were stored at −80°C. To measure the concentrations of various cytokines, 50 μl of clarified samples was tested in duplicate using the Bio-plex protein array system. The concentration of each cytokine detected in the PIKA-treated group was expressed as the fold increase over the concentration in the PBS control sample. The bars and error bars represent the means and standard errors for each group. Note that, due to the differences in the expression levels of the various cytokines, the scales of the y axes are different. (B) Groups of 5 mice were vaccinated with two 100-ng doses of the unadjuvanted or adjuvanted vaccine i.n. Influenza virus-specific serum antibody titers were determined on day 56 by ELISA using a BPL-inactivated H5N1 vaccine as the coating antigen. Each filled circle represents the titer for an individual mouse, and each line represents the mean for the group of mice. (C and D) The mice were challenged with 1 × 105 TCID50 of wt VN04 virus on day 56. The viral titers on day 4 p.i. in nasal turbinates (C) and the lung (D) are expressed as log10 TCID50/g of tissue. An asterisk indicates that the difference between two groups was statistically significant (P < 0.05). The data were obtained from a single experiment.
DISCUSSION
Due to unpredictable antigenic variation of the surface glycoproteins of influenza viruses, the production of a matched pandemic influenza virus vaccine cannot be initiated until a pandemic virus is identified. This criterion imposes serious constraints on our preparation for future pandemics. Also, because the production capacity for vaccines is not sufficient to meet global needs during a pandemic, a vaccine shortage is likely. The incorporation of adjuvants for antigen sparing is an important strategy for pandemic preparedness (26, 34, 40). However, there is a need for a new generation of adjuvants, because traditional adjuvants, such as alum, do not provide significant antigen sparing and are not effective adjuvants for H5N1 and 2009 pandemic H1N1 influenza virus vaccines (3, 9, 51). In the current study, we evaluated the ability of PIKA, a dsRNA compound, to potentiate the immunogenicity of an H5N1 subunit vaccine. Our results showed a significant increase in influenza virus-specific antibody responses when the vaccine was coadministered with PIKA by different routes. Although the adjuvanting properties of other dsRNA compounds, such as Ampligen, have been described previously (15-18), the degree of antigen sparing was not addressed, and qualitative assessment of the immune responses induced by dsRNA-adjuvanted vaccines versus unadjuvanted vaccines was not discussed. Our data show that the immune responses achieved by the adjuvanted vaccine were robust enough to protect mice from challenge with wt H5N1 viruses, and we extended these observations by showing that PIKA can provide significant antigen sparing. Compared with unadjuvanted vaccine, comparable antibody titers and protective efficacy against wt virus infection were achieved with 15-fold less antigen (1.5 μg versus 0.1 μg) when the vaccine was administered intranasally with PIKA. In addition, the breadth of the immune response induced by the adjuvanted vaccine was sufficient to protect mice from a heterologous H5N1 virus challenge. Because vaccines prepared in a pandemic might not be optimally matched with the virus that circulates, the ability to provide protection against antigenically heterologous viruses is an attractive feature. Using a number of routes and doses in BALB/c mice that have an inherent bias toward “Th2 responses,” we showed that the unadjuvanted H5N1 vaccine elicited an IgG1 (Th2)-dominant response with little IgG2a production. For the group that was inoculated i.n. with 1,500 ng of unadjuvanted vaccine, although serum ELISA titers were high, the mice had varied Th1/Th2 ratios (from 1:7 to 1:2.4), and 2 out of 5 mice had no detectable virus in the lungs on day 4 p.i. The mice that had higher Th1/Th2 ratios had virus present in the lungs, and mice that had Th1/Th2 ratios of <1:3.3 had no virus detectable in the lungs (Table 1). The results suggest that apart from a quantitative improvement in the magnitude of the humoral immune responses, modifying the isotype distribution of antibodies might be another strategy for improving antibody-mediated viral clearance. Our observation that PIKA is a Th1-biasing adjuvant is consistent with the observation made by Shen et al., who compared unadjuvanted hepatitis B virus vaccine in mice with PIKA-adjuvanted hepatitis B virus vaccine and demonstrated that the latter induced significantly higher numbers of gamma interferon (IFN-γ)-secreting cells (38). In our model, the inclusion of PIKA drove the anti-HA immune responses toward a more “antiviral-like” response, with more consistent induction of IgG2a, the main IgG subclass associated with antiviral responses (6, 10). The Fc portion of IgG2a antibodies can interact with complement components with high affinity (11, 32, 44), allowing more effective activation of effector functions, such as complement activation and antibody-dependent cell-mediated cytotoxicity (ADCC). Despite the presence of comparable serum ELISA antibody titers, we speculate that the higher Th1/Th2 ratio induced by the adjuvanted vaccine, compared with unadjuvanted vaccine, resulted in more effective viral clearance. In addition to humoral responses, others have shown that the inclusion of dsRNA compounds can enhance the induction of cellular immune responses. Trumpfheller et al. demonstrated that the inclusion of poly(I:C) into a vaccine is beneficial for the induction of antigen-specific CD4+ T lymphocytes in mice (43), and Stahl-Henning et al. demonstrated that when dsRNA compounds were used as an adjuvant, Th1 responses were potentiated in rhesus macaques (39). We believe that the inclusion of PIKA in influenza virus vaccines would be beneficial, especially for the elderly population, because lower vaccine efficacy in the elderly population is thought to be related to dysregulation of the Th1/Th2 balance in immunosenescence (8, 13). As suggested by other studies (36, 45), the inclusion of an adjuvant that potentiates a Th1 bias may improve the efficacy of a vaccine for the elderly.
It is of interest that although the immune responses induced in mice inoculated with a single dose of 15 ng of the adjuvanted H5N1 vaccine were below the detection limit of the assay, the antibody titers increased rapidly after the second dose of vaccine. We observed similar results with a seasonal influenza virus vaccine (25). These data lead us to speculate that antigen-specific B cells would have been primed during the primary immunization, likely at mucosal sites, and they expand rapidly after the second dose of vaccine. Priming a population with a suboptimal dose of a prepandemic influenza virus vaccine followed by a booster dose might provide rapid induction of immunity and protection without interfering with serological surveillance efforts early in a pandemic.
Our data show that although the production of certain cytokines, such as IL-1, KC, and IFN-β, was not affected (25a; this study), there was a general trend of diminished cytokine production in response to PIKA stimulation in mice lacking functional TLR3. In addition, the antibody titers induced by the adjuvanted vaccine in the TLR3−/− mice were lower than those in TLR3+/+ mice, and TLR3−/− vaccinated mice had significantly higher viral titers in their lungs after challenge with the wt H5N1 virus. These observations suggest that, although PIKA can interact with a number of receptors, such as TLR3, MDA-5, and RIG-I, TLR3 is still important for optimal bioactivity of PIKA in vivo. Our results are consistent with the work described by Kumar et al. showing that cooperative activation of TLR and cytoplasmic RNA helicase pathways is important for the adjuvanting property of poly(I:C) (23). More specifically, IFN-β promoter stimulator-1 (IPS-1)-deficient mice and Toll/IL-1R domain-containing adaptor-inducing IFN-β (TRIF)-deficient mice showed 80% and 60% lower ovalbumin-specific antibodies than wild-type mice when poly(I:C) was administered as an adjuvant with ovalbumin (23). The adjuvanting property of poly(I:C) was lost in double-knockout (IPS-1/TRIF−/−) mice. Interestingly, although PIKA and Ampligen are chemically related, Ampligen uses TLR3 exclusively for its adjuvanting activity (43), while PIKA maintains a certain level of adjuvanting property in TLR3−/− mice, suggesting they might be processed differently in vivo.
Our data show that shortly after intranasal administration, PIKA induced a rapid infiltration of APCs, with increased expression of costimulatory molecules on the cell surface and proinflammatory cytokine production in the lungs. The results are consistent with previous in vitro studies that showed that dsRNA compounds, such as poly(I:C) and PIKA, can induce maturation of dendritic cells in vitro (25, 31, 38). The ability to initiate DC maturation is an important characteristic for a good adjuvant (24, 50). The activation of DC by PIKA allows more effective antigen processing and presentation by APCs, together with increased expression of costimulatory molecules, leading to more effective activation of CD4+ T cells and B cells. Compared with traditional parenteral routes, vaccination via intranasal administration has been shown to be more effective in inducing mucosal immunity, such as IgA antibody (1, 47). We believe that the incorporation of an external adjuvant, such as PIKA, allows IgA-secreting antibody secretory cells (ASCs) to be established more readily in the respiratory tract; we have previously shown that the induction of influenza virus-specific ASCs in lungs is dependent on the inflammatory responses induced by live attenuated influenza virus vaccines (Lau et al., submitted for publication). The polymeric forms of IgA secreted by local ASCs can be actively transported by the transmembrane polymeric Ig receptor from the basolateral to the apical surfaces of mucosal epithelial cells, which are the initial site of influenza virus infection. IgA can neutralize virus by blocking viral attachment to cells in the respiratory tract or by intracellular neutralization of newly synthesized viral proteins within infected cells (29) and has been shown to be important in preventing influenza-induced pathology in the trachea (35). IgG also has been shown to be a mediator of viral clearance in lungs, and a number of studies have shown that IgG antibodies in the lungs or nasal washes are transudates (19, 46). As the concentration of IgG in the nasal wash correlates with serum IgG titers (35), optimal induction of serum antibodies by incorporation of an adjuvant would ensure that a gradient could be established between the serum and lung tissues, allowing sufficient IgG to transude across the epithelium to protect the lower respiratory tract.
The development of needle-free vaccines has been hindered by the lack of safe adjuvants, because traditional mucosal adjuvants, such as poly(I:C), have been associated with serious side effects in mice and humans (37, 49). Our results show that the new generation of modified dsRNA compounds are effective mucosal adjuvants, with few side effects in the animal models. We previously showed that mice that received three daily doses of 100 μg of PIKA intranasally showed no significant weight loss (25a). In addition, poly(I):poly(C12 U) (Ampligen) has a safe record following >75,000 human doses (17). PIKA is an effective adjuvant that can be administered by a number of routes without the side effects associated with poly(I:C). In a recent toxicity trial, PIKA showed no significant toxicity in mice, and a phase I clinical study showed that PIKA was well tolerated in humans (P. Brazier, unpublished data). Its safety profile, long shelf life, and antigen sparing make PIKA an attractive adjuvant to be considered for use in future pandemics to ensure maximum coverage with limited supplies of influenza virus vaccine.
Supplementary Material
Acknowledgments
We thank Jadon Jackson for excellent technical support for animal studies performed at the NIH.
This research was supported by funds from the Intramural Research Program of the NIAID, NIH, and the Future Systems Directorate, Ministry of Defense, the Republic of Singapore.
Footnotes
Published ahead of print on 10 June 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Atmar, R. L., W. A. Keitel, T. R. Cate, F. M. Munoz, F. Ruben, and R. B. Couch. 2007. A dose-response evaluation of inactivated influenza vaccine given intranasally and intramuscularly to healthy young adults. Vaccine 25:5367-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beigel, J. H., J. Farrar, A. M. Han, F. G. Hayden, R. Hyer, M. D. de Jong, S. Lochindarat, T. K. Nguyen, T. H. Nguyen, T. H. Tran, A. Nicoll, S. Touch, and K. Y. Yuen. 2005. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 353:1374-1385. [DOI] [PubMed] [Google Scholar]
- 3.Bresson, J. L., C. Perronne, O. Launay, C. Gerdil, M. Saville, J. Wood, K. Hoschler, and M. C. Zambon. 2006. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 367:1657-1664. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2009. Swine influenza A (H1N1) infection in two children-Southern California, March-April 2009. MMWR Morb. Mortal. Wkly. Rep. 58:400-402. [PubMed] [Google Scholar]
- 5.Cleret, A., A. Quesnel-Hellmann, J. Mathieu, D. Vidal, and J. N. Tournier. 2006. Resident CD11c+ lung cells are impaired by anthrax toxins after spore infection. J. Infect. Dis. 194:86-94. [DOI] [PubMed] [Google Scholar]
- 6.Coutelier, J. P., J. T. van der Logt, F. W. Heessen, G. Warnier, and J. Van Snick. 1987. IgG2a restriction of murine antibodies elicited by viral infections. J. Exp. Med. 165:64-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox, N. J., and K. Subbarao. 1999. Influenza. Lancet 354:1277-1282. [DOI] [PubMed] [Google Scholar]
- 8.Deng, Y., Y. Jing, A. E. Campbell, and S. Gravenstein. 2004. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J. Immunol. 172:3437-3446. [DOI] [PubMed] [Google Scholar]
- 9.Ehrlich, H. J., M. Muller, H. M. Oh, P. A. Tambyah, C. Joukhadar, E. Montomoli, D. Fisher, G. Berezuk, S. Fritsch, A. Low-Baselli, N. Vartian, R. Bobrovsky, B. G. Pavlova, E. M. Pollabauer, O. Kistner, and P. N. Barrett. 2008. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N. Engl. J. Med. 358:2573-2584. [DOI] [PubMed] [Google Scholar]
- 10.Fazekas, G., B. Rosenwirth, P. Dukor, J. Gergely, and E. Rajnavolgyi. 1994. IgG isotype distribution of local and systemic immune responses induced by influenza virus infection. Eur. J. Immunol. 24:3063-3067. [DOI] [PubMed] [Google Scholar]
- 11.Gessner, J. E., H. Heiken, A. Tamm, and R. E. Schmidt. 1998. The IgG Fc receptor family. Ann. Hematol. 76:231-248. [DOI] [PubMed] [Google Scholar]
- 12.Gillim-Ross, L., C. Santos, Z. Chen, A. Aspelund, C. F. Yang, D. Ye, H. Jin, G. Kemble, and K. Subbarao. 2008. Avian influenza H6 viruses productively infect and cause illness in mice and ferrets. J. Virol. 82:10854-10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin, K., C. Viboud, and L. Simonsen. 2006. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24:1159-1169. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg, M. E., M. H. Lai, G. F. Hartel, C. H. Wichems, C. Gittleson, J. Bennet, G. Dawson, W. Hu, C. Leggio, D. Washington, and R. L. Basser. 2009. Response to a monovalent 2009 influenza A (H1N1) vaccine. N. Engl. J. Med. 361:2405-2413. [DOI] [PubMed] [Google Scholar]
- 15.Ichinohe, T., A. Ainai, M. Tashiro, T. Sata, and H. Hasegawa. 2009. PolyI:polyC(12)U adjuvant-combined intranasal vaccine protects mice against highly pathogenic H5N1 influenza virus variants. Vaccine 27:6276-6279. [DOI] [PubMed] [Google Scholar]
- 16.Ichinohe, T., A. Kawaguchi, S. Tamura, H. Takahashi, H. Sawa, A. Ninomiya, M. Imai, S. Itamura, T. Odagiri, M. Tashiro, J. Chiba, T. Sata, T. Kurata, and H. Hasegawa. 2007. Intranasal immunization with H5N1 vaccine plus Poly I:Poly C(12)U, a Toll-like receptor agonist, protects mice against homologous and heterologous virus challenge. Microbes Infect. 9:1333-1340. [DOI] [PubMed] [Google Scholar]
- 17.Ichinohe, T., S. Tamura, A. Kawaguchi, A. Ninomiya, M. Imai, S. Itamura, T. Odagiri, M. Tashiro, H. Takahashi, H. Sawa, W. M. Mitchell, D. R. Strayer, W. A. Carter, J. Chiba, T. Kurata, T. Sata, and H. Hasegawa. 2007. Cross-protection against H5N1 influenza virus infection is afforded by intranasal inoculation with seasonal trivalent inactivated influenza vaccine. J. Infect. Dis. 196:1313-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichinohe, T., I. Watanabe, S. Ito, H. Fujii, M. Moriyama, S. Tamura, H. Takahashi, H. Sawa, J. Chiba, T. Kurata, T. Sata, and H. Hasegawa. 2005. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J. Virol. 79:2910-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito, R., Y. A. Ozaki, T. Yoshikawa, H. Hasegawa, Y. Sato, Y. Suzuki, R. Inoue, T. Morishima, N. Kondo, T. Sata, T. Kurata, and S. Tamura. 2003. Roles of anti-hemagglutinin IgA and IgG antibodies in different sites of the respiratory tract of vaccinated mice in preventing lethal influenza pneumonia. Vaccine 21:2362-2371. [DOI] [PubMed] [Google Scholar]
- 20.Itoh, Y., K. Shinya, M. Kiso, T. Watanabe, Y. Sakoda, M. Hatta, Y. Muramoto, D. Tamura, Y. Sakai-Tagawa, T. Noda, S. Sakabe, M. Imai, Y. Hatta, S. Watanabe, C. Li, S. Yamada, K. Fujii, S. Murakami, H. Imai, S. Kakugawa, M. Ito, R. Takano, K. Iwatsuki-Horimoto, M. Shimojima, T. Horimoto, H. Goto, K. Takahashi, A. Makino, H. Ishigaki, M. Nakayama, M. Okamatsu, D. Warshauer, P. A. Shult, R. Saito, H. Suzuki, Y. Furuta, M. Yamashita, K. Mitamura, K. Nakano, M. Nakamura, R. Brockman-Schneider, H. Mitamura, M. Yamazaki, N. Sugaya, M. Suresh, M. Ozawa, G. Neumann, J. Gern, H. Kida, K. Ogasawara, and Y. Kawaoka. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson, D. C., X. L. Tang, L. E. Brown, J. M. Murray, D. O. White, and G. W. Tregear. 1986. Antigenic determinants of influenza virus hemagglutinin. XII. The epitopes of a synthetic peptide representing the C-terminus of HA1. Virology 155:625-632. [DOI] [PubMed] [Google Scholar]
- 22.Joseph, T., J. McAuliffe, B. Lu, L. Vogel, D. Swayne, H. Jin, G. Kemble, and K. Subbarao. 2008. A live attenuated cold-adapted influenza A H7N3 virus vaccine provides protection against homologous and heterologous H7 viruses in mice and ferrets. Virology 378:123-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar, H., S. Koyama, K. J. Ishii, T. Kawai, and S. Akira. 2008. Cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J. Immunol. 180:683-687. [DOI] [PubMed] [Google Scholar]
- 24.Lau, Y. F., G. Deliyannis, W. Zeng, A. Mansell, D. C. Jackson, and L. E. Brown. 2006. Lipid-containing mimetics of natural triggers of innate immunity as CTL-inducing influenza vaccines. Int. Immunol. 18:1801-1813. [DOI] [PubMed] [Google Scholar]
- 25.Lau, Y. F., L. H. Tang, and E. E. Ooi. 2009. A TLR3 ligand that exhibits potent inhibition of influenza virus replication and has strong adjuvant activity has the potential for dual applications in an influenza pandemic. Vaccine 27:1354-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Lau, Y.-F., L.-H. Tang, E.-E. Ooi, and K. Subbarao. Activation of the innate immune system provides broad-spectrum protection against influenza A viruses with pandemic potential in mice. Virology, in press. [DOI] [PMC free article] [PubMed]
- 26.Leroux-Roels, I., A. Borkowski, T. Vanwolleghem, M. Drame, F. Clement, E. Hons, J. M. Devaster, and G. Leroux-Roels. 2007. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 370:580-589. [DOI] [PubMed] [Google Scholar]
- 27.Lewis, D. J., Z. Huo, S. Barnett, I. Kromann, R. Giemza, E. Galiza, M. Woodrow, B. Thierry-Carstensen, P. Andersen, D. Novicki, G. Del Giudice, and R. Rappuoli. 2009. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One 4:e6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipatov, A. S., E. A. Govorkova, R. J. Webby, H. Ozaki, M. Peiris, Y. Guan, L. Poon, and R. G. Webster. 2004. Influenza: emergence and control. J. Virol. 78:8951-8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazanec, M. B., C. S. Kaetzel, M. E. Lamm, D. Fletcher, and J. G. Nedrud. 1992. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. U. S. A. 89:6901-6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosca, F., E. Tritto, A. Muzzi, E. Monaci, F. Bagnoli, C. Iavarone, D. O'Hagan, R. Rappuoli, and E. De Gregorio. 2008. Molecular and cellular signatures of human vaccine adjuvants. Proc. Natl. Acad. Sci. U. S. A. 105:10501-10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navabi, H., B. Jasani, A. Reece, A. Clayton, Z. Tabi, C. Donninger, M. Mason, and M. Adams. 2009. A clinical grade poly I:C-analogue (Ampligen) promotes optimal DC maturation and Th1-type T cell responses of healthy donors and cancer patients in vitro. Vaccine 27:107-115. [DOI] [PubMed] [Google Scholar]
- 32.Neuberger, M. S., and K. Rajewsky. 1981. Activation of mouse complement by monoclonal mouse antibodies. Eur. J. Immunol. 11:1012-1016. [DOI] [PubMed] [Google Scholar]
- 33.Nichol, K. L., and J. J. Treanor. 2006. Vaccines for seasonal and pandemic influenza. J. Infect. Dis. 194(Suppl. 2):S111-S118. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson, K. G., A. E. Colegate, A. Podda, I. Stephenson, J. Wood, E. Ypma, and M. C. Zambon. 2001. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 357:1937-1943. [DOI] [PubMed] [Google Scholar]
- 35.Renegar, K. B., P. A. Small, Jr., L. G. Boykins, and P. F. Wright. 2004. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J. Immunol. 173:1978-1986. [DOI] [PubMed] [Google Scholar]
- 36.Riedl, K., R. Riedl, A. von Gabain, E. Nagy, and K. Lingnau. 2008. The novel adjuvant IC31 strongly improves influenza vaccine-specific cellular and humoral immune responses in young adult and aged mice. Vaccine 26:3461-3468. [DOI] [PubMed] [Google Scholar]
- 37.Robinson, R. A., V. T. DeVita, H. B. Levy, S. Baron, S. P. Hubbard, and A. S. Levine. 1976. A phase I-II trial of multiple-dose polyriboinosic-polyribocytidylic acid in patients with leukemia or solid tumors. J. Natl. Cancer Inst. 57:599-602. [DOI] [PubMed] [Google Scholar]
- 38.Shen, E., L. Li, L. Li, L. Feng, L. Lu, Z. Yao, H. Lin, and C. Wu. 2007. PIKA as an adjuvant enhances specific humoral and cellular immune responses following the vaccination of mice with HBsAg plus PIKA. Cell. Mol. Immunol. 4:113-120. [PubMed] [Google Scholar]
- 39.Stahl-Hennig, C., M. Eisenblatter, E. Jasny, T. Rzehak, K. Tenner-Racz, C. Trumpfheller, A. M. Salazar, K. Uberla, K. Nieto, J. Kleinschmidt, R. Schulte, L. Gissmann, M. Muller, A. Sacher, P. Racz, R. M. Steinman, M. Uguccioni, and R. Ignatius. 2009. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 5:e1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephenson, I., K. G. Nicholson, A. Colegate, A. Podda, J. Wood, E. Ypma, and M. Zambon. 2003. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine 21:1687-1693. [DOI] [PubMed] [Google Scholar]
- 41.Suguitan, A. L., Jr., J. McAuliffe, K. L. Mills, H. Jin, G. Duke, B. Lu, C. J. Luke, B. Murphy, D. E. Swayne, G. Kemble, and K. Subbarao. 2006. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 3:e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Treanor, J. J., J. D. Campbell, K. M. Zangwill, T. Rowe, and M. Wolff. 2006. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 354:1343-1351. [DOI] [PubMed] [Google Scholar]
- 43.Trumpfheller, C., M. Caskey, G. Nchinda, M. P. Longhi, O. Mizenina, Y. Huang, S. J. Schlesinger, M. Colonna, and R. M. Steinman. 2008. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc. Natl. Acad. Sci. U. S. A. 105:2574-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unkeless, J. C., and H. N. Eisen. 1975. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J. Exp. Med. 142:1520-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wack, A., B. C. Baudner, A. K. Hilbert, I. Manini, S. Nuti, S. Tavarini, H. Scheffczik, M. Ugozzoli, M. Singh, J. Kazzaz, E. Montomoli, G. Del Giudice, R. Rappuoli, and D. T. O'Hagan. 2008. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine 26:552-561. [DOI] [PubMed] [Google Scholar]
- 46.Wagner, D. K., M. L. Clements, C. B. Reimer, M. Snyder, D. L. Nelson, and B. R. Murphy. 1987. Analysis of immunoglobulin G antibody responses after administration of live and inactivated influenza A vaccine indicates that nasal wash immunoglobulin G is a transudate from serum. J. Clin. Microbiol. 25:559-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wareing, M. D., and G. A. Tannock. 2003. Route of administration is the prime determinant of IgA and IgG2a responses in the respiratory tract of mice to the cold-adapted live attenuated influenza A donor strain A/Leningrad/134/17/57. Vaccine 21:3097-3100. [DOI] [PubMed] [Google Scholar]
- 48.Winzler, C., P. Rovere, M. Rescigno, F. Granucci, G. Penna, L. Adorini, V. S. Zimmermann, J. Davoust, and P. Ricciardi-Castagnoli. 1997. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med. 185:317-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong, J. P., H. Yang, L. Nagata, M. Kende, H. Levy, G. Schnell, and K. Blasetti. 1999. Liposome-mediated immunotherapy against respiratory influenza virus infection using double-stranded RNA poly ICLC. Vaccine 17:1788-1795. [DOI] [PubMed] [Google Scholar]
- 50.Zeng, W., S. Ghosh, Y. F. Lau, L. E. Brown, and D. C. Jackson. 2002. Highly immunogenic and totally synthetic lipopeptides as self-adjuvanting immunocontraceptive vaccines. J. Immunol. 169:4905-4912. [DOI] [PubMed] [Google Scholar]
- 51.Zhu, F. C., H. Wang, H. H. Fang, J. G. Yang, X. J. Lin, X. F. Liang, X. F. Zhang, H. X. Pan, F. Y. Meng, Y. M. Hu, W. D. Liu, C. G. Li, W. Li, X. Zhang, J. M. Hu, W. B. Peng, B. P. Yang, P. Xi, H. Q. Wang, and J. S. Zheng. 2009. A novel influenza A (H1N1) vaccine in various age groups. N. Engl. J. Med. 361:2414-2423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.