Abstract
The human cytomegalovirus (HCMV) IE86 protein is essential for HCMV replication due to its ability to transactivate critical viral early promoters. In the current study, we performed a comprehensive mutational analysis between amino acids (aa) 535 and 545 of IE86 and assessed the impact of these mutations on IE86-mediated transcriptional activation. Using transient assays and complementing analysis with recombinant HCMV clones, we show that single amino acid mutations differentially impair the ability of IE86 to mediate transactivation of essential early gene promoters. The conserved tyrosine at amino acid 544 is critical for activation of the UL54 promoter in vitro and in the context of the viral genome. In contrast, mutation of the proline at position 535 disrupted activation of the UL54 promoter in transient assays but displayed activity similar to that of wild-type (WT) IE86 when assessed in the genomic context. To examine the underlying mechanism of this differential effect, glutathione S-transferase (GST) pulldown assays were performed, revealing that Y544 is critical for binding to the TATA binding protein (TBP), suggesting that this interaction is likely necessary for the ability of IE86 to activate the UL54 promoter. In contrast, mutation of either P535 or Y544 disrupted activation of the UL112-113 promoter both in vitro and in vivo, suggesting that interaction with TBP is not sufficient for IE86-mediated activation of this early promoter. Together, these studies demonstrate that IE86 activates early promoters by distinct mechanisms.
Human cytomegalovirus (HCMV), a member of the Herpesviridae, is a ubiquitous and opportunistic pathogen that under conditions of immune immaturity or suppression is a major cause of disease and mortality (39). The first subset of viral genes to be expressed upon HCMV infection are the immediate early (IE) gene products (39). The major IE (MIE) proteins IE72 and IE86 are produced as a result of differential splicing from the UL122-123 gene region (39). Expression of these gene products is controlled by the MIE promoter (MIEP), which in turn is regulated by a complex interplay between viral and cellular proteins (37, 58). For example, the IE72 and pp71 viral proteins enhance activation of the MIEP, leading to increased MIE gene products (10, 32, 60). In contrast, IE86 negatively regulates expression of the MIE gene products through direct binding to the cis repression sequence (CRS), located between the TATA box and the transcription start signal of the MIEP (9, 31, 46, 47). It is thought that IE86 inhibits transcription from the MIEP by blocking RNA polymerase recruitment and preventing assembly of the preinitiation complex (28).
The 579-amino acid IE86 protein encoded by the IE2 transcript is a potent transactivator of viral and cellular genes and is essential for virus replication (17, 36, 59). The essential nature of the IE86 protein is due in part to a requirement for IE86 to activate the promoters of viral genes required for replication, including the UL112-113 replication proteins and the UL54 DNA polymerase (17, 23, 41, 51, 55, 57). Early promoter activation by IE86 is thought to be mediated by direct binding of IE86 to DNA and/or protein interactions with cellular and viral transcription factors (14, 59). For example, IE86 interacts in vitro with components of the basal transcription machinery and transcriptional regulators, such as TATA binding protein (TBP), SP1, and CREB (7, 15, 27, 50, 59, 62). It has been suggested that IE86 mediates transcriptional activation at a step following TBP recruitment to the promoter (24). However, these analyses relied on artificial recruitment of TBP to a generic promoter construct. In a series of elegant studies, Lukac et al. demonstrated that IE86 activation required a functional TATA box and determined that IE86 had TAF-like functions (34, 35). These studies suggest that the interaction of IE86 with TBP and other basal promoter factors may be critical for transcriptional activation by this protein. In addition, IE86 interacts with histone modifiers, such as P/CAF and HDAC3, suggesting that the modulation of chromatin modification facilitates transcriptional activation by IE86 (6, 43, 48). Indeed, histone acetyltransferase (HAT) recruitment was found to be critical for the activation of viral early promoters (6). The IE86 protein also regulates early gene transcription by direct binding to DNA. For example, the promoter of the UL112-113 gene is regulated by an ATF/CREB binding site and an IE86 binding site (2, 51, 52). Deletion of the IE86 binding site causes a reduction of IE86-mediated transactivation of this promoter during infection (50, 51). The IE86 protein possesses a number of additional functions that are likely important for efficient virus replication, including the regulation of cell cycle progression, inhibition of host DNA synthesis, and modulation of cytokine expression (59).
As a result of the essential role of IE86 in virus replication, numerous studies have been performed to identify functional domains of the IE86 protein and assess the biological significance of these regions (reviewed in reference 59). However, most of these analyses relied on the use of large-scale deletions within the IE86 protein, raising the possibility that alterations in protein structure result that complicate interpretation of the data. Despite this caveat, a core domain in the carboxy-terminal region of IE86 from amino acids 450 to 544 that overlaps regions important for DNA binding, dimerization, and interaction with the basal transcription factors TBP and TFIIB has been identified (3, 59). Mutations within this domain disrupt both transactivation and autoregulation by IE86, suggesting that these two properties are linked (3). In support of this, research in our laboratory showed that insertion of 4 amino acids adjacent to the alanine residue at position 540 of IE86 caused an 8-fold decrease in activation of the UL54 promoter in transient assays, with a corresponding 10-fold increase in MIEP activation (57). Similarly, studies by White et al. examined mutations or deletions in IE86 between amino acids 356 to 359, 427 to 435, and 505 to 511 within HCMV bacterial artificial chromosome (BAC) constructs (61). The resultant viruses were nonviable, correlating with a lack of activation of the UL44 and UL112-113 early genes during infection as well as a loss of repression of the MIEP (61). However, this study lacked corresponding data regarding the functional properties of IE86 that were impacted by these specific mutations. A more recent study showed that simultaneous mutation of amino acids 535 and 537 within IE86 results in a loss of early gene activation, but the ability of IE86 to autoregulate the MIE promoter was retained (44), suggesting that in some cases, these properties are distinguishable. Further, this analysis demonstrated that the ability of IE86 to associate with viral early promoters correlated with transcriptional activation (44). However, this study did not directly assess the effects of single amino acid mutations on transcriptional regulation. Thus, it is possible that more than one functional property of IE86 was disrupted by the mutation. In the current work, we have performed a mutational analysis of 10 individual amino acids within the core domain to assess the impact on transcriptional activation of viral early promoters involved in virus replication. These studies reveal that in all cases, activation correlated directly with autoregulation by IE86. In addition, assessment of two specific mutants in the context of the viral genome demonstrated differential activation of the UL54 and UL112-113 early promoters, suggesting that more than one mechanism is involved in the regulation of these essential early genes by IE86. Further analysis showed that the interaction of IE86 with TBP appears to be critical for the activation of the UL54 early promoter.
MATERIALS AND METHODS
Cells and plasmids.
Primary human foreskin fibroblasts (HFF) were obtained from Clonetics Corporation and maintained as previously described (53). HeLa cells were obtained from ATCC and grown in Iscove's medium with 10% fetal bovine serum and 1% penicillin and streptomycin.
The pSVH plasmid used for expression of the MIE gene products (12) was modified by deletion of a KpnI restriction enzyme site to generate pSVHk. The pMCRS86 plasmid that contains the IE86 cDNA sequence under the control of the MIE promoter lacking the cis repression sequence was kindly provided by J. A. Nelson (Oregon Health Sciences University). The cDNA construct was corrected for IE86 mutations by the replacement of a SmaI-Bsu36I fragment from pSVHk as previously described (4). Mutagenesis of the IE86 coding sequence in the pSVHk and pMCRS86 constructs was achieved using the QuikChange site-directed PCR mutagenesis kit XL (Stratagene). Primer sequences for individual mutations are shown in Table 1. All constructs were confirmed by DNA sequencing using the BigDye Terminator version 3.1 cycle sequencing kit and analyzed on an ABI Primer 3130 genetic analyzer (Applied Biosystems). The pSVOd plasmid used as an empty vector control has been described previously (38). The reporter plasmids pMIEP-Luc andpUL54-Luc have also been described elsewhere (22, 53). The pUL112-113-Luc reporter plasmid was generated by PCR amplification of p729CAT (55) kindly provided by D. H. Spector (University of California, San Diego, CA) and subsequent cloning of the HindIII-to-BglII promoter fragment into the pGL3-Basic plasmid (Promega).
TABLE 1.
Primers used for site-directed PCR mutagenesis
| Primer | Primer sequencea | Mutation |
|---|---|---|
| P535A-2 | 5′-GTGGGTTCATGCTGGCTATCTACGAGACGGC-3′ | Pro 535→Ala |
| I536A | 5′-GGTTCATGCTGCCTGCCTACGAGACGGCCA-3′ | Ile 536→Ala |
| Y537A | 5′-CATGCTGCCTATCGCCGAGACGGCCACG-3′ | Try 537→Ala |
| E538A | 5′-GTTCATGCTGCCTATCTACGCGACGGCCACGAAGG-3′ | Asn 538→Ala |
| T539A | 5′-CCTATCTACGAGGCGGCCACGAAGGC-3′ | Try 539→Ala |
| A540V | 5′-TATCTACGAGACGGTCACGAAGGCCTACGC-3′ | Ala 540→Val |
| T541A | 5′-CTATCTACGAGACGGCCGCGAAGGCCTA-3′ | Try 541→Ala |
| K542A | 5′-TATCTACGAGACGGCCACGGCGGCCTACGC-3′ | Asn 542→Ala |
| A543V | 5′-GGCCACGAAGGTCTACGCCGTGGG-3′ | Ala 543→Val |
| Y544A | 5′-CCACGAAGGCCGCCGCCGTGGGGC-3′ | Try 544→Ala |
| A545V | 5′-CGAAGGCCTACGTCGTGGGGCAGTTTGA-3′ | Ala 545→Ala |
Only forward primer sequence shown for each primer set, and mutations in IE2 are underlined.
Luciferase assays and Western blot analysis.
HFF cells were transfected with the indicated plasmids using either Lipofectamine 2000 (Invitrogen) or the DEAE-dextran technique (12, 42). At 48 h after transfection, cell extracts were harvested and assessed for luciferase activity as described previously (53). Significance was determined by a P value of <0.05 using a two-tailed Student t test. Western blot analysis of the cell extracts was performed using either the polyclonal peptide antibody 1218 that recognizes the IE86 protein (56) or the MAB810 monoclonal antibody (Millipore) essentially as previously described (11). In some cases, IRDye-labeled secondary antibodies (IRDye 800CW goat anti-mouse IgG; Licor) were used, and the proteins detected using the Odyssey Infrared imaging system (Licor). Protein loading was controlled for by Western analysis using the actin antibody (A1978; Sigma).
RNA extraction and real-time reverse-transcription (RT)-PCR.
HFFs were transfected with 10 μg of total plasmid DNA by the DEAE-dextran method (12). Samples were harvested 48 h posttransfection using the RNeasy kit protocol (Qiagen). Cells transfected with 10 μg of BAC constructs by the calcium phosphate method (26) were harvested at the indicated times, and RNA was isolated using Trizol reagent (Invitrogen). In either case, samples were treated with DNase I (Qiagen), and cDNA was generated using 1 to 5 μg of total RNA primed with 250 ng of random hexamers (Superscript; Invitrogen). Real-time PCR was then performed using the iQ SYBR green supermix (Invitrogen) using a Bio-Rad iCycler system and analyzed using the PFAFFL mathematical model (45). Primers used to amplify the IE2, UL112-113, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) genes have been described previously (61). The UL54 gene was amplified using the following primers: forward, 5′-CAGCCGCTGCAGAACCTCTTTC; and reverse 5′-CTGCTCGTTGGTGTAAGGCG.
HCMV BAC mutagenesis.
BAC constructs were generated essentially as previously described (13, 40). To minimize the possibility of revertants, mutagenesis of IE86 was repeated using the following primer sequences (mutations in IE86 are underlined, and forward primers shown): P535A-3, GTG GGT TCA TGC TGG CGA TCT ACG AGA CGG, and Y544A-2, CCA CGA AGG CCG CGG CCG TGG GGC. Deletion of the HCMV UL122-123 exon 5 region coding for IE86-specific sequences was accomplished as previously described (13, 40) using the following primers containing a 20-bp overhang with homology to the C terminus of IE86 between amino acids 449 and 579: 5′-CCA TGG CCC TCT CCA CTC CCT TCC TCA TGG AGC ACA CCA TGG CTC TTG TTG GCT AGT GCG TA-3′ and 5′-CAC TAT GTA CAA GAG TCC ATG TCT CTC TTT CCA GTT TTT CTC TGC CAG TGT TAC AAC CAA-3′. This PCR fragment was gel purified and transformed into electro- and recombination-competent DY380 cells containing the HCMV Towne-BAC to generate the HCMV ΔExon5 BAC. Positive clones containing successful deletion of amino acids 449 to 579 and replacement of this region with the kanamycin resistance marker were confirmed by growth on LB agar plates containing kanamycin and chloramphenicol, PCR analysis, and restriction enzyme digestion.
To generate pIE2-Zeo for homologous recombination with the HCMV ΔExon5 BAC, the Zeocin (Zeo) gene was first amplified from pCMV/Zeo (Invitrogen) using BamHI-LoxP-Zeo-LoxP-BglII forward and reverse primers 5′-AGA TCT ATA ACT TCG TAT AGC ATA CAT TAT ACG AAG TTA TGG AAC GGA CCG TGT TGA C-3′ and 5′-GGA TCC ATA ACT TCG TAT AGC ATA CAT TAT ACG AAG TTA TCA AGT TTC GAG GTC GAG GTG-3′, respectively, and cloned into pCR4-TOPO (Invitrogen). Subsequently, wild-type (WT) or mutant IE86 fragments were amplified from pMCRS86 using PstI-435-IE2-579-BamHI primers 5′-CTG CAG AAC CTG GCC CTC TCC ACT C-3′ and 5′-GGA TCC ACT TAC TGA GAC TTG TTC CTC AGG TCC-3′ (restriction sites are underlined), and the resultant fragment was cloned into the pZeo-TOPO construct between the PstI and BamHI sites. The IE2-Zeo region was then amplified from pIE2-Zeo using the following primers: 5′ CAA CCT GGC CCT CTC ACT CCC TTC CTC ATG GAG CAC ACC ATG CCC GTG ACA CAT 3′ and 5′ CGG GGA ATC ACT ATG TAC AAG AGT CCA TGT CTC TCT TTC CAG TTT TTC AGA TCT ATA ACT TCG TAT AAT G 3′. This PCR fragment was gel purified and transformed into electro- and recombination-competent DY380 cells containing the HCMV ΔExon5 BAC.
Positive clones were confirmed by growth on LB agar plates containing Zeocin and chloramphenicol, PCR analysis, Southern blot analysis, and restriction enzyme digestion. Southern blot analysis was performed as previously described (8). The following primers were used for PCR confirmation of HCMV BAC constructs: DS IE2 primer, 5′ GAT GTC TCG CAG GGT GGG TAG ATG 3′; US IE2 primer, 5′ GCA TGT TCC GCA ACA CCA ATC G 3′; internal IE2 primer 1, 5′ AAG ACC TGG ACA CCC TGA GCC TG 3′; and internal IE2 primer 2, 5′ CAG GCT CAG GGT GTC CAG GTC TTC 3′.
Transfection of BAC DNA and growth curve analysis.
Large-scale BAC DNA purifications were performed using Clontech's Nucleobond BAC 100 kit according to the manufacturer's instructions. For BAC transfections in HFFs, 10 μg of BAC DNA and 1 μg of pCMVpp71 DNA (32) were transfected into HFF by the calcium phosphate method (26). A plasmid expressing Cre recombinase (1 μg) was also transfected with the BACs in DNA and virus replication assays (63). Medium was changed every 4 or 5 days until plaque outgrowth occurred. Cells were monitored for 3 or more weeks for plaque formation. Reconstituted virus was harvested from the supernatant of BAC-transfected cells when 90 to 100% of cells displayed cytopathic effects (CPE). Viral DNA replication was assessed by harvesting BAC-transfected cells at various time points using Trizol reagent (Invitrogen). Virus replication assays were performed as described in Lorz et al. (33). Briefly, 20 μl of viral supernatant was incubated with 160 μl of 10 mg/ml proteinase K for 1 h at 56°C. DNA was denatured by incubation at 95°C for 5 min. Real-time PCR analysis was performed as described above using equal amounts of total DNA (100 ng). The primer sequences used for amplification of gB have been described previously (44).
GST pulldown assays.
Wild-type and mutant IE86 proteins were generated using the TnT coupled reticulocyte lysate system (Promega). A construct designed to express the TATA-binding protein (TBP) as a GST fusion protein was generated by amplifying the TBP sequence from cDNA generated from HFFs using the following primers: hTBP-f, 5′-ATGGATCAGAACAACAGCCTGCC; and hTBP-r, 5′-GCCATTACGTCGTCTTCCTGAATC. The resultant fragment was subcloned as an EcoRI fragment into pGEX-6P, and the fidelity of the construct confirmed by DNA sequencing. GST fusion proteins were isolated from Escherichia coli BL21 cultures by sonication in buffer A (20 mM HEPES [pH 7.2], 100 mM KCl, 0.2 mM EDTA, 20% glycerol, 1% Triton X-100) followed by purification using glutathione Sepharose (GE Healthcare). Binding assays were performed in binding buffer (25 mM HEPES [pH 7.5], 12.5 mM magnesium chloride, 20% glycerol, 0.1% NP-40, 150 mM potassium chloride, 0.15 mg/ml bovine serum albumin, 1 mM dithiothreitol [DTT]) for 2 h at 4°C. Complexes were washed six times in NETN buffer (20 mM Tris [pH 8.0], 100 mM NaCl, 0.5% NP-40, 0.1 mM EDTA), and the bound proteins resolved by SDS-PAGE on 12.5% acrylamide gels. Images were visualized using a Typhoon 9410 variable-mode imager. Protein quantification was performed using ImageQuant TL software.
RESULTS
Mutational analysis of the IE86 core region.
Previous studies in our laboratory determined that the insertion of 4 amino acids at position 540 in the carboxy terminus of IE86 resulted in the loss of both MIE promoter repression and early promoter activation (57), suggesting that this region was critical for IE86 functions. Later studies identified a “core” domain within IE86 from amino acid 450 to 544 that is essential for the ability of IE86 to regulate gene expression (3). Consistent with this notion, this region of IE86 is highly conserved across cytomegaloviruses (43). To more closely address the role of this domain in IE86-mediated transcriptional regulation, we performed a comprehensive mutational analysis between amino acids 535 and 545. Each residue within this region was individually mutated to either an alanine or valine, and the ability of the IE86 mutant in the context of the entire IE gene region to regulate two essential viral early promoters was assessed (Fig. 1 A and B). This analysis revealed that mutations of amino acids 535, 536, 537, 543, and 544 resulted in a significant decrease in the ability of IE86 to activate the UL112-113 and UL54 promoters compared to the wild-type construct. These results are consistent with the role of the core region of IE86 in the transcriptional regulation of viral early genes (3). Interestingly, mutation of the threonine residue at position 541 demonstrated a significant decrease in UL54 promoter activation but had minimal effects on the ability to regulate the UL112-113 promoter. This finding demonstrates that a single amino acid mutation may have differential effects on early gene promoter activation, suggesting that individual early promoters may be regulated by distinct mechanisms.
FIG. 1.
Effect of IE86 mutations on UL54, UL112-113, and MIE promoter regulation. The pUL54-Luc (A), pUL112-113-Luc (B), or pMIEP-Luc (C) reporter construct was cotransfected into primary fibroblasts with the pSVHk vector that expresses either wild-type IE86 or the indicated mutant in the context of the MIE gene region. Cell extracts were assessed for luciferase activity 48 h after transfection. Data are the average and standard deviation (SD) of results of a minimum of two experiments performed in duplicate, normalized to promoter activity in the presence of wild-type IE86. (D) Total protein was harvested at 48 h posttransfection and separated on a 10% SDS-PAGE gel. Western blot analysis was performed using a peptide antibody that recognizes the IE86 protein. An antibody to actin was included to normalize for protein loading. Symbols: *, significantly different from wild type, P < 0.05; C, level of promoter activity in the presence of the pSV0d empty vector control plasmid.
The IE86 protein regulates its own expression by binding to the CRS of the MIE promoter (21, 31, 46). To assess the extent of correlation between IE86 early promoter activation and autoregulatory functions, we determined the effects of the IE86 mutations on MIEP activity (Fig. 1C). This analysis revealed that mutations of amino acid 535, 536, 537, 543, or 544 resulted in a loss in IE86 autorepressive properties. Indeed, these mutants exhibited a significantly greater enhancement of MIEP promoter activity than might be expected from a simple loss of repression, suggesting that mutation of amino acid 535, 536, 537, 543, or 544 leads to MIEP activation by IE86. These results also indicate that mutations which disrupt transactivation of the UL112-113 and UL54 early promoters likely disrupt the ability of IE86 to autoregulate the MIEP. To confirm the effects of these mutations on IE86 autorepression, we assessed IE86 protein levels in the transfected cells using an antibody that specifically recognizes the IE86 protein (Fig. 1D). This analysis shows that the wild-type IE86 protein expressed in the context of the entire IE gene region is barely detectable, consistent with previous analysis using this antibody (56). In contrast, the majority of mutants that exhibited enhanced MIEP activation also expressed higher levels of the IE86 protein. In particular, mutation of either amino acid 537 or 544 resulted in dramatically increased expression of IE86. However, in some cases the level of IE86 protein did not correlate well with the observed transcriptional activity. For example, mutation of amino acid 543 results in enhanced activation of the MIEP but minimal increases in IE86 protein levels. These findings suggest that other IE proteins expressed by the pSVH construct may influence the transcriptional activity of IE86 (56).
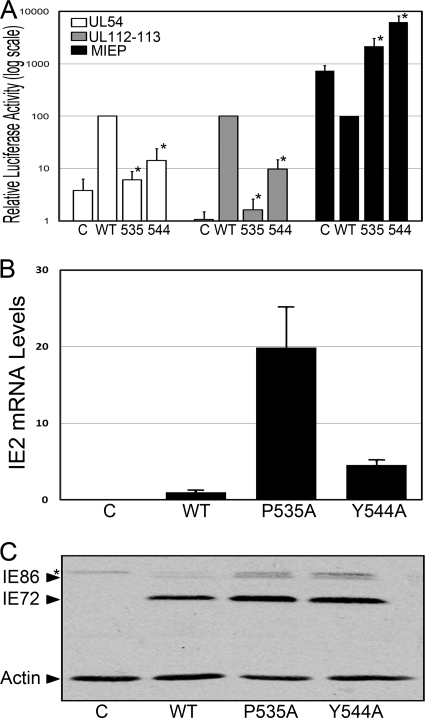
In order to determine the effects specific for the IE86 protein, we assessed the IE86 mutants in the pMCRS86 vector that expresses IE86 cDNA under the control of a mutated MIE promoter designed to prevent autoregulation of the transfected plasmid (Fig. 2). To refine our analysis, these studies were performed with two representative mutants, P535A and Y544A, that correspond to highly conserved residues (44). Consistent with our examination in the context of the entire genomic region, mutations in IE86 at amino acids 535 and 544 impaired transcriptional regulation of the UL54 and UL112-113 promoters in the absence of other viral gene products (Fig. 2A). These findings suggest that the changes in early promoter regulation were due to the mutations in the IE86 protein. Significantly, analysis of the ability of the P535A and Y544A mutants to regulate the MIE promoter revealed not just a lack of repression but an enhancement of promoter activity, suggesting that multiple mechanisms likely contribute to the regulation of the MIEP by the IE86 protein.
FIG. 2.
Effect of IE86 mutations on promoter activation, IE2 mRNA, and IE86 protein expression. (A) The pMIEP-Luc, pUL54-Luc, or UL112-113-Luc reporter construct was cotransfected into primary fibroblasts with pMCRS86 vector expressing either wild-type IE86 or the indicated mutant. Cell extracts were assessed for luciferase activity 48 h after transfection. Data are the average and SD of results of a minimum of two experiments performed in duplicate, normalized to promoter activity in the presence of wild-type IE86, and presented on a log scale to assist in the comparison of promoter activation levels. Symbols: *, significantly different from wild type, P < 0.05; C, level of promoter activity in the in the presence of the pSV0d empty vector control plasmid. (B and C) Primary fibroblasts were transfected with the pSVHk vector expressing either wild-type IE86 or the indicated mutant. (B) Total RNA was harvested at 48 h posttransfection, and real-time RT-PCR analysis performed to determine IE2 mRNA levels. Analyses were performed in duplicate, and relative expression normalized to GAPDH mRNA levels was expressed as mean ± SD. (C) Total protein was harvested at 48 h posttransfection and separated on a 12.5% SDS-PAGE gel. Western blot analysis was performed using an antibody that recognizes IE72 and IE86. An antibody to actin was included to normalize for protein loading. Symbol: *, nonspecific band.
To determine whether the mutations introduced affected IE86 protein stability, we assessed the accumulation of IE2 mRNA and IE86 protein levels in transfected cells by real-time RT-PCR and Western blot analysis, respectively. We observed a 20-fold enhancement of IE2 transcript levels over that observed with the wild-type construct in cells transfected with the IE86 P535A mutant and a 5-fold enhancement with the IE86 Y544A mutant (Fig. 2B). These findings are consistent with the lack of autorepression observed with these two mutants (Fig. 2A). Western blot analysis revealed that both mutants displayed enhanced IE86 protein levels compared to the wild type-construct (Fig. 2C). However, despite a 4-fold higher level of the IE2 P535A mutant transcript compared to the IE2 Y544A mutant, we observed similar levels of the IE86 protein, suggesting that the presence of a proline residue at position 535 may be important to maintain IE86 protein structure and/or stability. However, we cannot rule out the possibility that other posttranscriptional mechanisms, such as translational efficiency, contribute to the altered ratio of IE86 transcript and protein levels.
Assessment of IE86 mutants in the context of the viral genome.
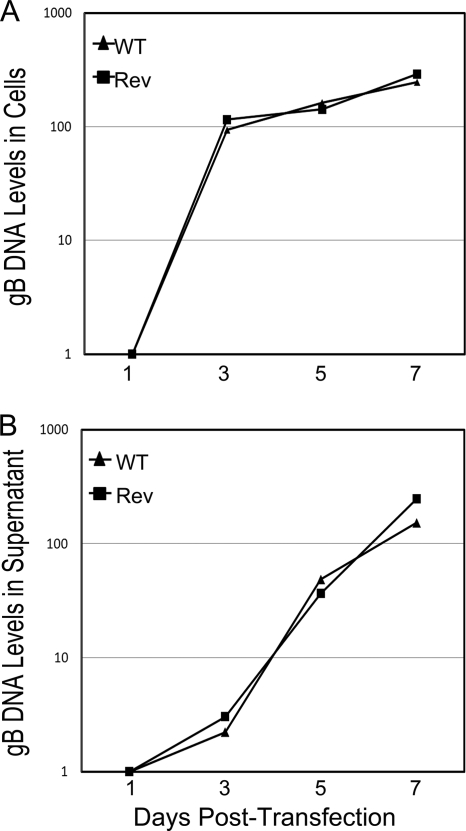
To more closely investigate the transcriptional regulatory properties of IE86 that are essential for the activation of early genes, we assessed the effect of mutations at amino acids 535 and 544 on transcriptional regulation in the context of the viral genome. To accomplish this, we generated equivalent mutations in the HCMV Towne BAC (36). The constructs were confirmed by PCR, Southern blot analysis, and restriction enzyme digestion (Fig. 3). During the generation of these constructs, additional sequences not present in the original HCMV Towne BAC were introduced. To ensure that these sequences did not interfere with viral growth, we compared the levels of viral DNA synthesis after transfection of the WT BAC and a WT revertant BAC construct (WT-Rev) into primary fibroblasts using primers that amplify the gB region of the genome (Fig. 4 A). These studies showed identical levels of viral DNA synthesis for the WT and WT-Rev BAC constructs. We also performed single-step growth curve analysis of virus recovered after BAC transfections (33) and confirmed that there was no significant difference in replication between these two viruses (Fig. 4B). These results demonstrate that the additional sequences inserted into the BAC constructs did not affect the replication of the HCMV BAC-derived virus.
FIG. 3.
Analysis of mutant HCMV BACs. (A) Schematic of the MIE gene region of the mutant BACs. The Zeocin resistance cassette containing a SmaI site is represented by the gray box. The location of primer sequences used to confirm appropriate recombination are also indicated by arrows. (B) Restriction enzyme analysis of HCMV BACs was performed using SalI and HindIII. Following digestion, the fragmented DNA was run on a 0.8% agarose gel. (C) PCR analysis of HCMV BACs was performed using the indicated primers, and the gel fragments were separated on a 1.2% agarose gel. The HCMV WT BAC results in an approximately 0.6-kb fragment, with the Zeocin resistance cassette increasing the size of this fragment to 1.1 kb. (D) Southern blot analysis of HCMV BACs digested with SmaI showing the presence of the additional SmaI site in the WT-Rev, P535A, and Y544A BAC constructs due to the presence of the Zeocin resistance cassette.
FIG. 4.
Replication kinetics of HCMV WT and WT-Rev virus. WT-Rev and mutant HCMV BACs were transfected into primary fibroblasts by calcium phosphate transfections. (A) DNA replication was assessed by isolating genomic DNA at the indicated times following transfection and assessment of gB DNA levels by real time PCR. (B) Growth kinetics were assessed by isolating genomic DNA from the culture supernatant at the indicated times following transfection and assessment of gB DNA levels by real-time PCR.
Our analysis using transient transfection assays suggested that mutations at either amino acid 535 or 544 inhibited the ability of IE86 to activate the UL54 and UL112-113 promoters (Fig. 1). Since the products of both of these genes are essential for virus replication, we assessed the capability of BAC constructs containing these mutations to yield viable virus. Following transfection of the BAC DNA into HFFs, cultures were monitored and maintained for up to 6 weeks, with no indication of virus replication. These experiments were performed multiple times, and efficient delivery of the BAC DNA into the cells was confirmed by PCR. These results confirm that the mutations in IE86 at amino acids 535 and 544 prevent virus replication.
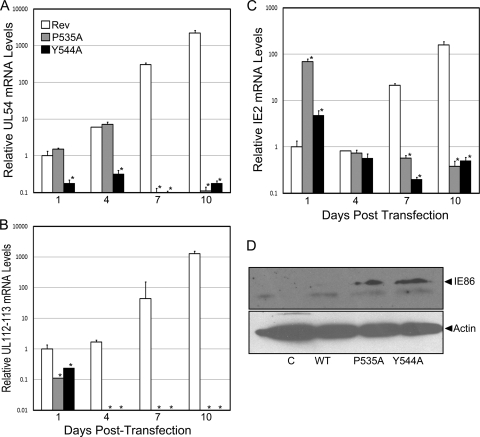
To determine the stage at which virus replication was blocked, UL54 and UL112-113 mRNA transcript levels in BAC-transfected cells were assessed by real-time RT-PCR (Fig. 5). Surprisingly, cells transfected with the BAC containing the mutation of proline 535 contained levels of the UL54 transcript similar to that observed with the WT-Rev construct at days 1 and 4 posttransfection (Fig. 5A), suggesting that the P535A mutation within IE86 has little effect on the ability of the UL54 promoter to be activated in this context. This result was in marked contrast to our in vitro analysis showing that IE86 containing the P535A substitution is unable to activate the UL54 promoter in transient assays (Fig. 1). At later times after transfection, the levels of UL54 transcripts in cells transfected with the IE86 P535A mutant BAC declined significantly compared to that of WT-Rev, consistent with the null phenotype of this virus. Analysis of the substitution of the tyrosine at position 544 in IE86 revealed that this mutation abrogated activation of the UL54 promoter (Fig. 5A), in line with the activity of this mutant when assessed in transient assays (Fig. 1). These studies therefore revealed distinct differences in the abilities of recombinant viruses containing mutations in IE86 at amino acid 535 and 544 to regulate the UL54 early gene, despite similar phenotypes when assessed in transient transfection studies. When UL112-113 transcript levels were assessed, we observed minimal activation of this gene with either mutant at any time after transfection of the BAC construct (Fig. 5B). These data suggest that both mutations disrupt a functional property of IE86 that is essential for UL112-113 promoter activation. Further, they suggest that efficient expression of the UL112-113 gene region is at least one determinant of the outcome of HCMV infection.
FIG. 5.
Regulation of viral gene expression in BAC-transfected cells. Total RNA was isolated from BAC-transfected cells at the indicated times posttransfection and analyzed by real-time RT-PCR using primers specific for the UL54 (A), UL112-113 (B), and IE2 (C) transcript levels. Data are expressed as mean ± SD from results of two independent experiments performed in duplicate. Symbol: *, significantly different from wild type, P < 0.05. (D) Total protein was harvested at 48 h posttransfection and separated on a 10% SDS-PAGE gel. Western blot analysis was performed using a peptide antibody that recognizes the IE86 protein. An antibody to actin was included to normalize for protein loading.
We next determined the effects of mutations in IE86 at amino acids 535 and 544 on the ability to autorepress the MIEP. Following transfection of the wild-type revertant BAC, IE2 transcript levels remain stable at 1 and 4 days posttransfection and then begin to increase at 7 and 10 days postinfection due to the progression of virus infection (Fig. 5C). We initially observed, similar to results observed in transient transfection assays (Fig. 1C), a lack of MIE promoter repression in cells transfected with BAC DNA containing IE86 mutations at amino acid 535 or 544, resulting in a 65- or 4-fold increase in IE2 mRNA levels, respectively (Fig. 5C). Subsequently, the level of IE2 transcripts gradually declined, consistent with the block in virus replication observed with these two mutations. To confirm that the increase in IE2 transcripts correlated with IE86 protein levels, we performed Western blot analysis on cells harvested at 2 days posttransfection (Fig. 5D). This analysis showed enhanced levels of the IE86 protein in cells transfected with the BAC DNA containing IE86 mutations at amino acid 535 or 544 and is consistent with the in vitro analysis of these mutants (Fig. 1 and 2).
Assessment of IE86 functional properties.
The IE86 protein regulates viral gene expression by binding to viral promoters and/or interactions with cellular and viral transcription factors (59). Previous studies suggest that IE86 may be recruited to the UL54 promoter due to interactions with cellular factors, such as TBP (22, 23). To determine if the mutations in the C terminus of IE86 disrupt the interaction of IE86 with TBP, we performed a GST pulldown analysis (Fig. 6). Consistent with previous studies (7), our experiments show that wild-type IE86 interacts efficiently with TBP (Fig. 6B), as does the IE86 protein containing the P535A substitution. In contrast, IE86 containing a mutation of tyrosine 544 exhibited markedly reduced binding with TBP (Fig. 6B). These studies indicate that IE86 interaction with TBP correlates with the ability to activate the UL54 promoter in the context of the viral genome. In addition, these findings suggest that the interaction between IE86 and TBP is not sufficient for the activation of the UL112-113 promoter, as the P535A mutant is unable to activate this promoter in the context of the viral genome.
FIG. 6.
GST pulldown analysis of IE86 mutant proteins. (A) SDS-PAGE analysis of wild type and the indicated IE86 mutant proteins generated by in vitro transcription/translation (IVTT) reactions. Positive-control (+) and negative-control (−) IVTT reactions were included. (B) Wild-type or mutant IE86 proteins were incubated with bacterially expressed GST or GST-TBP. GST complexes were then purified by glutathione Sepharose beads, the bound proteins separated by SDS-PAGE, and the 35S-labeled IE86 proteins visualized on a Typhoon imager.
DISCUSSION
Several studies have investigated the functional domains of the HCMV IE86 protein that are important for transcriptional regulation of viral early genes (reviewed in reference 59). Many of these studies approached this challenge using multiple amino acid mutations or large deletions that could have confounding effects on IE86 protein structure. In the current study, we have performed a comprehensive mutational analysis of amino acids 535 to 545 of the IE86 sequence. These studies revealed that two clusters of amino acids within this region (aa 535 to 537 and aa 543 and 544) were particularly important for the ability of IE86 to activate viral early promoters as well as to regulate the MIEP. Strikingly, these residues correspond to sequences that are highly conserved in IE86 homologs from other species (44), with amino acids 535, 536, 537, and 543 found to be invariant. Surprisingly, we noted that mutation of other highly conserved residues within this domain of IE86 have minimal effects on transcriptional regulation in transient assays. For example, glutamic or aspartic acid residues at amino acid position 538 are retained across species (44). However, in our study, mutation of this residue to an alanine had no significant effect on the ability of IE86 to activate the UL54 or UL112-113 promoters. Similarly, amino acids 542 and 545 are highly conserved in primate cytomegaloviruses but were found not to be important for the regulation of these early promoters. One possibility is that these residues are important for other IE86 functions not assessed in transient assays. Another important observation from this initial analysis was the finding that mutation of amino acid 541 differentially affected transcriptional activation of the two early promoters that we assessed. This finding is consistent with previous analysis of an equivalent IE86 mutant (4) and may be due to effects on IE86 posttranslational modifications.
We considered the possibility that mutation of these highly conserved residues results in altered folding or conformation of IE86, accounting for the reduced activities. Indeed, when we compared the P535A and Y544A mutants, we observed 4-fold higher levels of the IE2 P535A transcript but similar steady-state protein levels. This suggests that substitution of an alanine for the proline at position 535 may result in decreased stability of IE86, consistent with an altered conformation. However, analysis of the mutants in the context of the IE86 cDNA construct revealed enhancement of MIEP activity. This suggests that these IE86 mutants are capable of activating the MIEP, possible via effects on chromatin modifying enzymes (6, 20, 25, 43, 48), and is inconsistent with major changes in the conformation of the IE86 protein. The ability of the IE86 mutants to enhance the MIEP is also indicative of a loss of the autoregulatory functions of this protein. Indeed, we consistently observed correlation between loss of IE86 autoregulation and transactivation of essential early gene promoters. In addition, assessment of the IE86 P535A and Y544A mutants in the context of the viral genome revealed a similar lack of MIEP repression. These results support other studies suggesting that IE86 domains involved in transcriptional activation and repression overlap (3, 57, 61). In contrast, a recent study evaluating a double mutation at amino acids 535 and 537 in the context of the HCMV BAC demonstrated that these two effects were separable (44). This discrepancy could be explained by the temporal differences at which RNA expression was assessed. Another possible explanation is that the tyrosine 537 mutation may partially compensate for the proline 535 mutation, with respect to autorepression due to localized effects on protein conformation.
Analysis in the context of the viral genome revealed that the proline at amino acid position 535 and the tyrosine residue at position 544 were essential for the generation of viable HCMV. This finding is consistent with reduced activation of essential viral early gene promoters observed in transient assays. Surprisingly, assessment of the IE86 P535A mutant revealed levels of UL54 mRNA similar to that of WT-Rev following transfection of the BAC clones. This implies that other viral proteins are important for activation of the UL54 promoter and/or that sufficient functions of IE86 are retained to enable activation of this promoter in this context. At later times after transfection we observed a decrease in UL54 transcript levels that likely reflects a lack of progression of the infection due to a failure to activate other essential viral early genes, such as the UL112-113 gene region. The levels of UL112-113 transcripts were approximately 10-fold lower than that of the wild type at 1 day posttransfection and were essentially undetectable by day 4. A similar result was obtained when UL112-113 transcript levels were assessed in cells transfected with the BAC containing the IE86 Y544A mutant and is in line with results of transient assays showing that both mutations disrupt IE86 activation of this promoter. Previous studies showed that the UL112-113 promoter is controlled by ATF/CREB and IE86 binding sites (49). It is possible that these mutations disrupt the ability of IE86 to bind DNA, resulting in lower levels of Ul112-113 transcripts. This is also consistent with the lack of autoregulatory activity observed with both mutants.
The phenotype of the HCMV clone containing the Y544A substitution within IE86 is consistent with this mutation disrupting a function of IE86 that is critical for the activation of viral early promoters, such as the ability of IE86 to associate with the basal transcriptional machinery (7, 15). Indeed, our analysis revealed that mutation of amino acid 544 causes a dramatic reduction in TBP binding. This finding is consistent with studies showing that IE86 has TAF-like functions and adds further support to the notion that IE86-TBP interactions are critical for the transcriptional activation of viral early promoters (24, 34, 35). Although the TBP interaction domain of IE86 does not map to this region (7), other studies suggest that dimerization of IE86 is important for IE86-TBP interactions (54). Since amino acid 544 overlaps the dimerization domain of IE86 (1), it is possible that this mutation inhibits IE86 dimerization. However, it should also be noted that these studies were performed in vitro and may not reflect interactions that occur in the context of virus infection. For example, it is known that IE86 phosphorylation and sumoylation have significant effects on IE86-mediated transcriptional activation (5, 16, 18, 19, 29, 30). We are currently examining the recruitment of viral and cellular factors to viral early promoters in the context of viral infection in order to further address this question. In summary, our current analysis has revealed two important findings: (i) IE86-mediated activation of viral essential early promoters occurs via distinct mechanisms and (ii) the interaction between IE86 and TBP is critical for the activation of the UL54 early promoter.
Acknowledgments
We acknowledge Zhen Zhang for his invaluable technical advice in the development of the BAC clones and Lisa Bolin for her intellectual and technical contributions.
This study was supported by Public Health Service grant AI38372 (J.A.K.) from the National Institutes of Health.
Footnotes
Published ahead of print on 16 June 2010.
REFERENCES
- 1.Ahn, J. H., C. J. Chiou, and G. S. Hayward. 1998. Evaluation and mapping of the DNA binding and oligomerization domains of the IE2 regulatory protein of human cytomegalovirus using yeast one and two hybrid interaction assays. Gene 210:25-36. [DOI] [PubMed] [Google Scholar]
- 2.Arlt, H., D. Lang, S. Gebert, and T. Stamminger. 1994. Identification of binding sites for the 86-kilodalton IE2 protein of human cytomegalovirus within an IE2-responsive viral early promoter. J. Virol. 68:4117-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asmar, J., L. Wiebusch, M. Truss, and C. Hagemeier. 2004. The putative zinc finger of the human cytomegalovirus IE2 86-kilodalton protein is dispensable for DNA binding and autorepression, thereby demarcating a concise core domain in the C terminus of the protein. J. Virol. 78:11853-11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrasa, M. I., N. Harel, Y. Yu, and J. C. Alwine. 2003. Strain variations in single amino acids of the 86-kilodalton human cytomegalovirus major immediate-early protein (IE2) affect its functional and biochemical properties: implications of dynamic protein conformation. J. Virol. 77:4760-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berndt, A., H. Hofmann-Winkler, N. Tavalai, G. Hahn, and T. Stamminger. 2009. Importance of covalent and noncovalent SUMO interactions with the major human cytomegalovirus transactivator IE2p86 for viral infection. J. Virol. 83:12881-12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant, L. A., P. Mixon, M. Davidson, A. J. Bannister, T. Kouzarides, and J. H. Sinclair. 2000. The human cytomegalovirus 86-kilodalton major immediate-early protein interacts physically and functionally with histone acetyltransferase P/CAF. J. Virol. 74:7230-7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caswell, R., C. Hagemeier, C. J. Chiou, G. Hayward, T. Kouzarides, and J. Sinclair. 1993. The human cytomegalovirus 86K immediate early (IE) 2 protein requires the basic region of the TATA-box binding protein (TBP) for binding, and interacts with TBP and transcription factor TFIIB via regions of IE2 required for transcriptional regulation. J. Gen. Virol. 74:2691-2698. [DOI] [PubMed] [Google Scholar]
- 8.Chau, N. H., C. D. Vanson, and J. A. Kerry. 1999. Transcriptional regulation of the human cytomegalovirus US11 early gene. J. Virol. 73:863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherrington, J. M., E. L. Khoury, and E. S. Mocarski. 1991. Human cytomegalovirus ie2 negatively regulates alpha gene expression via a short target sequence near the transcription start site. J. Virol. 65:887-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherrington, J. M., and E. S. Mocarski. 1989. Human cytomegalovirus ie1 transactivates the alpha promoter-enhancer via an 18-base-pair repeat element. J. Virol. 63:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciocco-Schmitt, G. M., Z. Karabekian, E. W. Godfrey, R. M. Stenberg, A. E. Campbell, and J. A. Kerry. 2002. Identification and characterization of novel murine cytomegalovirus M112-113 (e1) gene products. Virology 294:199-208. [DOI] [PubMed] [Google Scholar]
- 12.Depto, A. S., and R. M. Stenberg. 1989. Regulated expression of the human cytomegalovirus pp65 gene: octamer sequence in the promoter is required for activation by viral gene products. J. Virol. 63:1232-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. U. S. A. 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortunato, E. A., and D. H. Spector. 1999. Regulation of human cytomegalovirus gene expression. Adv. Virus Res. 54:61-128. [DOI] [PubMed] [Google Scholar]
- 15.Hagemeier, C., S. Walker, R. Caswell, T. Kouzarides, and J. Sinclair. 1992. The human cytomegalovirus 80-kilodalton but not the 72-kilodalton immediate-early protein transactivates heterologous promoters in a TATA box-dependent mechanism and interacts directly with TFIID. J. Virol. 66:4452-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harel, N. Y., and J. C. Alwine. 1998. Phosphorylation of the human cytomegalovirus 86-kilodalton immediate-early protein IE2. J. Virol. 72:5481-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heider, J. A., W. A. Bresnahan, and T. E. Shenk. 2002. Construction of a rationally designed human cytomegalovirus variant encoding a temperature-sensitive immediate-early 2 protein. Proc. Natl. Acad. Sci. U. S. A. 99:3141-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heider, J. A., Y. Yu, T. Shenk, and J. C. Alwine. 2002. Characterization of a human cytomegalovirus with phosphorylation site mutations in the immediate-early 2 protein. J. Virol. 76:928-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann, H., S. Floss, and T. Stamminger. 2000. Covalent modification of the transactivator protein IE2-p86 of human cytomegalovirus by conjugation to the ubiquitin-homologous proteins SUMO-1 and hSMT3b. J. Virol. 74:2510-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu, C. H., M. D. Chang, K. Y. Tai, Y. T. Yang, P. S. Wang, C. J. Chen, Y. H. Wang, S. C. Lee, C. W. Wu, and L. J. Juan. 2004. HCMV IE2-mediated inhibition of HAT activity downregulates p53 function. EMBO J. 23:2269-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jupp, R., S. Hoffmann, A. Depto, R. M. Stenberg, P. Ghazal, and J. A. Nelson. 1993. Direct interaction of the human cytomegalovirus IE86 protein with the cis repression signal does not preclude TBP from binding to the TATA box. J. Virol. 67:5595-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerry, J. A., M. A. Priddy, T. Y. Jervey, C. P. Kohler, T. L. Staley, C. D. Vanson, T. R. Jones, A. C. Iskenderian, D. G. Anders, and R. M. Stenberg. 1996. Multiple regulatory events influence human cytomegalovirus DNA polymerase (UL54) expression during viral infection. J. Virol. 70:373-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerry, J. A., M. A. Priddy, and R. M. Stenberg. 1994. Identification of sequence elements in the human cytomegalovirus DNA polymerase gene promoter required for activation by viral gene products. J. Virol. 68:4167-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, J. M., Y. Hong, K. T. Jeang, and S. Kim. 2000. Transactivation activity of the human cytomegalovirus IE2 protein occurs at steps subsequent to TATA box-binding protein recruitment. J. Gen. Virol. 81:37-46. [DOI] [PubMed] [Google Scholar]
- 25.Klucher, K. M., M. Sommer, J. T. Kadonaga, and D. H. Spector. 1993. In vivo and in vitro analysis of transcriptional activation mediated by the human cytomegalovirus major immediate-early proteins. Mol. Cell. Biol. 13:1238-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohler, C. P., J. A. Kerry, M. Carter, V. P. Muzithras, T. R. Jones, and R. M. Stenberg. 1994. Use of recombinant virus to assess human cytomegalovirus early and late promoters in the context of the viral genome. J. Virol. 68:6589-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang, D., S. Gebert, H. Arlt, and T. Stamminger. 1995. Functional interaction between the human cytomegalovirus 86-kilodalton IE2 protein and the cellular transcription factor CREB. J. Virol. 69:6030-6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, G., J. Wu, P. Luu, P. Ghazal, and O. Flores. 1996. Inhibition of the association of RNA polymerase II with the preinitiation complex by a viral transcriptional repressor. Proc. Natl. Acad. Sci. U. S. A. 93:2570-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, H. R., and J. H. Ahn. 2004. Sumoylation of the major immediate-early IE2 protein of human cytomegalovirus Towne strain is not required for virus growth in cultured human fibroblasts. J. Gen. Virol. 85:2149-2154. [DOI] [PubMed] [Google Scholar]
- 30.Lee, J. M., H. J. Kang, H. R. Lee, C. Y. Choi, W. J. Jang, and J. H. Ahn. 2003. PIAS1 enhances SUMO-1 modification and the transactivation activity of the major immediate-early IE2 protein of human cytomegalovirus. FEBS Lett. 555:322-328. [DOI] [PubMed] [Google Scholar]
- 31.Liu, B., T. W. Hermiston, and M. F. Stinski. 1991. A cis-acting element in the major immediate-early (IE) promoter of human cytomegalovirus is required for negative regulation by IE2. J. Virol. 65:897-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, B., and M. F. Stinski. 1992. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J. Virol. 66:4434-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorz, K., H. Hofmann, A. Berndt, N. Tavalai, R. Mueller, U. Schlotzer-Schrehardt, and T. Stamminger. 2006. Deletion of open reading frame UL26 from the human cytomegalovirus genome results in reduced viral growth, which involves impaired stability of viral particles. J. Virol. 80:5423-5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukac, D. M., N. Y. Harel, N. Tanese, and J. C. Alwine. 1997. TAF-like functions of human cytomegalovirus immediate-early proteins. J. Virol. 71:7227-7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukac, D. M., J. R. Manuppello, and J. C. Alwine. 1994. Transcriptional activation by the human cytomegalovirus immediate-early proteins: requirements for simple promoter structures and interactions with multiple components of the transcription complex. J. Virol. 68:5184-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchini, A., H. Liu, and H. Zhu. 2001. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 75:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meier, J. L., and M. F. Stinski. 1996. Regulation of human cytomegalovirus immediate-early gene expression. Intervirology 39:331-342. [DOI] [PubMed] [Google Scholar]
- 38.Mellon, P., V. Parker, Y. Gluzman, and T. Maniatis. 1981. Identification of DNA sequences required for transcription of the human alpha 1-globin gene in a new SV40 host-vector system. Cell 27:279-288. [DOI] [PubMed] [Google Scholar]
- 39.Mocarski, E. S., Jr., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott, Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 40.Netterwald, J., S. Yang, W. Wang, S. Ghanny, M. Cody, P. Soteropoulos, B. Tian, W. Dunn, F. Liu, and H. Zhu. 2005. Two gamma interferon-activated site-like elements in the human cytomegalovirus major immediate-early promoter/enhancer are important for viral replication. J. Virol. 79:5035-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pari, G. S., and D. G. Anders. 1993. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J. Virol. 67:6979-6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pari, G. S., and Y. Xu. 2004. Gene transfer into mammalian cells using calcium phosphate and DEAE-dextran. Methods Mol. Biol. 245:25-32. [DOI] [PubMed] [Google Scholar]
- 43.Park, J. J., Y. E. Kim, H. T. Pham, E. T. Kim, Y. H. Chung, and J. H. Ahn. 2007. Functional interaction of the human cytomegalovirus IE2 protein with histone deacetylase 2 in infected human fibroblasts. J. Gen. Virol. 88:3214-3223. [DOI] [PubMed] [Google Scholar]
- 44.Petrik, D. T., K. P. Schmitt, and M. F. Stinski. 2007. The autoregulatory and transactivating functions of the human cytomegalovirus IE86 protein use independent mechanisms for promoter binding. J. Virol. 81:5807-5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pizzorno, M. C., and G. S. Hayward. 1990. The IE2 gene products of human cytomegalovirus specifically down-regulate expression from the major immediate-early promoter through a target sequence located near the cap site. J. Virol. 64:6154-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pizzorno, M. C., P. O'Hare, L. Sha, R. L. LaFemina, and G. S. Hayward. 1988. trans-activation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J. Virol. 62:1167-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reeves, M., J. Murphy, R. Greaves, J. Fairley, A. Brehm, and J. Sinclair. 2006. Autorepression of the human cytomegalovirus major immediate-early promoter/enhancer at late times of infection is mediated by the recruitment of chromatin remodeling enzymes by IE86. J. Virol. 80:9998-10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodems, S. M., C. L. Clark, and D. H. Spector. 1998. Separate DNA elements containing ATF/CREB and IE86 binding sites differentially regulate the human cytomegalovirus UL112-113 promoter at early and late times in the infection. J. Virol. 72:2697-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartz, R., B. Helmich, and D. H. Spector. 1996. CREB and CREB-binding proteins play an important role in the IE2 86-kilodalton protein-mediated transactivation of the human cytomegalovirus 2.2-kilobase RNA promoter. J. Virol. 70:6955-6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz, R., M. H. Sommer, A. Scully, and D. H. Spector. 1994. Site-specific binding of the human cytomegalovirus IE2 86-kilodalton protein to an early gene promoter. J. Virol. 68:5613-5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scully, A. L., M. H. Sommer, R. Schwartz, and D. H. Spector. 1995. The human cytomegalovirus IE2 86-kilodalton protein interacts with an early gene promoter via site-specific DNA binding and protein-protein associations. J. Virol. 69:6533-6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen, W., E. Westgard, L. Huang, M. D. Ward, J. L. Osborn, N. H. Chau, L. Collins, B. Marcum, M. A. Koach, J. Bibbs, O. J. Semmes, and J. A. Kerry. 2008. Nuclear trafficking of the human cytomegalovirus pp71 (ppUL82) tegument protein. Virology 376:42-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sommer, M. H., A. L. Scully, and D. H. Spector. 1994. Transactivation by the human cytomegalovirus IE2 86-kilodalton protein requires a domain that binds to both the TATA box-binding protein and the retinoblastoma protein. J. Virol. 68:6223-6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Staprans, S. I., D. K. Rabert, and D. H. Spector. 1988. Identification of sequence requirements and trans-acting functions necessary for regulated expression of a human cytomegalovirus early gene. J. Virol. 62:3463-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stenberg, R. M., A. S. Depto, J. Fortney, and J. A. Nelson. 1989. Regulated expression of early and late RNAs and proteins from the human cytomegalovirus immediate-early gene region. J. Virol. 63:2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stenberg, R. M., J. Fortney, S. W. Barlow, B. P. Magrane, J. A. Nelson, and P. Ghazal. 1990. Promoter-specific trans activation and repression by human cytomegalovirus immediate-early proteins involves common and unique protein domains. J. Virol. 64:1556-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stinski, M. F., and H. Isomura. 2008. Role of the cytomegalovirus major immediate early enhancer in acute infection and reactivation from latency. Med. Microbiol. Immunol. 197:223-231. [DOI] [PubMed] [Google Scholar]
- 59.Stinski, M. F., and D. T. Petrik. 2008. Functional roles of the human cytomegalovirus essential IE86 protein. Curr. Top. Microbiol. Immunol. 325:133-152. [DOI] [PubMed] [Google Scholar]
- 60.Stinski, M. F., and T. J. Roehr. 1985. Activation of the major immediate early gene of human cytomegalovirus by cis-acting elements in the promoter-regulatory sequence and by virus-specific trans-acting components. J. Virol. 55:431-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White, E. A., C. L. Clark, V. Sanchez, and D. H. Spector. 2004. Small internal deletions in the human cytomegalovirus IE2 gene result in nonviable recombinant viruses with differential defects in viral gene expression. J. Virol. 78:1817-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu, J., J. O'Neill, and M. S. Barbosa. 1998. Transcription factor Sp1 mediates cell-specific trans-activation of the human cytomegalovirus DNA polymerase gene promoter by immediate-early protein IE86 in glioblastoma U373MG cells. J. Virol. 72:236-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu, D., G. A. Smith, L. W. Enquist, and T. Shenk. 2002. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J. Virol. 76:2316-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]