Abstract
A synthetic feline TRIM5-cyclophilin A fusion protein (feTRIMCyp) was generated and transduced into feline cells. feTRIMCyp was highly efficient at preventing infection with human (HIV) and feline (FIV) immunodeficiency virus pseudotypes, and feTRIMCyp-expressing cells resisted productive infection with either FIV-Fca or FIV-Pco. The restriction of FIV infection by feTRIMCyp was reversed by the cyclosporine (Cs) derivatives NIM811 and Debio-025 but less so by Cs itself. FeTRIMCyp and FIV infections of the cat offer a unique opportunity to evaluate TRIMCyp-based approaches to genetic therapy for HIV infection and the treatment of AIDS.
Human immunodeficiency virus type 1 (HIV-1) infection is inhibited immediately after viral entry by the α isoform of the tripartite-motif-containing protein TRIM5 (TRIM5α) (8, 23, 29, 32). The C terminus of TRIM5α contains a PRY/SPRY (B30.2) domain (12, 25), and this domain mediates binding of TRIM5α to the viral capsid (30, 33). The N-terminal tripartite motif, or RBCC (RING, B-box, and coiled coil) domain, possesses an E3 ubiquitin ligase domain (RING) (39), and ubiquitination recruits incoming virions to the proteasome, where they are degraded. While inhibition of the proteasome prevents degradation of the viral core and enables reverse transcription to proceed, the process of infection does not complete (7, 38), indicating an additional proteasome-independent antiviral function for TRIM5α. Accelerated uncoating of the viral capsid from the incoming virion may underlie this proteasome-independent restriction activity (24, 33).
Cyclophilin A (CypA) associates with the HIV-1 capsid (16) and is present in viral particles (10, 34). CypA is a ubiquitous cytoplasmic protein that catalyzes the cis/trans isomerization of peptidyl-prolyl bonds, and following binding to HIV-1 capsid, the peptidyl-prolyl bond linking residues G89 and P90 is isomerized (2). The specific association of target cell CypA with the incoming HIV-1 capsid is required for viral infectivity (3-5, 11, 31). However, the specificity of the CypA-capsid interaction has been utilized by several species of primates to target TRIM5 to the lentiviral capsid. Insertion of a CypA cDNA between exons 7 and 8 of TRIM5 in the New World monkey Aotus trivirgatus (owl monkey) generated a TRIM5-CypA fusion (TRIMCyp) with potent lentiviral restriction activity (20, 29). Moreover, gene fusions have been detected in three species of Old World macaques, Macaca mulatta (rhesus macaque) (19, 37), Macaca nemestrina (pig-tailed macaque) (6, 14, 19, 35), and Macaca fascicularis (crab-eating macaque) (6), resulting from insertion of a CypA cDNA into the untranslated region of exon 8. In the Old World macaques, splicing of the mRNA transcript fuses the end of exon 6 to the CypA splice acceptor. The potency of the lentiviral restriction by the primate TRIMCyp proteins offers a novel approach to HIV-1 gene therapy; transduction of either bone marrow stem cells or peripheral blood CD4+ T cells with vectors bearing TRIMCyp fusion proteins should render the cells resistant to HIV infection and replication. To circumvent a potential immune response by the recipient against Aotus or Macaca TRIMCyps, a synthetic human TRIM5-CypA fusion protein was generated and shown to confer robust resistance to HIV-1 replication (18).
Feline immunodeficiency virus (FIV) infection of the domestic cat (Felis catus) offers a well-characterized small-animal model for HIV infection (9, 21, 36). Moreover, with ∼0.5 million FIV-infected cats in the United Kingdom alone, there is both a suitable study population and the advanced clinical facilities that would be required to investigate cutting-edge therapies for immunodeficiency-causing lentiviruses, benefiting both human and veterinary medicine alike. Toward this end, we asked whether a feline-specific TRIMCyp could be engineered and whether it would display the potent lentiviral restriction activity of the primate proteins. To date, we have found no evidence of a naturally occurring TRIMCyp fusion protein in either the Felis, Panthera, or Puma lineages (17). Moreover, the domestic cat lacks a full-length TRIM5 gene due to the presence of a premature stop codon in the feline TRIM5 exon homologous to human TRIM5 exon 8 (17). However, felid cells express an abundant message for the TRIM5 RBCC (17). As the feline TRIM5 RBCC is encoded by exons 2 to 6, we elected to fuse the start codon of feline CypA to the last codon of exon 6. The feline TRIM5 RBCC was reamplified from feT5-CXCR (17) using primers feT5a-1 (5′-GCGGATCCATGGCTTCTGAACTCCTGAAAT-3′) and feT5a-2 (5′-CACGATGGGGTTGACCATTTTTTTAAAGGCTTGT ATTAT-3′). Feline CypA was amplified from cDNA derived from the domestic cat primary T-cell line Mya-1 using primers directed to the predicted feline CypA (GenBank AANG01610851), fCypA R69 5′ Nde (5′-AACATATGGTCAACCCCATCGTG-3′) and feCypA 3′ Mlu (5′-AAACGCGTTTAGATTTGTCCACAGTCA-3′). The amplicon was cloned into the prokaryotic expression vector pOPTH using NdeI and MluI restriction sites and subsequently reamplified using primers feCypA-1 (5′-ATAATACAAGCCTTTAAAAAAATGGTCAACCCCATCGTG-3′) and feCypA-2 (5′-GCGTCGACTTAGATTTGTCCACAGTCAGC-3′). Feline CypA binding to FIV capsid (CA) was confirmed by isothermal titration calorimetry (ITC), as previously described (26). FIV capsid N-terminal domain (feCAN), feCypA, and human CypA (huCypA) were overexpressed in Escherichia coli and purified by Ni-nitrilotriacetic acid (NTA), gel filtration, and ion-exchange chromatography. Titrations were carried out at 10°C, and binding isotherms fit to a standard one-state model to give the stoichiometry (N), enthalpy change (ΔH), and equilibrium association constant (KA), from which the change in Gibbs energy (ΔG) and entropy (ΔS) and the equilibrium dissociation constant (KD) were derived. FeCAN bound feCypA and huCypA with KDs of 6.2 μM and 7.2 μM, respectively, compared to a value of 5.3 μM for HIV-1 CA binding to huCypA (previous reports indicated a KD of 5 to 15 μM for HIV-1 and huCypA [26, 40]). Mutation of HIV-1 CA proline-rich loop residues G89 and P90 abrogated CypA binding in HIV-1 (40). To test whether CypA interacted with FIV in a similar manner, the P90A mutant that was previously shown to prevent CypA packaging within FIV virions (15) was tested for feCypA binding. No detectable interaction was found, suggesting that, like HIV-1, the proline-rich loop is critical in feCAN-CypA interactions.
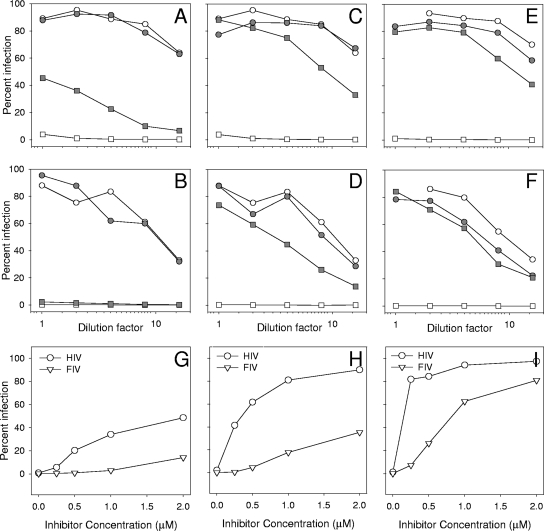
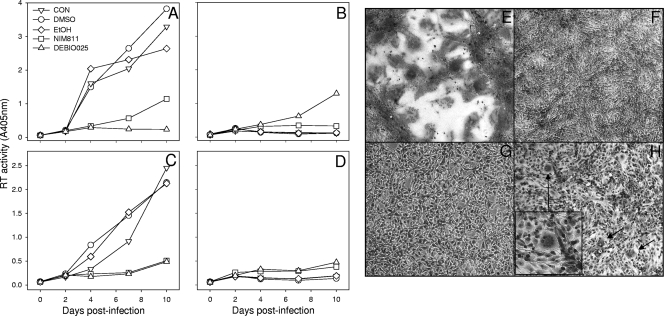
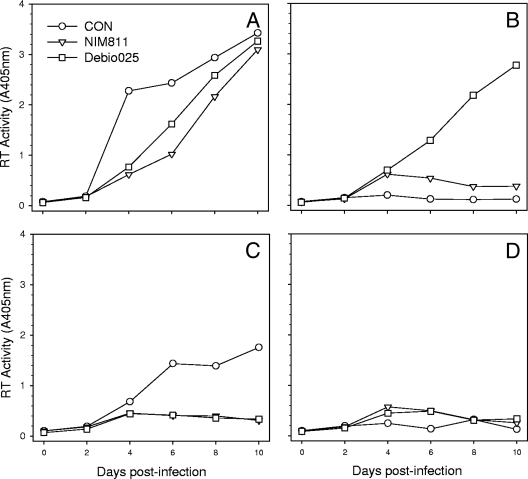
The TRIM5 RBCC and CypA amplification products were annealed and used as templates to generate a TRIMCyp gene fusion by reamplification with feT5a-1 and feCypA-2. The TRIMCyp fusion was cloned into BamHI and SalI sites of the retroviral vector pDON-AI-2neo (Takara Bio Europe S.A.S., Saint-Germain-en-Laye, France), and the nucleic acid sequence of the TRIMCyp fusion was confirmed (GenBank accession number HM246715). Crandell feline kidney cells (CrFK, line ID10) were transduced with murine leukemia virus pseudotypes bearing the TRIMCyp fusion, and stable lines were selected in G418. Stable expression of the feline TRIMCyp fusion rendered ID10 cells resistant to infection with either the HIV(VSV) (vesicular stomatitis virus envelope glycoprotein) or FIV(VSV) pseudotype (Fig. 1). The specificity of the inhibitory effect was confirmed by the addition of specific antagonists of the CypA-capsid interaction; either Cs (Fig. 1A and B) or its nonimmunosuppressive derivatives NIM811 (28) (Fig. 1C and D) and Debio-025 (27) (Fig. 1E and F). While 2.0 μM Cs displayed a modest reversal of the inhibition of HIV pseudotype entry by TRIMCyp (Fig. 1A), it was unable to reverse the inhibition of FIV pseudotype infection (Fig. 1B). In contrast, the Cs derivatives NIM811 (Novartis, Basel, Switzerland) and Debio-025 (Debiopharm, Lausanne, Switzerland) at 2.0 μM reversed the inhibition of infection with both HIV (Fig. 1C and E) and FIV (Fig. 1D and F) pseudotypes. Titrating the CypA antagonists confirmed the differential sensitivities of the HIV and FIV pseudotypes to reversal of the TRIMCyp restriction; restriction of HIV was readily reversed by NIM811 (Fig. 1H) and Debio-025 (Fig. 1I), while Debio-025 (Fig. 1L) alone reversed the restriction of FIV to near control levels of infection. The data suggest that the feline TRIMCyp fusion targets the HIV and FIV capsids specifically during viral entry and that inhibition of FIV is extremely potent. Next, we asked whether the TRIMCyp fusion would inhibit productive infection with replication-competent lentiviruses from the domestic cat FIV-Fca (Fig. 2A and B) or puma FIV-Pco (Fig. 2C and D). Replication of both FIV-Fca and FIV-Pco was blocked completely by expression of the TRIMCyp fusion protein (Fig. 2B and D), while both viruses replicated well in cells transduced with vector only (Fig. 2A and C). FIV-Fca replication was accompanied by the formation of prominent syncytia (Fig. 2E). The CypA antagonists NIM811 and Debio-025 (2.5 μM) blocked replication with FIV-Fca and FIV-Pco in the control cells, indicating an important role for CypA in the replication of these viruses in CrFK cells and consistent with previous studies suggesting a role for CypA in the replication of FIV (15). Debio-025 was more potent than NIM811 and blocked FIV-Fca replication completely. In contrast, both Debio-025 and, to a lesser extent, NIM811 countered the inhibition of viral growth by TRIMCyp. The most marked effect was with FIV-Fca, where Debio-025 restored viral growth to a level at which small syncytia could be visualized (Fig. 2H, arrows) and reverse transcriptase (RT) activity could be detected (Fig. 2B.). Thus, where endogenous CypA and ectopically expressed TRIMCyp are coexpressed, the CypA antagonists appear to tip the balance in favor of viral replication. In support of this hypothesis, we repeated the viral replication assay in the presence of a reduced antagonist concentration (2.0 μM) (Fig. 3). While growth of FIV-Pco in control cells was suppressed efficiently by both NIM811 and Debio-025 at 2.0 μM (Fig. 3C), growth of FIV-Fca was reduced modestly (Fig. 3A). However, in the presence of suboptimal antagonist, replication of FIV-Fca in the TRIMCyp-expressing cells was restored completely by Debio-025 and partially by NIM811 (Fig. 3B), with viral replication accompanied by prominent syncytium formation.
FIG. 1.
Restriction of lentiviral entry by feline TRIMCyp and its reversal by CypA antagonists. CrFK (ID10) cells were stably transduced with a retroviral vector bearing feline TRIMCyp (square) or vector only (circle). The cells were challenged with serial dilutions of HIV(VSV) (A, C, and E) or FIV(VSV) (B, D, and F) pseudotypes bearing a green fluorescent protein (GFP) marker gene. Seventy-two hours postinfection, GFP expression was quantified by flow cytometry. Infections were performed in the presence (filled symbols) or absence (open symbols) of medium supplemented with 2.5 μM CypA antagonists Cs (A and B), NIM811 (C and D), or Debio-025 (E and F) or their respective solvents dimethyl sulfoxide (DMSO) (Cs and NIM811) or ethanol (Debio-025). (G, H, and I) Sensitivity of HIV and FIV to Cs (G), NIM811 (H), and Debio-025 (I). TRIMCyp-expressing cells were incubated with inhibitor at 0, 0.5, 1.0, 1.5, or 2.0 μM prior to infection with HIV(VSV) or FIV(VSV) pseudotypes. Each point represents the mean of triplicate estimations.
FIG. 2.
Inhibition of viral replication by feline TRIMCyp and its reversal by CypA antagonists. CrFK (ID10) cells stably transduced with a retroviral vector bearing feline TRIMCyp (B and D) or vector only (A and C) were infected with FIV-Fca (Petaluma-F14 strain) (A and B) or FIV-Pco (CoLV strain) (C and D). Infections were performed in the presence or absence of medium supplemented with 2.5 μM CypA antagonist NIM811 or Debio-025 or their respective solvents, DMSO and ethanol (EtOH). Supernatants were collected and assayed for RT activity by nonisotopic RT assay (LentiRT; CavidTech, Sweden). CON, control. (E to H) Syncytium formation in CrFK cells infected with FIV-Fca. Cells expressing vector only (E and G) or TRIMCyp (F and H) and infected with FIV-Fca in the presence of Debio-025 (G and H) or ethanol solvent control (E and F) were fixed and stained at 10 days postinfection with 1.0% methylene blue-0.2% basic fuchsin in methanol. The arrows indicate small syncytia, magnified in the inset (H).
FIG. 3.
Restoration of viral replication in TRIMCyp-expressing cells by 2.0 μm NIM811 and Debio-025. CrFK (ID10) cells stably transduced with a retroviral vector bearing feline TRIMCyp (B and D) or vector only (A and C) were infected with FIV-Fca (Petaluma-F14 strain) (A and B) or FIV-Pco (CoLV strain) (C and D). Infections were performed in the presence or absence of medium supplemented with 2.0 μM NIM811 or Debio-025. Supernatants were collected and assayed for RT activity.
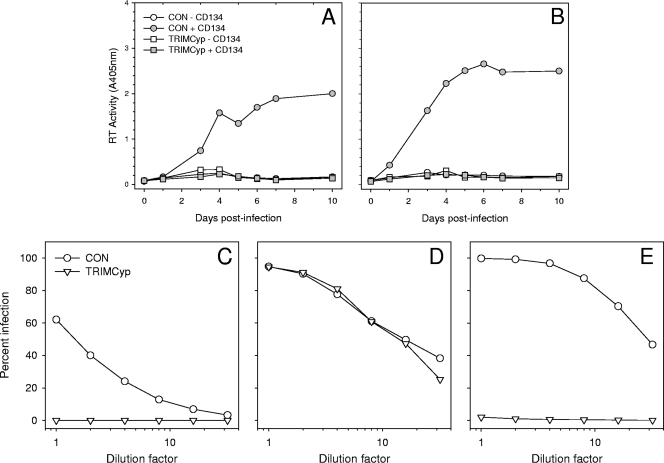
As FIV-Fca (Petaluma F14) and FIV-Pco (cougar lentivirus [CoLV]) are cell culture-adapted viral strains, we generated ID10 and ID10-TRIMCyp cells stably expressing CD134 to examine the effect of the feline TRIMCyp fusion on primary FIVs. Replication of the GL8 (Fig. 4A) and PPR (Fig. 4B) strains of FIV-Fca was blocked completely by the feline TRIMCyp fusion protein. Finally, we confirmed the specificity of TRIMCyp by comparing infection of feline TRIMCyp-expressing cells with FIV, HIV-1, and simian immunodeficiency virus (SIVmac). SIVmac CA does not possess CypA-binding activity, and accordingly, SIVmac pseudotypes infected feline TRIMCyp-expressing ID10 cells with efficiency similar to that with control cells (Fig. 4D), while parallel infections with either FIV (Fig. 4C) or HIV-1 (Fig. 4E) pseudotypes were blocked completely.
FIG. 4.
Growth of primary strains of FIV-Fca is blocked by feline TRIMCyp. (A and B) ID10 cells stably expressing feline TRIMCyp (TRIMCyp) or vector only (CON) and retransduced with vectors bearing either DSRed (− CD134) or a CD134-DSRed fusion (+ CD134) were infected with GL8 (A) or PPR (B), and viral replication was monitored by RT assay as for Fig. 2. (C to E) SIVmac infection is resistant to inhibition by feline TRIMCyp. ID10 control (CON) or TRIMCyp-expressing (TRIMCyp) cells were infected with SIVmac(VSV) GFP pseudotypes (D) and compared with FIV(VSV) (C) and HIV(VSV) (E) GFP pseudotypes. Infection was assessed 72 h postinfection by flow cytometry.
By designing a feline TRIM5-CypA gene fusion based on the naturally occurring TRIMCyp of A. trivirgatus, we postulated that a feline TRIMCyp with robust antilentiviral activity would result. In doing so we confirmed that the feline TRIM5 retains full ability to restrict both FIV and HIV-1; it simply lacks a capsid-targeting SPRY domain. The feline CypA targets the feline TRIM5 RBCC to the lentiviral capsid efficiently, generating a potent restriction factor of entirely feline origin. Indeed, feTRIMCyp ablated completely the ability of feline lentiviruses to grow in vitro. Accordingly, feTRIMCyp should facilitate in vivo analyses of the viability of gene therapy TRIMCyp without the potential pitfall of the host generating an immune response against xenoantigens. The TRIMCyp-based approach to lentiviral gene therapy offers advantages over other potential approaches to therapy; for example, by targeting viral entry, it denies the virus the opportunity to replicate, and thus the virus cannot generate escape mutants. As TRIMCyp does not target the function of an endogenously expressed molecule (recent targets for HIV gene therapy have included the coreceptor CCR5 [1, 13, 22]), it is unlikely to have side effects that are detrimental to the host. Treatment of the lentivirus-infected host using TRIMCyp fusions may be achieved by transduction of bone marrow-derived hematopoietic progenitor stem cells and repopulation of the host immune system following bone marrow ablation. However, transduction of peripheral-blood-derived CD4+ T cells and ex vivo expansion of the transduced cells prior to repopulation of the host immune system may be sufficient to overcome the immunodeficiency associated with AIDS. Accordingly, the successful in vitro studies described here offer strong support for clinical trials of feTRIMCyp both as a therapy for FIV infection and as a model for the gene therapy of HIV infections in humans.
Acknowledgments
We thank Jeremy Luban and Greg Towers for many helpful discussions.
This work was supported by funding from The Wellcome Trust to B.J.W. and M.J.H.
Footnotes
Published ahead of print on 16 June 2010.
REFERENCES
- 1.An, D. S., R. E. Donahue, M. Kamata, B. Poon, M. Metzger, S. H. Mao, A. Bonifacino, A. E. Krouse, J. L. Darlix, D. Baltimore, F. X. Qin, and I. S. Chen. 2007. Stable reduction of CCR5 by RNAi through hematopoietic stem cell transplant in non-human primates. Proc. Natl. Acad. Sci. U. S. A. 104:13110-13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosco, D. A., E. Z. Eisenmesser, S. Pochapsky, W. I. Sundquist, and D. Kern. 2002. Catalysis of cis/trans isomerization in native HIV-1 capsid by human cyclophilin A. Proc. Natl. Acad. Sci. U. S. A. 99:5247-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braaten, D., E. K. Franke, and J. Luban. 1996. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J. Virol. 70:3551-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braaten, D., E. K. Franke, and J. Luban. 1996. Cyclophilin A is required for the replication of group M human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus SIV(CPZ)GAB but not group O HIV-1 or other primate immunodeficiency viruses. J. Virol. 70:4220-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braaten, D., and J. Luban. 2001. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 20:1300-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan, G., Y. Kozyrev, and S. L. Hu. 2008. TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proc. Natl. Acad. Sci. U. S. A. 105:3569-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell, E. M., O. Perez, J. L. Anderson, and T. J. Hope. 2008. Visualization of a proteasome-independent intermediate during restriction of HIV-1 by rhesus TRIM5alpha. J. Cell Biol. 180:549-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz-Griffero, F., N. Vandegraaff, Y. Li, K. McGee-Estrada, M. Stremlau, S. Welikala, Z. Si, A. Engelman, and J. Sodroski. 2006. Requirements for capsid-binding and an effector function in TRIMCyp-mediated restriction of HIV-1. Virology 351:404-419. [DOI] [PubMed] [Google Scholar]
- 9.Elder, J. H., G. A. Dean, E. A. Hoover, J. A. Hoxie, M. H. Malim, L. Mathes, J. C. Neil, T. W. North, E. Sparger, M. B. Tompkins, W. A. Tompkins, J. Yamamoto, N. Yuhki, N. C. Pedersen, and R. H. Miller. 1998. Lessons from the cat: feline immunodeficiency virus as a tool to develop intervention strategies against human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 14:797-801. [DOI] [PubMed] [Google Scholar]
- 10.Franke, E. K., H. E. Yuan, and J. Luban. 1994. Specific incorporation of cyclophilin A into HIV-1 virions. Nature 372:359-362. [DOI] [PubMed] [Google Scholar]
- 11.Hatziioannou, T., D. Perez-Caballero, S. Cowan, and P. D. Bieniasz. 2005. Cyclophilin interactions with incoming human immunodeficiency virus type 1 capsids with opposing effects on infectivity in human cells. J. Virol. 79:176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry, J., M. T. Ribouchon, C. Offer, and P. Pontarotti. 1997. B30.2-like domain proteins: a growing family. Biochem. Biophys. Res. Commun. 235:162-165. [DOI] [PubMed] [Google Scholar]
- 13.Kumar, P., H. S. Ban, S. S. Kim, H. Wu, T. Pearson, D. L. Greiner, A. Laouar, J. Yao, V. Haridas, K. Habiro, Y. G. Yang, J. H. Jeong, K. Y. Lee, Y. H. Kim, S. W. Kim, M. Peipp, G. H. Fey, N. Manjunath, L. D. Shultz, S. K. Lee, and P. Shankar. 2008. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell 134:577-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao, C. H., Y. Q. Kuang, H. L. Liu, Y. T. Zheng, and B. Su. 2007. A novel fusion gene, TRIM5-Cyclophilin A in the pig-tailed macaque determines its susceptibility to HIV-1 infection. AIDS 21(Suppl. 8):S19-S26. [DOI] [PubMed] [Google Scholar]
- 15.Lin, T. Y., and M. Emerman. 2006. Cyclophilin A interacts with diverse lentiviral capsids. Retrovirology 3:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luban, J., K. L. Bossolt, E. K. Franke, G. V. Kalpana, and S. P. Goff. 1993. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell 73:1067-1078. [DOI] [PubMed] [Google Scholar]
- 17.McEwan, W. A., T. Schaller, L. M. Ylinen, M. J. Hosie, G. J. Towers, and B. J. Willett. 2009. Truncation of TRIM5 in Feliformia explains the absence of retroviral restriction in cells of the domestic cat. J. Virol. [DOI] [PMC free article] [PubMed]
- 18.Neagu, M. R., P. Ziegler, T. Pertel, C. Strambio-De-Castillia, C. Grutter, G. Martinetti, L. Mazzucchelli, M. Grutter, M. G. Manz, and J. Luban. 2009. Potent inhibition of HIV-1 by TRIM5-cyclophilin fusion proteins engineered from human components. J. Clin. Invest. 119:3035-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman, R. M., L. Hall, A. Kirmaier, L. A. Pozzi, E. Pery, M. Farzan, S. P. O'Neil, and W. Johnson. 2008. Evolution of a TRIM5-CypA splice isoform in old world monkeys. PLoS. Pathog. 4:e1000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nisole, S., C. Lynch, J. P. Stoye, and M. W. Yap. 2004. A Trim5-cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc. Natl. Acad. Sci. U. S. A. 101:13324-13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedersen, N. C., E. W. Ho, M. L. Brown, and J. K. Yamamoto. 1987. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency syndrome. Science 235:790-793. [DOI] [PubMed] [Google Scholar]
- 22.Perez, E. E., J. Wang, J. C. Miller, Y. Jouvenot, K. A. Kim, O. Liu, N. Wang, G. Lee, V. V. Bartsevich, Y. L. Lee, D. Y. Guschin, I. Rupniewski, A. J. Waite, C. Carpenito, R. G. Carroll, J. S. Orange, F. D. Urnov, E. J. Rebar, D. Ando, P. D. Gregory, J. L. Riley, M. C. Holmes, and C. H. June. 2008. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 26:808-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Caballero, D., T. Hatziioannou, F. Zhang, S. Cowan, and P. D. Bieniasz. 2005. Restriction of human immunodeficiency virus type 1 by TRIM-CypA occurs with rapid kinetics and independently of cytoplasmic bodies, ubiquitin, and proteasome activity. J. Virol. 79:15567-15572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perron, M. J., M. Stremlau, M. Lee, H. Javanbakht, B. Song, and J. Sodroski. 2007. The human TRIM5alpha restriction factor mediates accelerated uncoating of the N-tropic murine leukemia virus capsid. J. Virol. 81:2138-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponting, C., J. Schultz, and P. Bork. 1997. SPRY domains in ryanodine receptors (Ca(2+)-release channels). Trends Biochem. Sci. 22:193-194. [DOI] [PubMed] [Google Scholar]
- 26.Price, A. J., F. Marzetta, M. Lammers, L. M. Ylinen, T. Schaller, S. J. Wilson, G. J. Towers, and L. C. James. 2009. Active site remodeling switches HIV specificity of antiretroviral TRIMCyp. Nat. Struct. Mol. Biol. 16:1036-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ptak, R. G., P. A. Gallay, D. Jochmans, A. P. Halestrap, U. T. Ruegg, L. A. Pallansch, M. D. Bobardt, M. P. de Bethune, J. Neyts, E. De Clercq, J. M. Dumont, P. Scalfaro, K. Besseghir, R. M. Wenger, and B. Rosenwirth. 2008. Inhibition of human immunodeficiency virus type 1 replication in human cells by Debio-025, a novel cyclophilin binding agent. Antimicrob. Agents Chemother. 52:1302-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenwirth, B., A. Billich, R. Datema, P. Donatsch, F. Hammerschmid, R. Harrison, P. Hiestand, H. Jaksche, P. Mayer, and P. Peichl. 1994. Inhibition of human immunodeficiency virus type 1 replication by SDZ NIM 811, a nonimmunosuppressive cyclosporine analog. Antimicrob. Agents Chemother. 38:1763-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sayah, D. M., E. Sokolskaja, L. Berthoux, and J. Luban. 2004. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature 430:569-573. [DOI] [PubMed] [Google Scholar]
- 30.Sebastian, S., and J. Luban. 2005. TRIM5alpha selectively binds a restriction-sensitive retroviral capsid. Retrovirology 2:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokolskaja, E., D. M. Sayah, and J. Luban. 2004. Target cell cyclophilin A modulates human immunodeficiency virus type 1 infectivity. J. Virol. 78:12800-12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848-853. [DOI] [PubMed] [Google Scholar]
- 33.Stremlau, M., M. Perron, M. Lee, Y. Li, B. Song, H. Javanbakht, F. Diaz-Griffero, D. J. Anderson, W. I. Sundquist, and J. Sodroski. 2006. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc. Natl. Acad. Sci. U. S. A. 103:5514-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thali, M., A. Bukovsky, E. Kondo, B. Rosenwirth, C. T. Walsh, J. Sodroski, and H. G. Gottlinger. 1994. Functional association of cyclophilin A with HIV-1 virions. Nature 372:363-365. [DOI] [PubMed] [Google Scholar]
- 35.Virgen, C. A., Z. Kratovac, P. D. Bieniasz, and T. Hatziioannou. 2008. Independent genesis of chimeric TRIM5-cyclophilin proteins in two primate species. Proc. Natl. Acad. Sci. U. S. A. 105:3563-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willett, B. J., J. N. Flynn, and M. J. Hosie. 1997. FIV infection of the domestic cat: an animal model for AIDS. Immunol. Today 18:182-189. [DOI] [PubMed] [Google Scholar]
- 37.Wilson, S. J., B. L. Webb, L. M. Ylinen, E. Verschoor, J. L. Heeney, and G. J. Towers. 2008. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc. Natl. Acad. Sci. U. S. A. 105:3557-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, X., J. L. Anderson, E. M. Campbell, A. M. Joseph, and T. J. Hope. 2006. Proteasome inhibitors uncouple rhesus TRIM5alpha restriction of HIV-1 reverse transcription and infection. Proc. Natl. Acad. Sci. U. S. A. 103:7465-7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamauchi, K., K. Wada, K. Tanji, M. Tanaka, and T. Kamitani. 2008. Ubiquitination of E3 ubiquitin ligase TRIM5 alpha and its potential role. FEBS J. 275:1540-1555. [DOI] [PubMed] [Google Scholar]
- 40.Yoo, S., D. G. Myszka, C. Yeh, M. McMurray, C. P. Hill, and W. I. Sundquist. 1997. Molecular recognition in the HIV-1 capsid/cyclophilin A complex. J. Mol. Biol. 269:780-795. [DOI] [PubMed] [Google Scholar]