Abstract
Regulatory CD4+ T cells have been shown to be important in limiting immune responses, but their role in respiratory viral infections has received little attention. Here we observed that following respiratory syncytial virus (RSV) infection, CD4+ Foxp3+ CD25+ natural regulatory T-cell numbers increased in the bronchoalveolar lavage fluid, lung, mediastinal lymph nodes, and spleen. The depletion of CD25+ natural regulatory T cells prior to RSV infection led to enhanced weight loss with delayed recovery that was surprisingly accompanied by increased numbers of activated natural killer cells in the lung and bronchoalveolar lavage fluid on day 8 postinfection. Increased numbers of neutrophils were also detected within the bronchoalveolar lavage fluid and correlated with elevated levels of myeloperoxidase as well as interleukin-6 (IL-6) and gamma interferon (IFN-γ). CD25+ natural regulatory T-cell depletion also led to enhanced numbers of proinflammatory T cells producing IFN-γ and tumor necrosis factor alpha (TNF-α) in the lung. Despite these increases in inflammatory responses and disease severity, the viral load was unaltered. This work highlights a critical role for natural regulatory T cells in regulating the adaptive and innate immune responses during the later stages of lung viral infections.
Regulatory T cells (Tregs) are a subset of CD4+ T cells that are capable of regulating and suppressing the immune system (15). A number of Treg subsets have now been described, including naturally occurring Tregs (nTregs), which develop in the thymus, and inducible Tregs, which are induced in the periphery following encounters with antigen-loaded dendritic cells. The precise inhibitory mechanisms used by Tregs are not fully elucidated but can involve direct cell-cell contact or the secretion of various cytokines such as interleukin-10 (IL-10) and transforming growth factor β (TGF-β) (24).
The importance of Tregs in autoimmunity, allergy, and, more recently, bacterial and viral infections has been demonstrated (2, 10). Tregs can be both beneficial and detrimental to the host during infection, controlling excessive host immune responses, e.g., herpes simplex virus (14), but potentially enhancing pathogen survival and in some cases allowing the long-term persistence of a pathogen, e.g., Plasmodium yoelii (8). However, far less is known about the role that nTregs play during acute viral lung infections.
Respiratory syncytial virus (RSV) is the most important cause of acute respiratory tract viral infections in infants and the leading cause of viral bronchiolitis and infantile hospitalizations in the developed world (22). RSV disease is caused in part by a large inflammatory infiltrate into the lungs that is comprised of both natural killer (NK) cells and T cells. In mice, RSV infection leads to the early recruitment of NK cells into the lungs and airways during the first few days (9). This is followed by the recruitment of CD4 and CD8 effector T cells, the levels of which peak between days 7 and 10 postinfection (p.i.) (18, 23). However, the role of nTregs has yet to be fully explored. Using the CB6F1 hybrid mouse model for RSV infection, Ruckwardt et al. showed previously that Treg depletion delays CD8+ T cell responses in the lung and can modulate the disparities between dominant and subdominant epitopes (20).
Here we demonstrate that RSV infection leads to increased nTreg numbers in the lung, bronchoalveolar lavage (BAL) fluid, mediastinal lymph node (MLN), and spleen. The depletion of CD25+ nTregs resulted in enhanced disease severity that was characterized by increases in weight loss, the recruitment of innate cells to the bronchoalveolar lavage (BAL) fluid and lung, and increased levels of CD4+ and CD8+ T cells producing gamma interferon (IFN-γ). Despite this enhanced immune response, Treg depletion did not affect viral load in the lungs, and although recovery was delayed, it was not prevented. These data indicate that nTregs play a critical role in regulating the adaptive and innate immune responses to acute infection and in resolving inflammation following viral clearance.
MATERIALS AND METHODS
Mice and virus stocks.
Eight-week-old female BALB/c mice (Charles River Laboratories, Inc., United Kingdom) were maintained under pathogen-free conditions in accordance with institutional and United Kingdom Home Office guidelines. Plaque-purified human RSV (type A2 strain from the ATCC) was grown in HEp-2 cells with RPMI 1640 medium supplemented with 2% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Mouse infection and treatment.
Mice were infected intranasally (i.n.) with 100 μl of 6 × 105 PFU of RSV while under light anesthesia. Following infection, weight change was monitored daily. For Treg depletion, mice were treated with 250 μl of 1 mg/ml anti-CD25 (clone PC61) antibody, isotype control antibody (clone GL113) (kind gifts from A. Gallimore, Cardiff University), or phosphate-buffered saline (PBS) via intraperitoneal (i.p.) injection on days −3 and −1 prior to infection.
Cell collection and preparation.
After infection, animals were sacrificed by i.p. pentobarbitone injection. BAL was performed by the inflation of the lungs via the trachea three times with 1 ml of 12 mM lidocaine in Eagle's minimum essential medium. An aliquot of BAL fluid cells was transferred onto a microscope slide (Thermo Scientific, United Kingdom) using a cytospin centrifuge and stained with hematoxylin and eosin (H&E) (Reagena, Gamidor, United Kingdom) for cellular differentiation, and the rest of the cells were prepared for flow cytometry. BAL fluid cell-free supernatant was retained for enzyme-linked immunosorbent assay (ELISA). One half of the lung tissue was chopped finely using blades and digested in collagenase (50 μg/ml) for 30 min at 37°C, and red blood cell lysis was performed by using ACK lysing buffer (0.15 M NH4Cl, 1 mM KHCO3, 1 mM Na2 EDTA [pH 7.2]) for 5 min at room temperature. Total cells were quantified and prepared for flow cytometry. The second half of the lung tissue was homogenized and used for an RSV infectious focus assay and for quantitative PCR (qPCR) for the RSV L gene to measure viral load.
Flow cytometric analysis.
Prepared cells were blocked with Fc block (anti-CD16/32; BD, United Kingdom), and cell surface staining was performed on live cells resuspended in PBS containing 1% bovine serum albumin, 0.1% azide, and 5 μM EDTA (flow buffer). For intracellular Foxp3 staining, cells were fixed and permeabilized according to the manufacturer's instructions (eBioscience, United Kingdom). For intracellular IFN-γ and tumor necrosis factor alpha (TNF-α) analysis, cells were stimulated for 4 h with phorbol 12-myristate 13-acetate (PMA) (50 mg/ml) and ionomycin (500 ng/ml) (Sigma, United Kingdom) or the M2 peptide (Pro-Immune, United Kingdom) and interleukin-2 (IL-2) (50 U/ml) (R&D Systems, United Kingdom), as described previously (3), in the presence of GolgiPlug (BD, United Kingdom). The following antibodies were used and purchased from BD (United Kingdom), unless otherwise stated: anti-CD3-phycoerythin (PE)-Cy7, anti-CD4-peridinin chlorophyll protein (PerCP), anti-CD8-Pacific blue, anti-CD25-PE, anti-DX5-PE, anti-CD69-fluorescein isothiocyanate (FITC), anti-TNF-α-PE, anti-IFN-γ-FITC, and anti-Foxp3-allophycocyanin (APC) (eBioscience, United Kingdom). Cells were run on a Cyan ADP LX 9 color flow cytometer (Dako, United Kingdom), and data were analyzed by using the Dako Summit analysis program.
Cytokine ELISA.
Cytokines levels were quantified by ELISA according to the manufacturer's instructions. IL-6 was assessed by using antibody pairs from R&D Systems (United Kingdom), myeloperoxidase (MPO) was assessed by using a mouse MPO ELISA kit containing precoated plates from Hycult Biotechnology, and IFN-γ was assessed by using paired antibodies from BD (United Kingdom). Briefly, Immunosorb ELISA plates (Nunc, United Kingdom) were coated with capture antibody overnight at 4°C. Wells were washed and blocked with 1% bovine serum albumin in PBS for 1 h at room temperature. A sample or standard was added for 2 h at 37°C, and bound cytokine was detected by using biotinylated anti-cytokine antibody, avidin horseradish peroxidase, and tetramethylbenzidine. Color development was stopped with 2 M H2SO4, and optical densities were read at 450 nm. The concentration of the cytokine was determined from the standard curve.
Quantification of viral RNA.
Total RNA was extracted from lung tissue by using RNA STAT-60 (AMS Biotechnology Ltd., United Kingdom), and cDNA was generated by using random hexamers and Ominscript reverse transcriptase (Qiagen, United Kingdom). qPCR was performed for the RSV L gene using 900 nM forward primer (5′-GAACTCAGTGTAGGTAGAATGTTTGCA-3′), 300 nM reverse primer (5′-TTCAGCTATCATTTTCTCTGCCAAT-3′), and 175 nM probe (5′-6-carboxyfluorescein-TTTGAACCTGTCTGAACATTCCCGGTT-6-carboxytetramethylrhodamine-3′) with an ABI Prism 7500 sequence detection system as previously described (5). RSV L gene copies were normalized to the 18S rRNA housekeeping gene.
Statistical analysis.
Results are expressed as means ± standard errors of the means (SEM). Statistical analysis was performed by using analysis of variance (ANOVA) followed by the Bonferroni test if P values were significantly different using the Graphpad Prism software. Significance was noted when the P value was <0.05.
RESULTS
Primary RSV infection leads to an increase in nTregs.
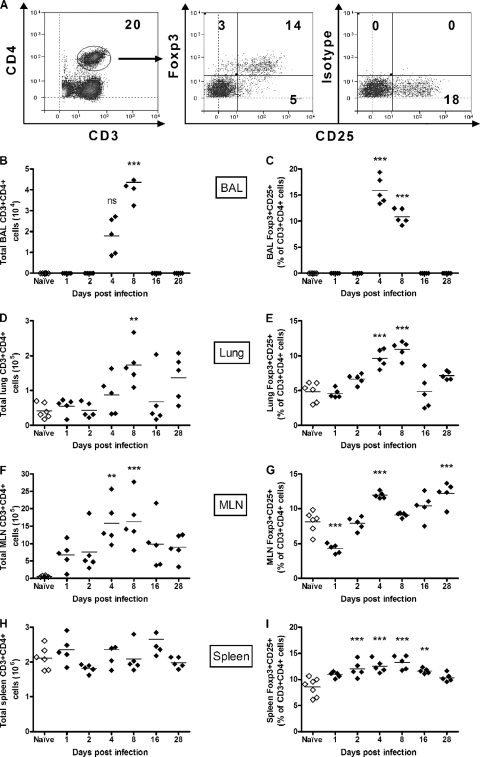
BALB/c mice were infected intranasally (i.n.) with 6 × 105 PFU of RSV, and weight was monitored daily. Mice lost weight from day 4 postinfection (p.i.), with the peak of weight loss being detected at day 6 p.i. (see Fig. S1A in the supplemental material). The percentage of CD3+ CD4+ Foxp3+ CD25+ nTregs in the bronchoalveolar lavage (BAL) fluid, lungs, mediastinal lymph node (MLN), and spleen was determined by flow cytometry with naïve mice at days 1, 2, 4, 8, 16, and 28 p.i. (Fig. 1 A). Following infection, both total CD3+ CD4+ T cells and CD4+ Foxp3+ CD25+ nTregs were detected in the BAL fluid on days 4 and 8 p.i., with no CD4+ Foxp3+ CD25+ nTregs being detected in the BAL fluid of naïve mice (Fig. 1B and C). This corresponded to a significant increase in the number of cells detected in the BAL fluid from day 4 onwards (Fig. S1B). In lungs of naïve mice, CD4+ Foxp3+ CD25+ nTregs comprised 4 to 5% of CD3+ CD4+ T cells, in line with previous findings for peripheral nTreg proportions (21). Infection with RSV led to a gradual increase in the percentage of lung CD4+ Foxp3+ CD25+ nTregs from day 2 p.i. onward, peaking at day 8 p.i. (Fig. 1E). This correlated with a rise in CD3+ CD4+ T cell numbers in the lung starting at day 4 p.i. (Fig. 1D) as well as a general influx of cells into the lung (Fig. S1C). As with the lungs, there were increased numbers of CD3+ CD4+ T cells detected in the MLN following infection (Fig. 1F). The peak of CD4+ Foxp3+ CD25+ nTregs in the MLN occurred earlier (day 4 p.i.), prior to the peak seen for the lung (Fig. 1G). Interestingly, on day 28 p.i., there was a second significant peak in the percentage of CD4+ Foxp3+ CD25+ nTregs in the MLN. For the spleen, we did not detect any differences in the total numbers of CD3+ CD4+ cells (Fig. 1H), although there was a significant increase in the percentage of CD4+ Foxp3+ CD25+ nTregs at days 2, 4, 8, and 16 p.i. (Fig. 1I). The percentage of CD4+ Foxp3+ CD25− cells showed a pattern similar to that of CD4+ Foxp3+ CD25+ nTregs in each tissue throughout the infection. However, the percentage of this population remained at a low level and below 4% for each of the tissues studied, except for the BAL fluid at days 4 and 8 p.i. (Fig. S2A to S2D). From this, we observed that primary RSV infection leads to increased frequencies of CD4+ Foxp3+ CD25+ nTregs in the BAL fluid, lungs, MLN, and spleen.
FIG. 1.
Identification and enumeration of nTregs during RSV infection. Adult BALB/c mice were infected i.n. with RSV or left naïve. At the indicated time points p.i., mice were sacrificed, and BAL fluid, lung, MLN, and spleen cells were stained for CD3, CD4, CD25, and Foxp3 and analyzed by flow cytometry. (A) Representative plots show CD4+ T cells, Foxp3+ CD25+ nTregs, and the isotype control staining for Foxp3 in the lung at day 8 p.i. (B to I) The total numbers of CD4+ T cells and the percentages of Foxp3+ CD25+ nTregs were measured in the BAL fluid (B and C), the lung (D and E), the MLN (F and G), and the spleen (H and I). Symbols represent individual animals, and the horizontal bars represent the means (n ≥ 5). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (for RSV-infected compared with naïve control mice). The graph represents data from one of two similar independent experiments. ns, not significant.
Anti-CD25 antibody treatment prior to infection results in enhanced RSV disease and the recruitment of innate effector cells.
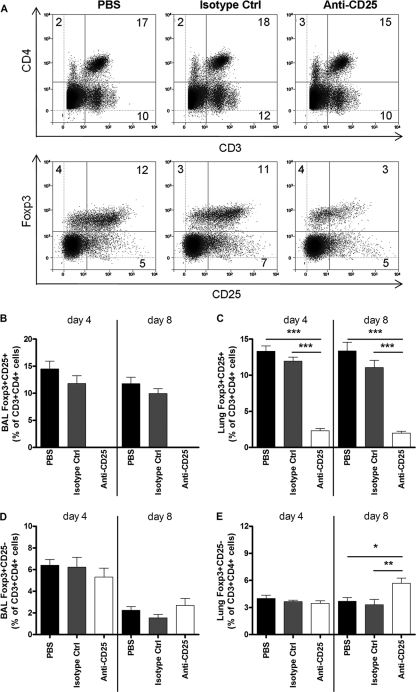
To determine the effects of depleting CD4+ Foxp3+ CD25+ nTregs prior to RSV infection, BALB/c mice were treated with either anti-CD25 antibody (PC61), isotype control antibody (GL113), or PBS 3 days and 1 day prior to RSV infection. This treatment resulted in the depletion of 73 to 75% of CD4+ Foxp3+ CD25+ nTregs from the lungs and complete depletion from the BAL fluid (Fig. 2 A, B, and C). Treatment with anti-CD25 antibody did not affect the percentage of CD4+ Foxp3+ CD25− cells detected at day 4 p.i. in the BAL fluid or lung compared to control groups (Fig. 2D). However, at day 8 p.i. the percentage of CD4+ Foxp3+ CD25− cells in the anti-CD25-treated group was significantly higher for the lung but not the BAL fluid (Fig. 2E).
FIG. 2.
Effective nTreg depletion using anti-CD25 antibody. Adult BALB/c mice were injected i.p. with PBS, isotype control antibody, or anti-CD25 antibody on days −3 and −1 prior to RSV infection. Twenty-four hours after the second injection, mice were infected i.n. with 6 × 105 PFU of RSV. Mice were sacrificed on day 4 or 8 p.i., and cells were stained as described in the legend of Fig. 1. (A) Representative plots show lung CD4+ T cells and Foxp3+ CD25+ nTregs from each treatment group on day 4 p.i. (B to E) The percentages of CD4+ Foxp3+ CD25+ nTregs and CD4+ Foxp3+ CD25− cells in the BAL fluid (B and D) and the lung (C and E) were determined. Bars represent means ± SEM (n = 5). *, P < 0.05; **, P < 0.01 (for the anti-CD25-depleted group compared to the PBS and isotype control groups). The graphs represent data from one of two similar independent experiments.
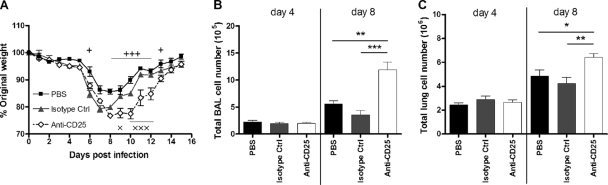
All infected mice started to lose weight from day 6 p.i., but weight loss was greatest for the anti-CD25-treated group, peaking at over 20% of the original body weight on day 8 p.i. Perhaps more striking was the sustained weight loss at over 20% for 3 days in the anti-CD25 treated group compared to the control groups, which began to recover the day after (Fig. 3 A). The depletion of CD4+ Foxp3+ CD25+ nTregs also led to a significant increase in the number of viable cells detected in the BAL fluid and the lungs compared to controls on day 8 but not day 4 p.i. (Fig. 3B and C).
FIG. 3.
Enhanced RSV disease following nTreg depletion. (A) Following treatment with PBS, isotype control antibody, or anti-CD25 antibody prior to RSV infection, weight change was monitored daily and plotted as a percentage of the weight on the day of infection. (B and C) The total viable BAL fluid (B) and lung (C) cell numbers were enumerated by using light microscopy. Symbols and bars represent means ± SEM (n = 5). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (for the anti-CD25-depleted group compared to the PBS and isotype control groups). +, P < 0.05; +++, P < 0.001 (for PBS versus anti-CD25 depletion). ×, P < 0.05; ×××, P < 0.001 (for isotype control versus anti-CD25 depletion). The graphs represent data from one of two similar independent experiments.
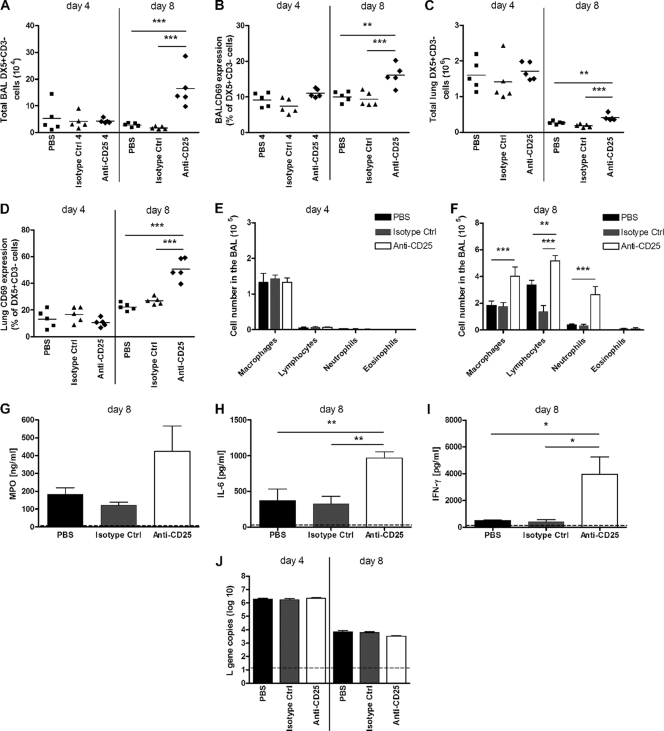
Having demonstrated that nTregs are important in limiting RSV-induced disease, we investigated their influence on the recruitment of innate effector cells. It was previously demonstrated that NK cells and neutrophils are recruited during RSV infection in both humans (16) and mice (18). During a primary RSV infection in mice, the peak of NK cells occurs on day 4 p.i. in the lung before declining (18). Following treatment with anti-CD25 antibody, the numbers of NK cells detected on day 4 p.i. were similar for all groups for both the BAL fluid and lung. However, surprisingly, at day 8 p.i. treatment with anti-CD25 antibody led to a significant increase in the number of NK cells detected within the BAL fluid and lung (Fig. 4 A and C). Moreover, the NK cells detected on day 8 in the CD25-depleted group were found to be more activated in the BAL fluid and lung, as indicated by an increased percentage of CD69 expression compared to NK cells in the control groups. This is in contrast to the NK cells detected on day 4 p.i., where CD69 expression levels were similar between the groups (Fig. 4B and D). In addition, on day 8 p.i. there was also a 5-fold increase in the number of neutrophils in the BAL fluid of CD25-depleted mice compared to controls (Fig. 4F). Neutrophils are usually detected during the early stages of infection on days 1 and 2 (see Fig. S1D in the supplemental material). RSV-infected non-CD25-depleted mice had very low numbers of neutrophils on day 4 (Fig. 4E) and day 8 (Fig. 4F) p.i. Furthermore, levels of macrophages and lymphocytes in the BAL fluid were also increased on day 8 p.i. in CD25-depleted mice but to a lesser degree, and there was no effect on eosinophil recruitment (Fig. 4F). In the BAL fluid of naïve mice that were CD25 depleted, only macrophages were detected (data not shown). The enhanced numbers of NK cells and neutrophils were accompanied by elevated levels of myeloperoxidase (MPO), the proinflammatory cytokine IL-6, and IFN-γ in the BAL fluid on day 8 p.i. (Fig. 4G, H, and I). However, despite the presence of elevated numbers of innate antiviral immune cells, nTreg depletion did not alter the viral load on day 4 or 8 p.i., as measured by qPCR (Fig. 4J) and an immunoplaque assay (data not shown). It therefore appears that nTregs limit weight loss and innate effector cell recruitment to the lung following RSV infection without affecting the control of viral replication.
FIG. 4.
nTreg depletion leads to sustained innate immune responses. Adult BALB/c mice received anti-CD25 antibody, isotype control antibody, or PBS prior to RSV infection. (A to D) Mice were sacrificed on days 4 and 8 p.i., and lung cells were analyzed by flow cytometry for NK cell (CD3− DX5+) numbers and activation (CD69+) in the BAL fluid (A and B) and lung (C and D). (E and F) BAL fluid cells were taken, and H&E staining was performed to carry out differential cell counts for macrophages, lymphocytes, neutrophils, and eosinophils on days 4 (E) and 8 (F) p.i. (G to I) Levels of MPO (G), IL-6 (H), and IFN-γ (I) in the BAL fluid were measured by ELISA on day 8 p.i. (J) RSV L gene copy numbers were determined by qPCR of lung tissue on days 4 and 8 p.i. The dotted line represents the detection limit of the assays. Symbols represent individual animals, and the horizontal bars represent the means. Bars represent means ± SEM (n = 5). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (for PBS- and isotype control-treated groups compared to the anti-CD25-depleted group). The graphs represent data from one of two similar independent experiments.
Depletion of nTregs prior to RSV infection leads to increased adaptive T cell responses in the lung.
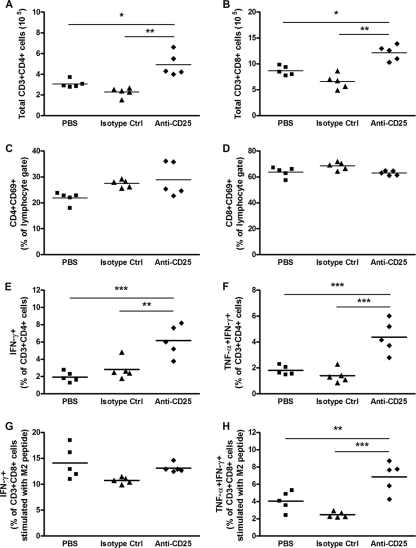
Previous studies using murine models of primary RSV infection showed that T cell numbers increase from day 4 p.i. and peak at around day 7 or 8 p.i. (18). Following anti-CD25 antibody treatment, the numbers of both CD4+ and CD8+ T cells in the lung rose significantly on day 8 p.i. compared to control groups (Fig. 5 A and B). The percentages of activated CD4+ and CD8+ T cells, as measured by CD69 expression, were similar for all three groups, indicating that cell activity was not altered by anti-CD25 antibody treatment (Fig. 5C and D). Despite comparable levels of activation, ex vivo stimulation of lung cells with PMA and ionomycin resulted in more CD4+ and CD8+ T cells producing IFN-γ in the CD25-depleted group than in controls (Fig. 5E, and see Fig. S3B and S3C in the supplemental material). Furthermore, following ex vivo stimulation, there were more CD4+ T cells in the CD25-depleted mice that produced both IFN-γ and TNF-α (Fig. 5F and Fig. S3B), while no difference was seen in CD8+ T cells (Fig. S3B and S3D). There was also no difference in the percentage of CD4+ or CD8+ T cells producing TNF-α alone (data not shown). To assess the RSV-specific response, lung cells were stimulated using the immunodominant RSV-M2 major histocompatibility complex (MHC) class I peptide (SYIGSINNI) in combination with IL-2, as described previously (3). CD8+ T cells from anti-CD25-depleted mice stimulated with this peptide coproduced significantly higher levels of TNF-α and IFN-γ (Fig. 5H), while no change was detected in the percentages of CD8+ T cells producing IFN-γ alone (Fig. 5G) or TNF-α alone (data not shown). Therefore, the depletion of CD4+ Foxp3+ CD25+ nTregs increases the recruitment of proinflammatory T cells to the lung following RSV infection.
FIG. 5.
Increased numbers of proinflammatory T cells following nTreg depletion. Adult BALB/c mice were given anti-CD25-depleting antibody, isotype control antibody, or PBS prior to RSV infection. Total lung cells were isolated at day 8 p.i. and analyzed by flow cytometry. (A to D) CD4 (CD3+ CD4+) (A) and CD8 (CD3+ CD8+) (B) T-cell numbers and activation (CD69+) (C and D) were determined. Lung cells were stimulated ex vivo for 4 h with PMA and ionomycin, or the RSV-specific M2 peptide and IL-2. Intracellular cytokine staining for IFN-γ and TNF-α was performed. (E and F) CD4 T cells producing IFN-γ alone (E) or coproducing TNF-α and IFN-γ (F) following PMA and ionomycin stimulation. (G and H) CD8 T cells producing IFN-γ alone (G) or coproducing TNF-α and IFN-γ (H) following RSV-specific M2 peptide stimulation. Symbols represent individual animals, and the horizontal bars represent the means (n ≥ 5). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (for PBS- and isotype control-treated groups compared to the anti-CD25-depleted group). The graphs represent data from one of two similar independent experiments.
DISCUSSION
While the role of effector T cells during RSV infection has been well studied, less is known about the role of regulatory T cells. We initially showed that during the course of a primary RSV infection, numbers of CD4+ Foxp3+ CD25+ nTregs increased in the BAL fluid, MLN, lung, and spleen, with peaks on day 4 p.i. in the BAL fluid and MLN, on day 8 in the lung, and from days 2 to 8 p.i. in the spleen. These results confirm previous findings by Ruckwardt et al. albeit with some differences. In their model, the peak of CD4+ Foxp3+ CD25+ nTreg accumulation appeared on day 6 p.i. in the lung, with only slight increases in nTreg numbers in the MLN and no increase detected in the spleen (20). Furthermore, we have shown for the first time the dynamic changes of CD4+ Foxp3+ CD25+ nTregs that occur in the BAL fluid during RSV infection.
To study the role of CD4+ Foxp3+ CD25+ nTregs in RSV infection, we effectively depleted these cells by anti-CD25 antibody treatment prior to infection. Interestingly, depletion using this antibody appeared to be more effective at removing CD4+ Foxp3+ CD25+ nTregs in the BAL fluid than in the lung. This may be due to the fact that there are fewer cells in the BAL fluid overall and, therefore, fewer CD4+ Foxp3+ CD25+ nTregs to remove. Depletion resulted in increased weight loss and delayed recovery. Weight loss was accompanied by increases in numbers of innate immune cells in the BAL fluid and lung on day 8 p.i. This novel finding indicates that CD4+ Foxp3+ CD25+ nTregs play a critical role in limiting either the recruitment, proliferation, or clearance of innate cells from the lung following RSV infection. Several studies have indeed demonstrated that Tregs are able to inhibit neutrophils and NK cells (6, 13). Therefore, a lack of Tregs in the lung could lead to a reduced apoptosis of newly activated neutrophils, and this may have led to their continued presence and accumulation in the BAL fluid and lung on day 8 p.i. Several clinical studies have demonstrated that neutrophils are the predominant cell type found in BAL fluid from infants hospitalized for severe RSV bronchiolitis (16). In addition, we have previously shown that NK cells can also contribute to RSV disease (7). It is possible that the severity of RSV disease seen in these infants is due to an imbalance or reduction in Treg numbers or function, which leads to an inability to regulate the innate immune response during infection.
The enhanced RSV disease in the absence of CD4+ Foxp3+ CD25+ nTregs could also be attributed to increases in both CD4 and CD8 T cell responses in the lung, since Tregs are known to control effector T cell proliferation (17). Interestingly, in the absence of CD4+ Foxp3+ CD25+ nTregs, there were increases in numbers of CD4 and CD8 T cells producing IFN-γ and, possibly more importantly, in numbers of CD4 T cells coproducing TNF-α and IFN-γ. Furthermore, RSV-specific CD8 T cell responses with the coproduction of TNF-α and IFN-γ, induced by restimulation with the RSV-M2 peptide, were also increased in the absence of nTregs. Taken together, our findings indicate that CD4+ Foxp3+ CD25+ nTregs control antiviral, presumably proinflammatory, CD4 and CD8 T cell responses. Our observations differ to some degree from results of studies by Ruckwardt et al., who found an increased RSV-specific production of IFN-γ but no coproduction of IFN-γ and TNF-α in CD8 T cells from CD25-depleted RSV-infected mice (20). These differences may result from the use of different mouse strains and different CD25 depletion regimens. An increased recruitment of both RSV-specific CD8 and CD4 T cells has been associated with increased disease severity (1, 23); however, their role in human disease is controversial (4, 25). It is likely, in the context of a primary RSV infection, that the lack of nTregs does play some role in the increased pathology observed.
Given the increased immune and inflammatory responses, we predicted that the viral load would be reduced following Treg depletion. However, the copy numbers of the RSV L gene on day 4, the peak of RSV replication, and day 8 p.i. in the lung remained unchanged. This is comparable with data reported previously by Ruckwardt et al., who found no difference at day 4, 5, or 8 p.i. but did observe an increase in the viral load at days 6 and 7 p.i. in CD25-depleted mice (20). Similar to our findings, Tregs do not seem to affect bacterial load either, as has been shown previously with models of lung infections with Mycobacterium tuberculosis or Mycobacterium bovis bacillus Calmette-Guérin (19). Together with our findings discussed above, these observations indicate that nTregs specifically regulate inflammatory immune responses in RSV infection without inhibiting antiviral responses involved in the control of RSV replication and its clearance.
Our study approach may have limitations in that the anti-CD25 antibody treatment used has the potential to deplete newly activated effector T cells, which upregulate CD25 as well as Foxp3+ CD25+ Tregs. However, by depleting naïve mice prior to infection, we aimed to specifically target nTregs. Supporting this, we found that levels of the early activation marker CD69 remained similar on both CD4 and CD8 T cells from CD25-depleted mice and controls. Importantly, CD4 and CD8 T cell numbers were increased in CD25-depleted mice, as was their effector T cell function, such as the production of IFN-γ. The recent development of “knock-in” mice expressing a diphtheria toxin receptor and green fluorescent protein, under the control of the Foxp3 locus, allows the specific, inducible depletion of Foxp3+ cells (11, 12) and may prove useful in further refining our understanding of Treg functions in respiratory viral infections.
In summary, we have shown that the local CD4+ Foxp3+ CD25+ nTreg population, from draining lymph nodes to bronchoalveolar lavage fluid, expands following RSV infection of the lung. These CD4+ Foxp3+ CD25+ nTregs appear to dampen both innate and adaptive immune responses, and in their absence, immunopathology and RSV disease are enhanced, and recovery is delayed. This highlights the importance of CD4+ Foxp3+ CD25+ nTregs in regulating virus-induced lung inflammation, and through studying regulatory mechanisms further, we may better understand why some infants suffer severe, prolonged illness with secondary complications, while the majority recover rapidly from RSV infection. It may be that exploiting this pathway could allow us to develop new therapeutic strategies targeted at reducing immune-mediated damage of the lungs.
Supplementary Material
Acknowledgments
We thank Awen Gallimore for providing the hybridomas for the anti-CD25 and isotype control antibodies and Rebecca Beavil at the MRC/AUK Centre for Allergic Mechanisms in Asthma Protein Facility for producing the antibody.
This work was supported by Wellcome Trust Programme grant 087805/Z/08/Z (United Kingdom).
Footnotes
Published ahead of print on 23 June 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Alwan, W. H., W. J. Kozlowska, and P. J. M. Openshaw. 1994. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med. 179:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkaid, Y. 2007. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 7:875-888. [DOI] [PubMed] [Google Scholar]
- 3.Chang, J., and T. J. Braciale. 2002. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat. Med. 8:54-60. [DOI] [PubMed] [Google Scholar]
- 4.Collins, P. L., and B. S. Graham. 2008. Viral and host factors in human respiratory syncytial virus pathogenesis. J. Virol. 82:2040-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culley, F. J., J. Pollott, and P. J. Openshaw. 2002. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J. Exp. Med. 196:1381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghiringhelli, F., C. Menard, M. Terme, C. Flament, J. Taieb, N. Chaput, P. E. Puig, S. Novault, B. Escudier, E. Vivier, A. Lecesne, C. Robert, J. Y. Blay, J. Bernard, S. Caillat-Zucman, A. Freitas, T. Tursz, O. Wagner-Ballon, C. Capron, W. Vainchencker, F. Martin, and L. Zitvogel. 2005. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 202:1075-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harker, J. A., A. Godlee, J. L. Wahlsten, D. C. Lee, L. G. Thorne, D. Sawant, J. S. Tregoning, R. R. Caspi, A. Bukreyev, P. L. Collins, and P. J. Openshaw. 2010. Interleukin 18 coexpression during respiratory syncytial virus infection results in enhanced disease mediated by natural killer cells. J. Virol. 84:4073-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hisaeda, H., Y. Maekawa, D. Iwakawa, H. Okada, K. Himeno, K. Kishihara, S. Tsukumo, and K. Yasutomo. 2004. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat. Med. 10:29-30. [DOI] [PubMed] [Google Scholar]
- 9.Hussell, T., and P. J. M. Openshaw. 1998. Intracellular interferon-gamma expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J. Gen. Virol. 79:2593-2601. [DOI] [PubMed] [Google Scholar]
- 10.Kearley, J., J. E. Barker, D. S. Robinson, and C. M. Lloyd. 2005. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 202:1539-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, J. M., J. P. Rasmussen, and A. Y. Rudensky. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8:191-197. [DOI] [PubMed] [Google Scholar]
- 12.Lahl, K., C. Loddenkemper, C. Drouin, J. Freyer, J. Arnason, G. Eberl, A. Hamann, H. Wagner, J. Huehn, and T. Sparwasser. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204:57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewkowicz, P., N. Lewkowicz, A. Sasiak, and H. Tchorzewski. 2006. Lipopolysaccharide-activated CD4+CD25+ T regulatory cells inhibit neutrophil function and promote their apoptosis and death. J. Immunol. 177:7155-7163. [DOI] [PubMed] [Google Scholar]
- 14.Lund, J. M., L. Hsing, T. T. Pham, and A. Y. Rudensky. 2008. Coordination of early protective immunity to viral infection by regulatory T cells. Science 320:1220-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maloy, K. J., and F. Powrie. 2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816-822. [DOI] [PubMed] [Google Scholar]
- 16.McNamara, P. S., P. Ritson, A. Selby, C. A. Hart, and R. L. Smyth. 2003. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch. Dis. Child. 88:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piccirillo, C. A., and E. M. Shevach. 2001. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J. Immunol. 167:1137-1140. [DOI] [PubMed] [Google Scholar]
- 18.Pribul, P. K., J. Harker, B. Wang, H. Wang, J. S. Tregoning, J. Schwarze, and P. J. Openshaw. 2008. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J. Virol. 82:4441-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn, K. M., R. S. McHugh, F. J. Rich, L. M. Goldsack, G. W. de Lisle, B. M. Buddle, B. Delahunt, and J. R. Kirman. 2006. Inactivation of CD4+ CD25+ regulatory T cells during early mycobacterial infection increases cytokine production but does not affect pathogen load. Immunol. Cell Biol. 84:467-474. [DOI] [PubMed] [Google Scholar]
- 20.Ruckwardt, T. J., K. L. Bonaparte, M. C. Nason, and B. S. Graham. 2009. Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities. J. Virol. 83:3019-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornton, A. M., and E. M. Shevach. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tregoning, J. S., and J. Schwarze. 2010. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 23:74-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tregoning, J. S., Y. Yamaguchi, J. Harker, B. Wang, and P. J. Openshaw. 2008. The role of T cells in the enhancement of RSV infection severity during adult reinfection of neonatally sensitized mice. J. Virol. 82:4115-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vignali, D. A., L. W. Collison, and C. J. Workman. 2008. How regulatory T cells work. Nat. Rev. Immunol. 8:523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welliver, T. P., R. P. Garofalo, Y. Hosakote, K. H. Hintz, L. Avendano, K. Sanchez, L. Velozo, H. Jafri, S. Chavez-Bueno, P. L. Ogra, L. McKinney, J. L. Reed, and R. C. Welliver, Sr. 2007. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 195:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.